Abstract
The overconsumption of folic acid has been associated with deleterious health effects; however, the extant body of research on this matter remains controversial. The principal objective of our investigation was to scrutinize the correlation between red blood cell (RBC) folate levels and appendicular skeletal muscle mass (ASM) among adult individuals. A total of 4117 adults aged over 20 years were included. The weighted prevalence of low muscle mass status (LMMS) was 14.50%. The correlation between RBC folate and ASM showed an inverted U-shaped curve. When the RBC folate concentration is below 500 nmol/L, ASM increases with increasing RBC folate concentration. However, when the RBC folate level exceeds 500 nmol/L, ASM decreases with increased RBC folate level. After correcting multiple confounding factors, a positive correlation was found between RBC folate and LMMS (p < 0.001). Compared with the RBC folate first quartile, the multivariable-adjusted ORs and 95% CIs of the second quartile, third quartile, and highest quartile were 1.08(0.81–1.44), 1.06(0.79–1.43), and 1.96(1.47–2.61), respectively. Our research suggested that excessive levels of RBC folate may be associated with an increased risk of LMMS in adults. Thus, being more cautious when considering folic acid supplementation is recommended.
Similar content being viewed by others
Introduction
It is well established that insufficient folate supply can markedly influence the proper development of the fetal neural tube and placental growth, thereby potentially leading to severe complications1,2. Furthermore, animal experiments and epidemiological inquiries have demonstrated that folate deficiency is a predisposing factor for conditions such as megaloblastic anemia, nervous system disorders, and cardiovascular ailments3,4,5. Therefore, in 1998, the United States instituted a policy mandating the fortification of grains with folic acid6. After three years of intervention, the report from the Centers for Disease Control and Prevention (CDC) in the United States indicated that the incidence of neonatal neural tube defects in 2001 significantly decreased to 10% before policy implementation7,8. However, certain epidemiological studies indicated that the consumption of elevated doses of folic acid may be implicated in the hastening of cancer progression, the onset of insulin resistance, the masking of vitamin B12 deficiency, and the induction of aortic calcification9,10,11,12,13,14, and research on the association between folic acid and adverse health outcomes is still limited. Consequently, it is imperative to underscore the necessity for additional research elucidating the potential adverse health ramifications associated with the excessive fortification of folic acid.
Both animal and cell experiments have proven that folic acid not only plays a crucial role in maintaining normal cell division and immune function but is also closely related to energy metabolism in the body, both of which may directly or indirectly affect muscle strength and mass15,16,17. Recently, studies on the relationship between folic acid and muscle strength have yielded different results in different populations such as the United Kingdom, Korea, Singapore, and the United States18,19,20,21, and similarly, the results of studies on the relationship between folic acid and muscle mass have been inconsistent22,23. In a survey of 58 elderly outpatient patients in the United States23, the results showed a positive correlation between folate intake and muscle mass. However, in elderly Japanese women, it was found that there was no statistical correlation between serum folate and muscle22. Previous studies on the relationship between folic acid and muscle mass and strength were predominantly based on serum folate and dietary folate18,19,20,21,22,23. Due to the low bioavailability of dietary folate and the short cycle representativeness of serum folate, so neither of these indicators can reflect the overall folate storage in the human body24,25. By contrast, RBC folate can represent the intake of folate throughout the entire life cycle of red blood cells25. Despite the significance of RBC folate, there is a clear lack of research on its relationship with muscle mass. Accordingly, in the present investigation, our focus will center on examining the correlation between RBC folate levels and muscle mass.
Results
The demographic characteristics of the study are delineated in Table 1. A total of 4117 individuals participated in this analysis, comprising 2049 males and 2068 females, with a weighted mean age of 44.0 ± 14.5 years. Among these participants, 597 adults were identified as presenting with LMMS, representing a weighted prevalence rate of 14.9%. Notably, age, serum folate, vitamin B12, waist circumference, and BMI demonstrated a positive correlation with ascending quartiles of RBC folate concentration. Conversely, homocysteine peaked in the second quartile of erythrocyte folate concentrations and then declined in the third quartile. In terms of categorical variables, individuals categorized as having high RBC folate levels exhibited a higher likelihood of being female, married, white, non-smoking, and drinking when compared to their counterparts with low RBC folate levels.
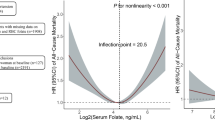
The association between RBC folate and ASM is illustrated in Figs. 1, which displays an inverted U-shape. At RBC folate concentrations below 500 nmol/L, ASM demonstrates an upward trajectory with increasing RBC folate concentration. Conversely, upon surpassing this threshold, ASM exhibits a declining trend. Analogously, the same outcomes were observed for RBC folate and ASMI. (Supplementary Fig. 1). Furthermore, considering the gender and age differences in ASM, we conducted a stratified analysis. In stratified analysis, the results indicated that this inverted U-shaped relationship between RBC folate and ASM does not change due to gender or age (Supplementary Fig. 2), which is similar to the stratification results of ASMI (Supplementary Fig. 3).
The relationship between RBC folate and LMMS is shown in Table 2. After adjusting for age and gender, compared with the lowest quartile of RBC folate, only the highest quartile of RBC folate was associated with LMMS (OR: 1.32, 95%CI: 1.04–1.67, p = 0.025), and this association persisted after adjusting for factors such as smoking, alcohol consumption, and other factors on the basis of age and gender, and this association persisted after additionally adjusting for ethnicity, education, family poverty levels, marital status, activity, alcohol drinking, smoking, vitaminB12 and waist on the basis of age and sex (OR: 1.97, 95%CI: 1.49–2.59, p < 0.001). Association of RBC folate with LMMS remained significant (OR: 1.96, 95%CI: 1.47–2.61, p < 0.001) after additional adjustment for hypertension, diabetes, high cholesterol, homocysteine in model 2. In addition, we also included the concentration of RBC folate as a continuous variable in the regression analysis and found that regardless of which model, the risk of LMMS increased for every 1 SD increase in RBC folate concentration (Model1: OR, 1.02, 95%CI, (1.01–1.03), p < 0.001; Model2: OR, 1.03, 95%CI, (1.02–1.04), p < 0.001; Model3: OR, 1.03, 95%CI, (1.02–1.04), p < 0.001.).
To explore whether the relationship between RBC folate and LMMS is affected by age and sex, we analyzed the interaction between age, sex, and RBC folate. The results showed that there was no interaction between RBC folate and the two factors (p for year interaction = 0.106, p for gender interaction = 0.717) (Table 3).
Discussion
Folate stands as a pivotal nutritional factor recognized for its potential to facilitate muscle development15,17. Nonetheless, preceding findings on the relationship between folate and indicators of muscular strength or mass have demonstrated inconsistency18,19,20,21,22,23. In our investigation, a notable observation emerged revealing an inverted U-shaped dose-response relationship between RBC folate levels and muscle mass. Specifically, when the RBC folate concentration fell below 500 nmol/L, an increment in ASM was noted with an escalating RBC folate concentration. However, entering into a high folate state, marked by a concentration of 745.5 nmol/L, was found to significantly elevate the risk of LMMS.
Previous research has predominantly focused on the relationship between folate and muscular strength, with multiple studies from the United Kingdom, Singapore, and South Korea underscores the significance of folate in maintaining skeletal muscle strength. Such investigations typically emphasize the role of folate in neuromuscular transmission, protein synthesis, and energy metabolism18,19,21. However, the study conducted by Zhang et al. indicates a decline in corrected grip strength when serum folate levels exceed 40 nmol/L among 9,079 adults aged ≥ 20 in the United States20. While these findings provide crucial insights into our understanding of the physiology of folate and muscles, research directly addressing the correlation between muscle mass and folate remains relatively limited22,23. Yeung et al. showed that serum folate levels are positively correlated with muscle mass in the elderly population of the United States23. Misora Ao et al. reported that there is no correlation between serum folate and muscle mass22. Our research results indicated an inverted U-shaped relationship between RBC folate and ASM, which is different from the findings of Yeung et al. and Misora Ao et al., possibly because the RBC folate used in this study was different from the serum folate they used, or because our study population was younger (4117 adults, age 44.0 ± 14.5 years old)22,23. In addition, we explored the interaction between RBC folate and age and gender. However, the stratified results showed that the trends of RBC folate and ASM were the same in both gender and age groups.
In the current discussion, we need to recognize that supplementing folic acid may involve a range of potential health risks. Regarding this, Marta Ebbing and colleagues observed that26 following a seven-year follow-up period, ischemic heart disease patients who consumed 0.8 mg/day of folic acid experienced a substantial increase in the incidence of lung cancer, cancer-related mortality, and overall mortality risk. Furthermore, a decade-long randomized controlled trial indicated a significant elevation in the risk of prostate cancer among males supplementing with 1 mg/day27. The folic acid intake in these studies notably approached or exceeded the upper tolerable intake level of 1000 ug/day28. However, we do not yet know how long or how large a dose of folic acid intake is needed to reach concentrations of RBC folate that may be potentially harmful. Therefore, before considering folic acid supplementation, various potential risks should be taken into account. In our study, we discovered a significant increase in the risk of LMMS when the concentration of red blood cell folate exceeded 745.5 nmol/L. Similarly, Zhou et al.‘s study presented that when the concentration of RBC folate exceeded 1809nmol/L, the risk of abdominal aortic calcification increased14. The dosage of RBC folate reported by our research is different from that in previous studies, which may due to be adverse health reactions that can affect the threshold of RBC folate29. Additionally, compared with the microbiological assay used in previous studies, the Bio-Rad assay used for measuring RBC folate in our study underestimated the concentration of RBC folate by 45%30. Accordingly, more accurate measurement methods are needed to explore the RBC folate threshold for different health effects. Our findings are along the lines of previous evidence suggesting that excessive RBC folate levels may be associated with decreased muscle mass. However, more direct evidence is needed in the future to confirm this speculation.
Although the mechanism between folic acid and muscle mass is not yet clear, the following possible mechanisms can help understand the relationship between folic acid and muscle mass. First, studies have shown that folate deficiency can affect the inhibition of S-adenosylmethionine in muscle cells, resulting in DNA hypomethylation, which can affect the proliferation and differentiation of muscle cells, and accelerate the aging and apoptosis of muscle cells15,24,31. Second, van Dijk M et al. have shown that the reduction of folic acid intake in the diet will reduce antioxidant capacity and increase oxidative stress, which is one of the risk factors for decreased muscle mass32,33. Moreover, folic acid intake can reduce high levels of homocysteine associated with poor physical performance, decreased muscle strength, and decreased muscle density34,35. However, previous studies have reported a U-shaped relationship between folate levels and homocysteine36,37, which is supported by the results of our study (Supplementary Fig. 3). Studies have shown that folate plays an important role in the conversion of homocysteine to S-adenosylmethionine - as a coenzyme in the methyl transfer reaction38. However, excessive folate levels may reduce methyl donor utilisation or impair the activity of methylenetetrahydrofolate reductase, which is responsible for homocysteine methylation36,37. Finally, it has been proven that the intake of folic acid can improve skeletal muscle blood flow, which is a key component in maintaining muscle mass39. Nevertheless, some studies have shown that excessive folate in red blood cells is associated with arteriosclerosis, which can hinder muscle blood supply and reduce muscle mass14,40.
This is the first study to investigate the relationship between RBC folate and muscle mass. The present study has several deficiencies as follows. First, the cross-sectional nature of our research made it difficult to determine a causal relationship between RBC folate and decreased muscle mass. Second, the data for this study were derived from the NHANES database for the years 2002–2006. This relatively early time frame may not fully reflect erythrocyte folate and muscle mass status in the current population. In addition, due to the time constraints of the database, we could not include other indicators that may reflect high levels of folate status (e.g., unmetabolized folate) in our analyses, which somewhat limits our ability to provide a comprehensive assessment of folate status. Eventually, although we adjusted for several potential factors, we cannot completely rule out the influence of residual confounding of unknown or unmeasured variables.
In conclusion, our study demonstrates that among American adults, the relationship between RBC folate and muscle mass exhibits an inverted U-curve. Specifically, when RBC folate concentrations exceed 745.5 nmol/L, there is a significant association with an increased risk of LMMS. This finding highlights the need for further investigation into the potential adverse health effects of elevated folate levels. Future research should delve into the broader health implications of high RBC folate concentrations, enhancing our understanding of the complex and potentially dual-edged nature of this essential nutrient.
Methods
Study population
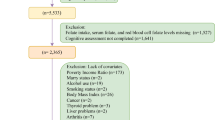
The present study utilized data derived from the National Health and Nutrition Examination Survey (NHANES) spanning the years 2003–2006, information about this cross-sectional study can be described in detail elsewhere[25]. Briefly, NHANES is a nationally representative cross-sectional study on the nutrition and health of respondents living in the United States. In total, 20,471 participants completed the interviews in the NHANES 2003–2006, in which the muscle mass of adult individuals over the age of 20 was evaluated. Individuals who met the following criteria in the analysis were excluded: (1) age < 20 years (n = 10451); (2) subjects with missing muscle mass data (n = 3125); (3) subjects who failed to obtain RBC folate data (n = 364); and (4) subjects who missed covariate data (n = 2415). Finally, a total of 4117 adults were retained for analysis. The specific exclusion process of the research object is shown in Fig. 2. All participants provided written informed consent and the protocol was approved by the Ethics Review Board of National Center for Health Statistics(Protocol #98–12, Protocol #2005–06).
Data collection
The demographic information of NHANES was collected by professionally trained staff using computer-assisted personal interview programs. The information collected included demographics, health status, lifestyle, and serum indicators such as physical activity, homocysteine, and vitamin B12. Other variables included ethnicity (to facilitate statistical analysis classifying Mexican and Hispanic as Hispanic, white, black, and other races41, educational attainment (below high school graduates, high school graduates, and above high school education level), and family poverty level (based on the poverty criteria, poverty to income ratio was divided into three categories: ≤1.30, 1.31–1.85, and > 1.85)14. By collecting the questionnaire data about the lifestyle of the researchers, at least 100 times of smoking in the previous year were defined as smokers, and at least 100 times of drinking in the previous year were classified as drinkers.
Laboratory tests and clinical definition
Whole blood and blood serum were processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, CDC, Atlanta, GA for analysis. Both folate (whole blood folate and serum folate) and vitamin B12 were measured by using the Bio-Rad Laboratories “Quantaphase II Folate/vitamin B12” radio assay kit. Homocysteine in plasma was measured by the Abbott homocysteine assay, a fully automated fluorescence polarization immunoassay from Abbott Diagnostics. Long-term estimated coefficients of variation (CV) for homocysteine, vitamin B12, serum and RBC folate measured or calculated by the above methods fluctuated within 3–7%. The concentration of RBC folate was calculated using the following formula:
Hypertension is defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg and/or currently used antihypertensive drugs42. Diabetes is defined as glycosylated hemoglobin ≥ 6.5% and/or currently treated with hypoglycemic drugs or insulin43. Hypercholesterolemia is defined as TC ≥ 6.2 mmol/l or current medication use44.
Body test data and low muscle mass status evaluation
The participant’s height and weight data were measured using a Toledo electronic weight scale and a Seca electronic stadiometer, respectively. The body mass index (BMI) was calculated by the following formula based on the measured height and weight data: weight(kg)/[height (m)]2. The waist circumference data of the participants were measured using an inelastic tape measure with a minimum graduation of 1 mm, at the highest point of the iliac crest, horizontally around the abdomen, and at the end of normal breathing, with an accuracy of 0.01 cm.
In this investigation, Dual-energy X-ray absorptiometry (DXA) was employed for comprehensive scanning of participants’ anatomical regions including the head, arms, legs, and trunk, providing data on bone mineral content (kg), fat content (kg), and lean body weight (kg). Participants were required to remove metallic accessories such as earrings and assume a supine position on the Hologic QDR 4500 A fan beam X-ray bone densitometer (Hologic Inc., equipped with Hologic Discovery software, version 12.1), with bare feet and arms extended alongside their bodies. The scanning procedure typically lasted approximately 3 min. Within the scope of this research, ASM represented the combined muscle mass of both the upper and lower limbs. In addition, to exclude the effect of height, the ASM was divided by the square of height to obtain the appendicular skeletal muscle mass index (ASMI). Low muscle mass status (LMMS) was established according to the diagnostic criteria proposed by EWGSOP. LMMS was defined as ASMI of less than 7.9 kg/m^2 in men and 5.8 kg/m^2 in women.
Statistical analysis
Data were presented as weighted mean ± standard deviations (SDs) for continuous variables and as frequency (weighted percentages) for categorical variables according to NHANES analytic guidelines. Baseline characteristics of the population were compared using one-way analysis of variance and Chi-square tests. RBC folate levels were used as quartile variables (Q1: <475.5 nmol/L; Q2: 457.5-579.8 nmol/L, Q3: 579.8-747.5 nmol/L, Q4: >747.5 nmol/L) to explore the relationship with LMMS. Conditional logistic regression analysis was performed to estimate odds ratios (ORs) and 95% CIs. Covariates included age, sex, race, education levels, marital status, family poverty levels, alcohol consumption, smoking status, activity, waist circumference, vitamin B12, homocysteine, hypertension, diabetes, and hyperlipidemia. In addition, this study analyzed the interaction between RBC folate and age and gender. The restricted cubic spline model was used for the correlation analysis. The covariates included in the analysis of the relationship between RBC folate and ASM using the restricted cubic spline method are the same covariates used in Model 3. A two-tailed p < 0.05 was considered significant. All analyses were performed using R version 4.2.1.
Data availability
The datasets analysed during the current study are available in the NHANES repository [https://www.cdc.gov/nchs/nhanes/index.htm].
References
van Gool, J. D., Hirche, H., Lax, H. & De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: a review. Reprod. Toxicol. 80, 73–84 (2018).
Viswanathan, M., Urrutia, R. P., Hudson, K. N., Middleton, J. C. & Kahwati, L. C. Folic acid supplementation to prevent neural tube defects: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 330, 460–466 (2023).
Liu, Y. et al. Associations of serum folate and vitamin B12 levels with Cardiovascular Disease Mortality among patients with type 2 diabetes. JAMA Netw. Open. 5, e2146124 (2022).
Balashova, O. A., Visina, O. & Borodinsky, L. N. Folate action in nervous system development and disease. Dev. Neurobiol. 78, 391–402 (2018).
Socha, D. S., DeSouza, S. I., Flagg, A., Sekeres, M. & Rogers, H. J. Severe megaloblastic anemia: vitamin deficiency and other causes. Cleve Clin. J. Med. 87, 153–164 (2020).
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B. Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press (US), 1998).
Shurtleff, D. B. Epidemiology of neural tube defects and folic acid. Cerebrospinal Fluid Res. 1, 5 (2004).
Centers for Disease Control and Prevention (CDC). Spina Bifida and anencephaly before and after folic acid mandate–United States, 1995–1996 and 1999–2000. MMWR Morb Mortal. Wkly. Rep. 53, 362–365 (2004).
Patel, K. R. & Sobczyńska-Malefora, A. The adverse effects of an excessive folic acid intake. Eur. J. Clin. Nutr. 71, 159–163 (2017).
Smith, A. D., Kim, Y. I. & Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 87, 517–533 (2008).
van Gool, J. D., Hirche, H., Lax, H. & Schaepdrijver, L. D. Fallacies of clinical studies on folic acid hazards in subjects with a low vitamin B12 status. Crit. Rev. Toxicol. 50, 177–187 (2020).
Mahmoud, A. M. & Ali, M. M. Methyl Donor micronutrients that modify DNA methylation and Cancer outcome. Nutrients. 11, 608 (2019).
Li, Z. et al. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr. 37, 1700–1706 (2018).
Zhou, L., Wen, X., Peng, Y., Guo, M. & Zhao, L. Red blood cell folate and severe abdominal aortic calcification: results from the NHANES 2013–2014. Nutr. Metab. Cardiovasc. Dis. 31, 186–192 (2021).
Hwang, S. Y. et al. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J. Cell. Physiol. 233, 736–747 (2018).
Wang, B. et al. Maternal folic acid supplementation modulates the growth performance, muscle development and immunity of Hu sheep offspring of different litter size. J. Nutr. Biochem. 70, 194–201 (2019).
Hwang, S. Y., Sung, B. & Kim, N. D. Roles of folate in skeletal muscle cell development and functions. Arch. Pharm. Res. 42, 319–325 (2019).
Gedmantaite, A. et al. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech. Ageing Dev. 189, 111269 (2020).
Lee, M. R. & Jung, S. M. Folic acid is related to muscle strength and vitamin A is related to Health-Related Quality of Life: results of the Korea National Health and Nutrition Examination Survey (KNHANES VII 2016–2018). Nutrients. 13, 3618 (2021).
Zhang, L., Sun, J., Li, Z. & Zhang, D. The relationship between serum folate and grip strength in American adults. Arch. Osteoporos. 16, 97 (2021).
Wee, A. K. H. Serum folate predicts muscle strength: a pilot cross-sectional study of the association between serum vitamin levels and muscle strength and gait measures in patients > 65 years old with diabetes mellitus in a primary care setting. Nutr. J. 15, 89 (2016).
Ao, M. et al. Relationship between Homocysteine, Folate, vitamin B12 and physical performance in the Institutionalized Elderly. J. Nutr. Sci. Vitaminol (Tokyo). 65, 1–7 (2019).
Yeung, S. S. Y. et al. Nutrient intake and muscle measures in geriatric outpatients. J. Am. Coll. Nutr. 40, 589–597 (2021).
Chen, M. Y. et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am. J. Clin. Nutr. 109, 1452–1461 (2019).
Zhou, L. et al. Associations of serum and red blood cell Folate with all-cause and Cardiovascular Mortality among Hypertensive patients with elevated homocysteine. Front. Nutr. 9, 849561 (2022).
Ebbing, M. et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 302, 2119–2126 (2009).
Figueiredo, J. C. et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J. Natl. Cancer Inst. 101, 432–435 (2009).
Orozco, A. M., Yeung, L. F., Guo, J., Carriquiry, A. & Berry, R. J. Characteristics of U.S. adults with Usual Daily Folic Acid Intake above the Tolerable Upper Intake Level: National Health and Nutrition Examination Survey, 2003–2010. Nutrients. 8, 195 (2016).
Identifying Research Needs for Assessing Safe Use of High Intakes of Folic Acid. National Toxicology Program. https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/completed/folate.
Yetley, E. A. et al. Biomarkers of folate status in NHANES: a roundtable summary. Am. J. Clin. Nutr. 94, 303S–312S (2011).
Majumder, A., Behera, J., Jeremic, N., Tyagi, S. C. & Hypermethylation Causes and consequences in skeletal muscle myopathy. J. Cell. Biochem. 118, 2108–2117 (2017).
van Dijk, M. et al. Reduced dietary intake of micronutrients with antioxidant properties negatively impacts muscle health in aged mice. J. Cachexia Sarcopenia Muscle. 9, 146–159 (2018).
Kim, J. W., Kim, R., Choi, H., Lee, S. J. & Bae, G. U. Understanding of Sarcopenia: from definition to therapeutic strategies. Arch. Pharm. Res. 44, 876–889 (2021).
Choi, J. H. et al. Association between Elevated Plasma Homocysteine and low skeletal muscle Mass in asymptomatic adults. Endocrinol. Metab. (Seoul). 37, 333–343 (2022).
Vidoni, M. L., Gabriel, P., Luo, K., Simonsick, S. T., Day, R. S. & E. M. & Relationship between Homocysteine and muscle strength decline: the Baltimore Longitudinal Study of Aging. J. Gerontol. Biol. Sci. Med. Sci. 73, 546–551 (2018).
Selhub, J., Morris, M. S. & Jacques, P. F. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc. Natl. Acad. Sci. U S A. 104, 19995–20000 (2007).
Faux, N. G. et al. Homocysteine, vitamin B12, and folic acid levels in Alzheimer’s disease, mild cognitive impairment, and healthy elderly: baseline characteristics in subjects of the Australian imaging Biomarker Lifestyle study. J. Alzheimers Dis. 27, 909–922 (2011).
Young, I. S. & Woodside, J. V. Folate and homocysteine. Curr. Opin. Clin. Nutr. Metab. Care. 3, 427–432 (2000).
Romero, S. A. et al. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am. J. Physiol. Heart Circ. Physiol. 313, H658–H666 (2017).
Hida, T. et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: the Yakumo study. Mod. Rheumatol. 28, 345–350 (2018).
Zhou, L. et al. Association between dietary sodium intake and non-alcoholic fatty liver disease in the US population. Public. Health Nutr. 24, 993–1000 (2021).
Messerli, F. H., Williams, B. & Ritz, E. Essential hypertension. Lancet. 370, 591–603 (2007).
Harreiter, J. & Roden, M. [Diabetes mellitus: definition, classification, diagnosis, screening and prevention (update 2023)]. Wien Klin. Wochenschr. 135, 7–17 (2023).
Civeira, F., Arca, M., Cenarro, A. & Hegele, R. A. A mechanism-based operational definition and classification of hypercholesterolemia. J. Clin. Lipidol. 16, 813–821 (2022).
Acknowledgements
All members of this research team are very grateful to all participants and staff involved in the NHANES project.
Funding
This work was supported by the Danone Dietary Nutrition Research and Education Fund [grant number DIC2019-10]. The funder had no role in the design and conduct of the study, data analysis, interpretation of data, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.Z. and Y.P. designed the current study; L.Z. performed data cleaning and analysis and wrote the manuscript; Y.P. contributed to data cleaning and interpretation; H.J. critically revised and edited the manuscript for important intellectual content; and all authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This data was retrieved from the public database of the National Health and Nutrition Examination Survey. The ethics approval was granted by the National Center for Health Statistics Ethics Review Committee. All procedures were carried out by relevant guidelines and regulations (Declaration of Helsinki). All individuals provided written informed consent before participating in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Peng, Y., Kong, Y. et al. The unique presentation of the relationship between red blood cell folate and appendicular skeletal muscle mass: a cross-sectional study. Sci Rep 14, 27263 (2024). https://doi.org/10.1038/s41598-024-76693-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76693-3