Abstract
Walking pace is a simple and functional form of exercise and a strong predictor of health, but little is known about its causal association with rheumatoid arthritis. This study aimed to investigate the causal effect of WP on the developing RA using Mendelian randomization analysis. The genetic variation associated with WP was selected as an instrumental variable from the latest genome-wide association studies. Summary-level data for the outcomes were obtained from the corresponding GWAS. The inverse-variance weighted method was used as the primary MR analysis. The results were further tested using a multivariable MR approach based on Bayesian model averaging. Confounders (BMI, SMK, HBP, TD) with close associations with RA were included in the analysis. An observational study with individual data from UK Biobank was performed to reinforce our findings. The MR results indicated the significant inverse associations of WP with the risk of RA (odds ratio (OR), 0.31; 95% confidence interval (CI), 0.15, 0.62; p = 1.05 × 10 −3). After adjusting for the risk factors, the associations for WP and RA did not change substantially. Observational study results demonstrated the same effect of WP on reducing the risk of RA. The Mendelian randomization analysis and observational study provide evidence suggesting that walking pace is a protective factor for rheumatoid arthritis. Given its simple measurement, walking pace may be a pragmatic target for interventions.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by persistent inflammation of the synovial joints, leading to joint pain, swelling, and deformity1,2. It affects approximately 1% of the global population and is more prevalent in women than men3. RA is associated with significant disability, reduced quality of life, and increased mortality, making it a substantial burden on individuals and healthcare systems worldwide4. The pathogenesis of RA involves a complex interplay of genetic, environmental, and immunological factors5. Genetic predisposition, particularly the involvement of human leukocyte antigen (HLA) genes, plays a significant role in disease susceptibility. However, environmental factors, such as smoking, obesity, and lifestyle behaviors, also contribute to the development and progression of RA6,7,8.
Walking is a fundamental human movement and a common form of physical activity that is accessible to individuals of all ages and fitness levels9,10. Walking pace, defined as the speed at which an individual walks, has gained attention as a simple and objective measure of physical function and overall health11. It has been recognized as a valuable indicator of cardiovascular fitness, mortality risk, and various health outcomes12,13. Research has shown that walking pace is associated with a range of health measures and outcomes. For instance, a faster walking pace has been linked to better cardiovascular health, including lower blood pressure, improved lipid profiles, and reduced risk of cardiovascular events14,15. Additionally, walking pace is a predictor of overall mortality, with slower walking speeds associated with increased mortality risk16,17,18. While the association between walking pace and various health outcomes has been extensively studied, its relationship with specific diseases, such as rheumatoid arthritis (RA), remains an area of interest. Identifying modifiable lifestyle factors that may influence the risk of developing RA is important for preventive strategies and improving patient outcomes.
Mendelian randomization (MR) is a powerful analytical approach that utilizes genetic variants as instrumental variables (IV) to investigate causal relationships between modifiable exposures and disease outcomes. It leverages the random assortment of genetic variants during meiosis, making it less prone to confounding and reverse causation biases compared to traditional observational studies19,20,21. In this study, we aim to employ MR analysis to investigate the potential causal association between walking pace and the risk of developing RA. By utilizing genetic instrumental variables, we can provide robust evidence regarding the potential role of walking pace in RA development, helping inform preventive strategies and interventions for individuals at risk of this debilitating autoimmune disease.
Method
Study design
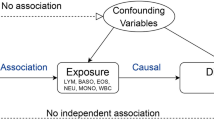
As shown in the flow chart in Fig. 1, We first extracted SNPs associated with WP from the GWAS data of 459,915 European individuals. In order to verify the correctness of the conclusion, another cohort with Ncase = 8255 and Ncontrol = 409,001 were chosen for repeat validation22. Then, using phenoscanner to identify and exclude confounding factors related to RA (p < 5 × 10−8), and selected the remaining SNPs that overlapped with RA-associated SNPs for the MR analysis23. Furthermore, to further validate the results of the MR analysis, we included other causal factors (BMI, SMK, HBP, TD) associated with RA and performed the MR-BMA to examine whether the causal relationship between WP and RA remained after adjusting for these factors24. Finally, we utilized individual data of WP and RA from the UK Biobank database, initially adjusting for gender and age, and subsequently further adjusting for the aforementioned risk factors to enhance the reliability of our findings.
Data source
The characteristics of the data sources in this study are presented in Supplementary Table S1. No additional ethical approval was required for the use of this database. The current study utilized publicly available summary-level data of WP obtained from the UK Biobank database of 459,915 European individuals. The replicated MR analysis were conducted by another cohort with Ncase = 8255 and Ncontrol = 409,001 obtained from Sakaue, et al.22. GWAS data of RA was obtained from a publicly available open-GWAS website (https://gwas.mrcieu.ac.uk/), which was from Finngen, comprising 153,457 participants of European ancestries. The data of confounders (BMI, SMK, HBP, TD) with close associations with RA were all obtained from UK Biobank databases, providing 1,845,984 participants of European ancestries.
Selection of genetic instruments
The necessary instrumental variables (IVs) were obtained through an initial screening of the related single nucleotide polymorphisms (SNPs). Genetic instruments were selected based on two criteria: (i) Pvalue of 5 × 10−8 and (ii) Clumping r2 of 0.001, and clumping distance cutoff < 10,000 kb. Phenoscanner were used to determine the trait of SNPs under the setting of Pvalue = 5 × 10−8, r2 = 0.8, and SNPs whose trait contain inflammation and inflammatory cells were removed. Moreover, the F-statistics for each SNP were calculated to assess the effect of intensity of the IVs, to minimize potential weak instrument bias, SNPs with F-statistics < 10 were excluded25. The screening flowsheet is shown in Fig. 1. The exposure and outcome data were harmonized to ensure that the effect of a SNP on the exposure and the effect of that SNP on the outcome correspond to the same allele. All information about harmonized SNPs is shown in Supplementary Table S2.
Unvariable MR estimates
The R software was utilized to estimate MR results through the application of “TwoSampleMR” packages. Effect estimates were reported in odds ratios (ORs) values when the outcome was dichotomous. Phenoscanner was used to detect and remove cofounder SNPs associated. IVW was utilized as the primary outcome, while MR-Egger, weighted mode, weighted median, and Simple mode enhanced the IVW estimates by generating more robust estimates in a wider range of circumstances26,27. The MR Egger test was employed to assess the pleiotropy effect, and Cochran’s Q test was applied to identify heterogeneity28. The flow diagram is illustrated in Fig. 1.
MR-BMA estimates
To overcome the limitations of traditional logistic regression methods and further validate the reliability of the results, we applied a novel analysis method based on Bayesian Model Averaging (BMA)24. The effectiveness of instruments in the model was examined using the Q-statistic. The Cook’s distance was used to quantify influential genetic variations29.
Observational analysis
UK Biobank was used for observational analysis. The project number was 80,532. RA participants was defined by using ICD codes (M06.9). The participants with full information including, BMI (field ID: 21001), ever smoked (field ID: 20160), blood pressure (field ID: 93, 94, 4079, 4080), and usual walking pace (field ID: 924) was included in this analysis. Finally, participants of European individuals in the UK Biobank (n = 43,770) including the RA group (n = 318) is presented in the Supplementary Table S3. According to the population characteristics provided, the logistic regression analysis was initially conducted, adjusting solely for age and gender. Subsequently, a multiple regression analysis was performed, incorporating other risk factors including BMI, SMK, and HBP.
Gene ontology (GO) and Kyoto Encyclopedia of genes and genomes (KEGG) pathwayenrichment analysis
Phenoscanner was employed to identify the genes where the SNPs, obtained by intersecting the summary data of WP and RA, were located for conducting the MR analysis. To further explore the biological role of WP on the development of RA, GO enrichment analysis was conducted on the identified genes. GO enrichment analysis has been used to annotate genes, gene products, and sequences30. GO terms are classified into three categories: cellular components, molecular functions, and biological processes. The KEGG database serves as a repository for the systematic analysis of gene function in numerous biochemical pathways31. The Database for Annotation, Visualization, and Integrated Discovery (DAVID) is a web-accessible program for classifying functionally associated genes into a manageable number of biological modules, followed by a systematic analysis of gene modules in a biological context32.
Software and statistics
All analysis were performed in R software (Version 4.2.3). Packages “TwoSampleMR” and “MRPRESSO” were used for Mendelian randomization analysis. Packages “enrichplot”, “clusterProfiler”and “ggplot2” were used for enrichment analysis and result visualization. Pvalue < 0.05 was considered to have potential causality.
Results
Unvariable MR estimates
From the GWASs for walking pace (WP), body mass index (BMI), smoking (SMK), high blood pressure (HBP), and type 2 diabetes (TD), we obtained 49, 394, 68, 189, 59 IVs that reached the genome-wide significance level (p < 5 × 10−8) (Supplementary Table S4). Unvariable MR was used to analyze the causal association between WP along with other potential risk factors (BMI, SMK, HBP, TD) with RA. The IVW approach was applied to appraise the causal effect. Results of the MR analysis were expressed as ORs of RA per standard deviation (SD) increase in each factor, as shown in Fig. 2A, indicating a causal relationship between WP (OR, 0.31; 95% CI, 0.15, 0.62; p = 1.05 × 10−3), BMI (OR, 1.33; 95% CI, 1.17, 1.51; p = 1.74 × 10 −5), SMK ( OR, 2.36; 95% CI, 1.15, 4.86; p = 1.97 × 10 −2), HBP (OR, 1.65; 95% CI, 1.11, 2.45; p = 1.32 × 10 −2), and RA, while no causal relationship was observed between TD (OR, 0.3; 95% CI, 0.07, 1.25; p = 9.73 × 10 −2) and RA. Furthermore, BMI, SMK, and HBP showed potential elevations in RA risk, whereas WP exhibited a potential protective effect against RA risk (Fig. 2A). Another cohort (Ncase = 8255, Ncontrol = 409001) was used to verify the protective effect of WP. The replicated MR analysis demonstrated that there was a protective effect of WP on RA (Supplementary Figure S1, Supplementary Table S5). To further validate the results of MR analysis, we employed an additional set of nine methods as supplementary evidence such as weighted mode and simple mode (Fig. 2B-E, Supplementary Figure S2-6). The results demonstrated that WP, BMI, SMK, and HBP still exhibited a causal association with RA among these ten methods. Summary information was presented in Supplementary Table S4.
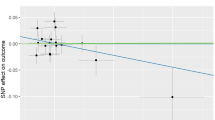
Multivariable MR-BMA analysis
Considering pleiotropy, we employed MR-BMA analysis to evaluate the causal effect between WP and RA to further test the robustness of the results. Three SNPs (SPHKAP, WNT2B, LINC00824) were identified with a Q statistic > 10. In the presence of these SNPs, there was a causal relationship between WP and RA applying MR-BMA analysis (OR, 0.39; 95% CI, 0.17, 0.84; p = 1.64 × 10−2) (Fig. 3AB). However, after excluding them as outliers, the causal relationship between WP and RA remained unchanged (OR, 0.44; 95% CI, 0.20, 0.96; p = 3.86 × 10−2) (Fig. 3CD). The results of Q-statistic and Cook’s distance are shown in Fig. 3 (the dots represented genes corresponding to invalid or influential instruments). Summary information was presented in Supplementary Table S6-8.
MR-BMA plots for outliers and influential genetic variants.(A) Diagnostic plots for invalid SNPs (outliers) (B) Diagnostic plots for influential genetic variants (C) Diagnostic plots for invalid SNPs after excluding invalid SNPs (rs2894446, rs3790604, rs4605363) (D) Diagnostic plots for influential genetic variants after excluding invalid SNPs (rs2894446, rs3790604, rs4605363). When Q-statistic > 10, the instrument for the corresponding gene is invalid and marked with a red box. When Cook’s distance > the threshold, the instrument for the corresponding gene is influential and marked with a red box.
Observational study
Participants of European individuals in the UK Biobank (n = 43,770) including the RA group (n = 318) and control group (n = 43,452), the specific demographic information, including age, gender, BMI, ever smoked, the percentage of HBP, and WP, was presented in the Supplementary Table S3. In the UK Biobank data, WP was categorized into three levels (1: slow, 2: moderate, 3: fast). Initially, the adjustment was performed only for age and sex, and logistic regression was employed to investigate the association, we found that the risk of RA decreased as the WP level increased. Furthermore, additional logistic regression analysis was conducted by incorporating the positive confounding factors (BMI, SMK, HBP) with the MR results. Interestingly, we observed that the conclusion regarding the protective effect of WP on RA remained unchanged even after including these confounding factors (Fig. 4). Summary information was presented in Supplementary Table S3, 9–10.
GO and KEGG enrichment analysis
GO terms enrichment analysis of WP on RA found significant enrichment of several crucial regulation pathways. Ten GO biological processes (e.g.,calcineurin-NFAT signaling, cascade calcineurin-mediated signaling, and inositol phosphate-mediated signaling) with Pvalue < 0.05 were observed to be involved in RA (Fig. 5A). KEGG pathway enrichment analysis with Pvalue < 0.05 revealed that genes where the SNPs obtained by intersecting WP and RA were primarily enriched in the Notch signaling pathway, Oocyte meiosis, ubiquitin-mediated proteolysis, and cellular senescence (Fig. 5B).
GO enrichment analysis and KEGG enrichment analysis of differential genes where the SNPs obtained by intersecting the summary data of WP and RA.(A) The top 10 GO enrichment analyses of biological processes, cellular components, and molecular functions. (B) KEGG enrichment analysis of differential genes where the SNPs obtained by intersecting the summary data of WP and RA.
Discussion
Our study found that WP was inversely associated with the risk of RA after adjusting for age and sex. The association between WP and RA persisted after adjusting for BMI, SMK, and HBP. Then a Mendelian randomization (MR) approach was employed to investigate the potential causal relationship between walking pace (WP) and the risk of developing rheumatoid arthritis (RA). Our findings suggested that walking pace may act as a protective factor against the development of RA.
Previous observational studies have indicated that higher physical activity (PA) levels correlate with reduced risk of RA. Specifically, these studies have demonstrated that PA, such as walking, can effectively reduce the burden of inflammation and improve functional ability33,34. Unfortunately, individuals with RA tend to exhibit a preference for sedentary behavior, which may be attributed to factors such as pain, fatigue, or fear of joint damage35,36,37. However, this behavior can lead to the exacerbation of RA-related symptoms, perpetuating a vicious cycle. These findings emphasized the necessity of PA among individuals with RA38,39. PA improves RA-related outcomes through various mechanisms, mediated by multiple factors, including the elevated activity of nitric oxide synthase (eNOS), increased blood flow, and the improvement of antioxidant mechanisms40. Furthermore, PA ameliorates pain by upregulating the expression of β-endorphin and inducing an anti-inflammatory phenotype41,42. In contrast, sun et al. used the MR analyses to suggest physical activity may not help to prevent RA43. Considering that PA is a multifaceted trait influenced by numerous genetic variants and environmental factors, it poses challenges in MR analysis due to its complexity. To some extent, it proves the limitations of MR when dealing with highly complex traits. For such complex traits, a multitude of SNPs may be identified, and whether these SNPs can truly represent these complex traits and be used for causal analysis remains debatable. Thus, the focus was narrowed to a specific aspect of physical activity, namely walking pace, to explore the causal relationship between WP and RA from GWAS data in our study.
It is worth noting that our MR analysis accounted for potential confounding factors, including BMI, SMK, HBP, and TD. Moreover, the analysis showed positive correlations between BMI, SMK, and HBP with RA, indicating their potential role as risk factors for the disease, which were consistent with previous studies44,45,46. Surprisingly, MR analysis did not find a causal relationship between TD and RA, similar conclusions were reached by Sun et al., who suggested that the results by MR may be influenced by population distribution45.
To reduce the potential impact of confounding factors on the MR estimates of WP and RA, MR-BMA analysis was employed to evaluate the causal effect between WP and RA after adjusting the confounding factors. The MR-BMA plots helped identify outliers and influential genetic variants. By excluding invalid SNPs and influential variants, we ensured the integrity and reliability of our MR analysis, and we found that the causal association between WP and RA remained unchanged regardless of whether outliers were removed or not.
To further explore the biological role of WP on the development of RA, GO enrichment analysis was conducted on the identified genes. GO biological processes (e.g.,calcineurin-NFAT signaling, cascade calcineurin-mediated signaling, and inositol phosphate-mediated signaling) were observed to be involved in RA, which has been supported by previous studies. For example, Park et al. found calcineurin-NFAT1 to 4 pathways are dysregulated in autoimmune diseases47, and Gu et al. indicated that the RA patients presented diverse dysfunctions in inositol phosphate metabolism48, suggesting that the effect of WP on RA is multifaceted.
While our study provides valuable insights, it is important to acknowledge its limitations. Firstly, gender and age are recognized as important factors influencing the risk of RA development. However, conducting further stratified analysis using publicly available summary-level GWAS data is challenging. In our study, we investigated RA as a whole, as individual patient-level data was not available, precluding subgroup analysis. Secondly, the GWAS summary data used in our study are predominantly focused on individuals of European, which may limit the generalizability of our findings to the whole population. Just as Timmins et al.49 conducted a Mendelian Randomization (MR) analysis with a mixed-race population and reached a different conclusion from ours, the LDSC analysis still suggests an association between walking and rheumatoid arthritis. Therefore, the differences in ancestry could be a factor that contributes to varying results to previous studies, potentially introducing bias. Thirdly, our unidirectional MR analysis focused on assessing the causal relationship between walking pace and RA. We did not investigate the two variables’ reverse causality or bidirectional association. Future studies could explore the reciprocal relationship between walking pace and RA to gain a more comprehensive understanding. As we are currently unable to obtain individual genotype data, we cannot conduct one-sample Mendelian Randomization at this time. We will attempt to obtain individual genotype data in the future in order to better confirm the reliability of our conclusions.
Conclusion
In conclusion, Mendelian randomization design is a valuable approach to studying the causal effects of WP on the risk of RA, our MR study suggested that walking pace acts as a protective factor against the development of rheumatoid arthritis. However further research is needed to fully elucidate the underlying mechanisms involved.
Abbreviations: CI, confidence interval; MR, Mendelian randomization; OR, odds ratio; RA, rheumatoid arthritis; SNP, single- nucleotide polymorphism.
Abbreviations: SNP, single- nucleotide polymorphism; MRA: Bayesian model averaging.
Data availability
The summary data in our work is publicly accessible. The individual data was obtained from UK Biobank. Data may also be available upon reasonable request to corresponding authors.
References
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M. & Gabriel, S. E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 62(6), 1576–1582. https://doi.org/10.1002/art.27425 (2010).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet (London England). 388(10055), 2023–2038. https://doi.org/10.1016/s0140-6736(16)30173-8 (2016).
Douglas, K. M. et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann. Rheum. Dis. 65(3), 348–353. https://doi.org/10.1136/ard.2005.037978 (2006).
Santo, R. C. E., Fernandes, K. Z., Lora, P. S., Filippin, L. I. & Xavier, R. M. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta-analysis. J. cachexia Sarcopenia Muscle. 9(5), 816–825. https://doi.org/10.1002/jcsm.12320 (2018).
Kojima, M. et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. 61(8), 1018–1024. https://doi.org/10.1002/art.24647 (2009).
Hu, Y. et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann. Rheum. Dis. 76(8), 1357–1364. https://doi.org/10.1136/annrheumdis-2016-210431 (2017).
Salliot, C., Nguyen, Y., Boutron-Ruault, M. C. & Seror, R. Environment and Lifestyle: their influence on the risk of RA. J. Clin. Med. 9(10). https://doi.org/10.3390/jcm9103109 (2020).
Buch, M. H., Eyre, S. & McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 17(1), 17–33. https://doi.org/10.1038/s41584-020-00541-7 (2021).
Kelly, P. et al. Walking on sunshine: scoping review of the evidence for walking and mental health. Br. J. Sports Med. 52(12), 800–806. https://doi.org/10.1136/bjsports-2017-098827 (2018).
Morris, J. N., Hardman, A. E. & Auckland Walking to health. Sports medicine (NZ); 23(5): 306–332. doi:https://doi.org/10.2165/00007256-199723050-00004. (1997).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54(24), 1451–1462. https://doi.org/10.1136/bjsports-2020-102955 (2020).
Murphy, M. H., Nevill, A. M., Murtagh, E. M. & Holder, R. L. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev. Med. 44(5), 377–385. https://doi.org/10.1016/j.ypmed.2006.12.008 (2007).
Manson, J. E. et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N. Engl. J. Med. 341(9), 650–658. https://doi.org/10.1056/nejm199908263410904 (1999).
Yates, T. et al. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur. Heart J. 38(43), 3232–3240. https://doi.org/10.1093/eurheartj/ehx449 (2017).
Tanasescu, M. et al. Exercise type and intensity in relation to coronary heart disease in men. Jama. 288(16), 1994–2000. https://doi.org/10.1001/jama.288.16.1994 (2002).
Celis-Morales, C. A. et al. Walking Pace is Associated with Lower Risk of all-cause and cause-specific mortality. Med. Sci. Sports. Exerc. 51(3), 472–480. https://doi.org/10.1249/mss.0000000000001795 (2019).
Elbaz, A. et al. Association of walking speed in late midlife with mortality: results from the Whitehall II cohort study. Age (Dordrecht Netherlands). 35(3), 943–952. https://doi.org/10.1007/s11357-012-9387-9 (2013).
Stamatakis, E. et al. Self-rated walking pace and all-cause, cardiovascular disease and cancer mortality: individual participant pooled analysis of 50 225 walkers from 11 population British cohorts. Br. J. Sports Med. 52(12), 761–768. https://doi.org/10.1136/bjsports-2017-098677 (2018).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23(R1), R89–98. https://doi.org/10.1093/hmg/ddu328 (2014).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Res. ed). 362, k601. https://doi.org/10.1136/bmj.k601 (2018).
Richmond, R. C. & Davey Smith, G. Mendelian randomization: concepts and scope. Cold Spring Harbor Perspect. Med. 12(1). https://doi.org/10.1101/cshperspect.a040501 (2022).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53(10), 1415–1424. https://doi.org/10.1038/s41588-021-00931-x (2021).
Staley, J. R. et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinf. (Oxford England). 32(20), 3207–3209. https://doi.org/10.1093/bioinformatics/btw373 (2016).
Zuber, V., Colijn, J. M., Klaver, C. & Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable mendelian randomization. Nat. Commun. 11(1), 29. https://doi.org/10.1038/s41467-019-13870-3 (2020).
Machiela, M. J. & Chanock, S. J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinf. (Oxford England). 31(21), 3555–3557. https://doi.org/10.1093/bioinformatics/btv402 (2015).
Wu, F., Huang, Y., Hu, J. & Shao, Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 18(1), 312. https://doi.org/10.1186/s12916-020-01778-5 (2020).
Yin, K. J. et al. No Genetic Causal Association between Periodontitis and Arthritis: a bidirectional two-sample mendelian randomization analysis. Front. Immunol. 13, 808832. https://doi.org/10.3389/fimmu.2022.808832 (2022).
Burgess, S., Scott, R. A., Timpson, N. J., Davey Smith, G. & Thompson, S. G. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30(7), 543–552. https://doi.org/10.1007/s10654-015-0011-z (2015).
Wang, X. et al. Appraising the Causal Association between systemic Iron status and heart failure risk: a mendelian randomisation study. Nutrients. 14(16). https://doi.org/10.3390/nu14163258 (2022).
Harris, M. A. et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 32 (Database issue), D258–261. https://doi.org/10.1093/nar/gkh036 (2004).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Huang, D. W. et al. The DAVID Gene Functional classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 8(9), R183. https://doi.org/10.1186/gb-2007-8-9-r183 (2007).
Metsios, G. S., Stavropoulos-Kalinoglou, A. & Kitas, G. D. The role of exercise in the management of rheumatoid arthritis. Expert Rev. Clin. Immunol. 11(10), 1121–1130. https://doi.org/10.1586/1744666x.2015.1067606 (2015).
Hu, H., Xu, A., Gao, C., Wang, Z. & Wu, X. The effect of physical exercise on rheumatoid arthritis: an overview of systematic reviews and meta-analysis. J. Adv. Nurs. 77(2), 506–522. https://doi.org/10.1111/jan.14574 (2021).
Tierney, M., Fraser, A. & Kennedy, N. Physical activity in rheumatoid arthritis: a systematic review. J. Phys. Act. Health. 9(7), 1036–1048. https://doi.org/10.1123/jpah.9.7.1036 (2012).
Thomsen, T. et al. Sedentary behaviour in patients with rheumatoid arthritis: a qualitative study. Int. J. Qualitative Stud. Health well-being. 10, 28578. https://doi.org/10.3402/qhw.v10.28578 (2015).
van Veldhuijzen, J. J. et al. Perceived barriers, facilitators and benefits for regular physical activity and Exercise in patients with rheumatoid arthritis: a review of the literature. Sports medicine (Auckland, NZ).; 45(10): 1401–1412 .doi: (2015). https://doi.org/10.1007/s40279-015-0363-2
Prioreschi, A., Makda, M. A., Tikly, M. & McVeigh, J. A. Habitual physical activity, sedentary Behaviour and Bone Health in Rheumatoid Arthritis. Int. J. Sports Med. 36(12), 1021–1026. https://doi.org/10.1055/s-0035-1550049 (2015).
Khoja, S. S., Almeida, G. J., Chester Wasko, M., Terhorst, L. & Piva, S. R. Association of Light-Intensity Physical Activity with Lower Cardiovascular Disease Risk Burden in Rheumatoid Arthritis. Arthritis Care Res. 68(4), 424–431. https://doi.org/10.1002/acr.22711 (2016).
Fichna, J., Janecka, A., Costentin, J. & Do Rego, J. C. The endomorphin system and its evolving neurophysiological role. Pharmacol. Rev. 59(1), 88–123. https://doi.org/10.1124/pr.59.1.3 (2007).
Metsios, G. S. et al. Individualised exercise improves endothelial function in patients with rheumatoid arthritis. Ann. Rheum. Dis. 73(4), 748–751. https://doi.org/10.1136/annrheumdis-2013-203291 (2014).
Mckenna, S. G. et al. OP0275-HPR the effects of exercise on depressive and anxiety symptoms in rheumatoid arthritis: a systematic review and meta-analysis. ;77(Suppl 2): 186–186 .https://doi.org/10.1136/annrheumdis-2018-eular. (2018). 1691%J Annals of the Rheumatic Diseases.
Sun, L. et al. Physical activity and the risk of rheumatoid arthritis: evidence from meta-analysis and mendelian randomization. Int. J. Epidemiol. 50(5), 1593–1603. https://doi.org/10.1093/ije/dyab052 (2021).
Chang, K. et al. Smoking and rheumatoid arthritis. Int. J. Mol. Sci. 15(12), 22279–22295. https://doi.org/10.3390/ijms151222279 (2014).
Inamo, J., Kochi, Y. & Takeuchi, T. Is type 2 diabetes mellitus an inverse risk factor for the development of rheumatoid arthritis? J. Hum. Genet. 66(2), 219–223. https://doi.org/10.1038/s10038-020-00837-2 (2021).
Qiu, S., Li, M., Jin, S., Lu, H. & Hu, Y. Rheumatoid arthritis and Cardio-Cerebrovascular Disease: a mendelian randomization study. Front. Genet. 12, 745224. https://doi.org/10.3389/fgene.2021.745224 (2021).
Park, Y. J., Yoo, S. A., Kim, M. & Kim, W. U. The role of calcium-Calcineurin-NFAT signaling pathway in Health and Autoimmune diseases. Front. Immunol. 11, 195. https://doi.org/10.3389/fimmu.2020.00195 (2020).
Gu, Y. et al. Plasma metabonomics study of rheumatoid arthritis and its Chinese medicine subtypes by using liquid chromatography and gas chromatography coupled with mass spectrometry. Mol. Biosyst. 8(5), 1535–1543. https://doi.org/10.1039/c2mb25022e (2012).
Timmins, I. R. et al. Genome-wide association study of self-reported walking pace suggests beneficial effects of brisk walking on health and survival. Commun. Biology. 3(1), 634. https://doi.org/10.1038/s42003-020-01357-7 (2020).
Acknowledgements
We thanked UK Biobank and IEU OpenGWAS project for providing access and strong support for our study and global scientific research.
Funding
The project was funded by Elite Medical Professionals Project of China-Japan Friendship Hospital (NO.ZRJY2024-GG05), National High Level Hospital Clinical Research Funding, National Natural Science Foundation of China (82172387, 82372371, 82372500), the Natural Science Foundation of Jiangsu Province (BK20230002), the Suzhou Science and Technology Development Plan (SKY2023035, SZM2023003).
Author information
Authors and Affiliations
Contributions
J.L. and Q.Z. designed the research; Y.Z., C.H. and Z.C. collected the data; Q.Z., W.X. and R.Z. analyzed the data; Q.Z., X.H. and R.A.H drew the figures; Q.Z., X.H. and Y.Z. wrote the manuscript. R.A.H., Y.Z.and Y.L reviewed the manuscript. J.L. and W. X. supervised the process of this study. All authors approve of the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The work has not been submitted elsewhere for publication, in whole or in part, and all the authors listed have approved the manuscript that is enclosed
Ethics Statement
The summary and individual data was approved by the related boards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Huang, X., Zhang, Y. et al. Walking pace is a protective factor for rheumatoid arthritis: a mendelian randomization study. Sci Rep 14, 24886 (2024). https://doi.org/10.1038/s41598-024-76666-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76666-6