Abstract
Despite the intensive research on gut microbiome-associated diseases over the past 20 years, pharmacological methods for effectively eliminating pathobionts remain unsatisfactory. This study investigated the therapeutic potential of bacteriophages against Enterococcus faecalis, in which bacterial tyrosine decarboxylase (TDC) converts orally administered levodopa (L-DOPA) to dopamine, in an MPTP mouse model of Parkinson’s disease (PD). E. faecalis bacteriophages PBEF62, PBEF66, and PBEF67 (4 × 1010 PFU total/200 µl/day), and E. faecalis cells (2 × 109 CFU/200 µl/day) were orally administered at 2-h intervals before every MPTP (i.p.) and/or L-DOPA (p.o.) treatments for 13 days. The relative abundances of E. faecalis cells and bacteriophages in the feces peaked at 4 and 12 h after administration and gradually decreased by 12 and 48 h, respectively. While the administration of E. faecalis cells eliminated the beneficial effect of L-DOPA on MPTP-induced behavioral deficits, as assessed by cylinder and rotarod tests, the co-administration of bacteriophages with bacterial cells restored this effect. The modulating effects of L-DOPA, E. faecalis, and bacteriophages on PD behavior were closely associated with choline acetyltransferase expression levels in the striatum but not with tyrosine hydroxylase in the substantia nigra of each group. Recurrence and extinction of PD behaviors following treatment with E. faecalis and/or bacteriophages were also coincident with the dopamine levels in the blood and brain tissues of PD mice. The effectiveness of L-DOPA was restored after the three types of E. faecalis bacteriophages selectively eliminated E. faecalis cells, along with the TDC gene copies and transcripts responsible for converting L-DOPA to dopamine in the gastrointestinal tract. In conclusion, a combination of bacteriophages PBEF62, PBEF66, and PBEF67 targeting E. faecalis demonstrates potential as a valuable supplement to L-DOPA therapy for PD.
Similar content being viewed by others
Introduction
Recent advances in metagenomics and bioinformatics based on next-generation sequencing technology have revealed that the gut microbiome coordinates host physiology and metabolism1. Thus, gut dysbiosis is closely associated with the etiology of a variety of chronic diseases ranging from obesity, diabetes mellitus, and inflammatory bowel disease to cardiovascular, immune, and psychiatric disorders2,3,4,5. Additionally, gut microbiome acts as an enteric pharmacist, chemically modifying xenobiotics such as chemical drugs, food additives, and environmental toxins. This process can sometimes reduce bioavailability and efficacy of the drugs in the gastrointestinal environment6. Certain commensal bacteria directly or indirectly interfere with the drugs to treat various diseases. For example, E. faecalis in the human intestine decarboxylates L-DOPA, the primary medication for Parkinson’s disease. The gut Actinobacterium Eggerthella lenta inactivates the cardiac drug digoxin, used to treat atrial fibrillation and chronic heart failure. Conversely, Bifidobacterium longum and Collinsella aerofaciens can enhance the antitumor effect in programmed death 1-based immunotherapy through unknown mechanisms7,8,9,10. However, while extensive efforts have focused on shaping gut pathbionts or pathogens that cause or aggravate diseases, as well as probiotics that treat or prevent them, there have been few efforts to develop effective methods for quickly and accurately modulating specific bacterial species or taxonomic groups in the intestine. Antibiotic treatment can alter the gut microbiota, resulting in a significant decrease in taxonomic abundance and diversity. However, antibiotics cannot precisely eliminate a particular bacterial species or group due to their broad spectrum. Moreover, excessive use of antibiotics leads to bacterial resistance, making the gut microbiome a major reservoir of antibiotic resistance genes11.
In the present study, we demonstrated the therapeutic potential of bacteriophages that selectively lyse E. faecalis cells capable of metabolizing L-DOPA as potent modulators of the gut microbiome in an MPTP-induced mouse model of PD. Tyrosine decarboxylase from E. faecalis can convert orally administered L-DOPA to dopamine, thereby reducing the effective concentrations of L-DOPA in the gastrointestinal tract and brain. This reduction attenuates the therapeutic effect of L-DOPA on MPTP-induced PD behaviors because dopamine cannot cross the blood-brain barrier12,13. To address this, we first examined whether the pharmacological effect of L-DOPA was compromised by E. faecalis cells colonizing the intestines of the MPTP-induced PD mouse model. We then investigated whether co-administration of three types of E. faecalis bacteriophages could reverse the pharmacological deactivation of L-DOPA caused by E. faecalis.
Methods
Animal, bacterium, and bacteriophages
Seven-week-old male C57BL/6N mice were purchased from Central Laboratories Animal, Inc. (Seoul, Republic of Korea). Mice were housed in 3–4 animals/cage with ad libitum access to water and food at 22 ± 2 °C in a humidity-controlled room (55 ± 15%) with a 12-h light/dark cycle (08:00–20:00 h light, 20:00–08:00 h dark). The mice were acclimatized for 7 days before the experiments. Enterococcus faecalis v583 (ATCC 700802, hereinafter written as E. faecalis) and bacteriophages PBEF62, PBEF66, and PBEF67 were purchased from ATCC (Manassas, VA, USA) and Bacteriophage Bank (Gyeonggi-do, Republic of Korea), respectively. E. faecalis was grown at 37 °C for 6 h in growth medium containing 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl. Bacteriophages were propagated in the exponentially growing E. faecalis culture at 37 °C for 16 h overnight. After overnight cultivation, the bacteriophages were centrifuged at 2500×g for 10 min to separate host cell debris from the bacteriophage. The supernatant was filtered through a 0.45-µm filter and stored at 4 °C as the stock solution. To demonstrate the potential of bacteriophages as an adjunctive therapy for PD treatment, a mouse model with PD-like behavioral symptoms was developed via i.p. injections of MPTP, following the protocol described by Tatton and Kish14. Briefly, MPTP (30 mg/kg, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was injected daily for the first five days. PD behavior was then assessed using cylinder and rotarod tests one week later. Concurrently, Enterococcus faecalis and L-DOPA were administered orally to induce bacterial L-DOPA metabolism in the gastrointestinal tract. E. faecalis cells were administered daily throughout the experiment to ensure colonization of the mouse intestine. This was necessary because the bacterium was not detected in the feces of naïve mice using 16 S rRNA gene-based PCR.
Animal groups
All mice were randomly divided into 10 groups: non-treated naïve group (NOR, n = 8); vehicle-treated control for MPTP + L-DOPA treatment (Vehicle (MPTP + L-DOPA), n = 8); MPTP-treated group (MPTP, n = 8); MPTP + L-DOPA (200 mg/kg/day)-treated group (MPTP + L-DOPA, n = 8); vehicle-treated control for E. faecalis treatment (Vehicle (MPTP + L-DOPA + EF), n = 8); MPTP + L-DOPA + E. faecalis (2 × 109 CFU/mouse/day)-treated group (MPTP + L-DOPA + EF, n = 8); E. faecalis-treated naïve group (EF only, n = 8); vehicle-treated control for phage treatment (Vehicle (MPTP + L-DOPA + EF + PhaG), n = 8); MPTP + L-DOPA + E. faecalis + bacteriophages (4 × 1010 PFU/mouse/day)-treated group (MPTP + L-DOPA + EF + PhaG, n = 8); and bacteriophage-treated naïve group (PhaG only, n = 8). The animal study evaluating the behavioral effects of treatments of L-DOPA, E. faecalis, and bacteriophages was conducted separately from the investigation that included body weight measurements and the collection of feces, brain, and blood samples. For the collection of blood and tissue samples, the animals were euthanized using isoflurane. To observe temporal changes in E. faecalis cells and bacteriophages in the gastrointestinal tract, E. faecalis (2 × 109 CFU/mouse/day) and three bacteriophages (4 × 1010 PFU in total) were orally administered once to naïve mice (n = 6), fecal samples (n ≥ 3 at each time point) were collected continuously for 48 h, and the amounts of bacterial cells and bacteriophages were measured using 16 S rRNA gene-based PCR and plaque assay, respectively.
Determination of bacteriophage titer
The titer of each phage, expressed as PFU, was determined using the double-layer agar technique described by Sambrook et al.15. Briefly, 100 µL of dilution of each phage sample was added to 100 µL of a E. faecalis suspension grown overnight at 37 °C. This mixed solution was added to 4 mL top agar, gently homogenized, and poured into a 90-mm Petri dish previously prepared with 10 mL bottom agar. The plates were gently swirled, dried for 10 min at room temperature, and then incubated at 37 °C overnight. The number of plaques on each plate was counted.
Plaque assay
For measuring E. faecalis bacteriophage titers in feces, 0.02 g of feces was dispersed in 500 µL of brain heart infusion (BHI) medium. After vortexing, the medium was centrifuged at 5000 rpm for 5 min, and the supernatant was transferred to a new tube. The supernatant (100 µL) was mixed with 2 µL MgSO4 and 98 µL of overnight culture of E. faecalis and then incubated at 37 °C for 20 min. After incubation, 200 µL of mixed solution was poured into 5 mL top agar and overlaid on a BHI agar plate. All agar plates were incubated at 37 °C overnight for 16 h before examination for numbering plaques.
Behavioral tests
All behavioral tests were performed during the light period, between 9 am to 6 pm. Prior to each test, the animals were acclimated to the testing room in their home cages for 30 min. The cylinder test was used to evaluate motor function in MPTP-induced PD mice. For this test, mice were placed in a transparent plastic cylinder (12 cm in diameter and 20 cm in height) for 4 min without prior habituation. Observers, who were blinded to the experimental conditions, recorded the number of paw lifts (wall contacts) made with both forelimbs for 3 min following a 1-min adaptation period. Lifts involving only one forelimb were not counted. Paw lift behavior was considered only when the mouse reared up on its hindlimbs, raised both forelimbs above shoulder level, and landed. The rotarod test was performed to evaluate behavioral impairment of fore and hind limb motor coordination and balance. A rod instrument (MED Associates, VT, USA) was used to record the fall time from the rod. One hour before the experiment, mice were trained for 2 min in accelerating rod speed mode. The time spent on the rod was recorded for a maximum of 480 s at successive rod speeds (0–35 rpm). For the rotarod test, any animal that fell within the first 30 s was immediately placed back on the apparatus for re-measurement. On each testing day (Day 11 and 12), L-DOPA was administrated two hours before after the behavioral tests to allow sufficient time for L-DOPA activity to take effect.
Immunohistochemistry
For brain sampling, the mice were euthanized with isoflurane (2%; BK PHARM Co., Goyang-si, Republic of Korea) and perfused with 0.1 M phosphate-buffered saline (PBS) followed by cold 10% neutral buffered formalin (HT501128, Sigma-Aldrich Chemical Co.), approximately 6 hours after L-DOPA administration on Day 12. The brains were removed, post-fixed overnight, and cryoprotected with 30% sucrose in 0.1 M PBS at 4 °C. The brains were frozen and cut into 30-µm slices. Sections were incubated with the primary antibodies mouse anti-TH (1:1000, sc-136100, Santa Cruz Biotechnology, Dallas, TX, USA) and sheep anti-ChAT (1:1000, AB1582, Chemicon, Temecula, CA, USA) and secondary antibodies biotinylated goat anti-mouse IgG (1:1000, BA-9200, Vector Laboratories, Burlingame, CA, USA) and rabbit anti-sheep IgG (PK-6106, Vector Laboratories, Burlingame, CA, USA) using the ABC kit (PK-6100, Vector Laboratories, Burlingame, CA, USA). Sections were then stained with 3,3′-diaminobenzidine substrate kit (SK-4100, Vector Laboratories). Histological images were obtained using a bright-field microscope (BX51, Olympus Japan Co., Tokyo, Japan).
PCR-based quantitation of E. faecalis cells and TDC in feces
To investigate time-dependent changes in E. faecalis cells and their TDC levels, genomic DNA was extracted from fecal samples using the SPINeasy DNA kit (MP Biomedicals, Irvine, CA, USA). The primers used were as follows: 16S rDNA gene for all bacteria (forward; 5′-TGGCTCAGGACGAACGCTGGCGGC-3′, reverse; 5′-CCTACTGCTGCCTCCCGTAGGAGT-3′; annealing temp.: 66 °C, 348 bp, 30 cycles)16, E. faecalis DDL (D-alanine–D-alanine ligase) gene (forward; 5′-ATCAAGTACAGTTAGTCTT-3′, reverse; 5′-ACGATTCAAAGCTAACTG-3′; 50 °C, 941 bp, 30 cycles)17, bacterial TDC (tyrosine decarboxylase) gene (forward; 5′- CGTTGTTGGTGTTGTTGGCACNACNGARGARG-3′, reverse; 5′-CCGCCAGCAGAATATGGAAYRTANCCCAT-3′; 56 °C, 350 bp, 30 cycles)18.
ELISA of L-DOPA and dopamine in blood and feces
Mice were euthanized with isoflurane (2%; BK PHARM Co.) for blood sampling, approximately 6 h after L-DOPA administration on that day. Blood was withdrawn by heart puncture and placed in tubes coated with 5 mM EDTA. The collected blood samples were centrifuged at 3000 rpm and 4 °C for 30 min and the plasma was stored at − 80 °C. Plasma and feces levels of L-DOPA (MBS9343615, MyBioSource, San Diego, CA, USA) and dopamine (MBS269234, MyBioSource) were measured using ELISA kits. ELISA plates were read at 450 nm using a microplate reader (VersaMax™ Tunable, Molecular Devices, Sunnyvale, CA, USA).
LC-MS/MS operating in multiple reaction monitoring (MRM)
L-DOPA and dopamine levels in brain tissues were analyzed using HPLC-tandem MS. For preparation of brain tissue samples, whole brain was rapidly dissected, snap-frozen in liquid nitrogen, and stored at − 80 °C. Brain samples were homogenized on ice using an ultrasonicator (SONICS VCX 130; Sonics & Materials Inc., Newtown, CT, USA) with the addition of type 1 water at a proportion of 20 mL/g tissue on ice. Homogenates were mixed with an equal volume of ice-cold 250 mM formic acid (33015; Sigma-Aldrich Chemical Co.) and vortexed for 20 s. The mixed samples were centrifuged at 14,000g and 4 °C for 10 min. After centrifugation, the supernatant was filtered with a 0.2 μm pore PVDF membrane syringe filter (Whatman, Maidstone, Kent, UK) and transferred to amber colored vials. Chromatographic separation was achieved using a Waters ACQUITY UPLC BEH C18 column (100 × 2.1 mm, 1.7 μm) combined with a Waters UPLC H-Class system (Waters, Milford, MA, USA). Gradient elution was performed using mobile phase A, 0.1% formic acid in water, and mobile phase B, 0.1% formic acid in acetonitrile. The flow rate and injection volume were set to 200 µL/min and 10 µL, respectively. All analytes were analyzed using an API 3200 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA). Samples were analyzed by Turbo V electrospray ionization in the positive ion MRM mode. Quantifier MRM transitions were achieved with m/z of 154 → 137 for dopamine and m/z 198 → 152 for L-DOPA. Qualifier MRM transitions were achieved with m/z 154 → 119 for dopamine and m/z 198 → 139 for LD. Quantifier and qualifier MRM transitions were selected as the most abundant and second most intense product ions, respectively. The mass spectrometric conditions were set as follows: ion spray voltage (IS) at 4500 V, curtain gas (CUR) at 20 psi, nebulizer gas (GS1) at 50 psi, auxiliary gas (GS2) at 50 psi, temperature (TEM) at 500 °C and collision-activated dissociation (CAD) gas at 5. The declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) were set to 20, 5, 15, and 3 for dopamine and 20, 9, 19/28 (QN/QL), and 11/5 (QN/QL) for L-DOPA, respectively. Data acquisition and analysis of all MRM chromatograms were performed using the Analyst 1.5 software (Applied Biosystems Inc.).
Statistical analysis
Statistical analyses were performed using the Prism ver. 8.0 software (GraphPad Software, San Diego, CA, USA). All measurements were performed by an independent investigator blind to the experimental conditions, and the results are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) or two-way ANOVA, succeeded by Tukey’s post-hoc tests for multiple comparisons were employed for statistical evaluations. A significance threshold of p < 0.05 was considered for all statistical analyses.
Ethics approval
The ethics and care of all animal experiments received approval from the Animal Care and Ethics Committees of Kyung Hee University (KHSASP-21-119) and the Institutional Animal Care and Use Committee (IACUC) of Korea, and all methods were in accordance the relevant protocols, guidelines and regulations. This was approved by the Korean Council on Animal Care and the Korean Animal Protection Law, 2007; Article 13 (Animal Experiments). All animal studies adhered to the ARRIVE 2.0 requirements, which included study design, animal numbers, randomization, and statistical methods.
Consent for publication
The paper was read and approved by all writers.
Results
In vitro examination of conversion of L-DOPA to dopamine by E. faecalis culture broth and lysis of E. faecalis cells by E. faecalis bacteriophages
The in vitro conversion of L-DOPA to dopamine by a pure culture broth of E. faecalis was verified using HPLC analysis and compared to that of an E. coli culture broth as a control. Additionally, the in vitro conversion of L-DOPA by supernatants of dissolved feces or extracts of the small and large intestines of mice administered a single dose of E. faecalis cells (2 × 109 CFU) was confirmed using the same methodology (data not shown).
Subsequently, the infection and lysis of E. faecalis cells by three types of E. faecalis bacteriophages—PBEF62, PBEF66, and PBEF67—were identified using an in vitro plaque assay (Fig. 1). When high-titer phage lysates (2.62 × 109, 3.90 × 107, and 7.0 × 107 plaque-forming units (PFU)) of three different E. faecalis bacteriophages were applied, plaques with hollow zones were clearly observed on the top-agar lawns of E. faecalis on nutrient agar plates. However, since the plaque shapes formed by the three types of pages were not distinct from one another, only the total number of plaques was counted. Additionally, it was confirmed that the supernatants of dissolved feces and mucus layers from the colon barrier surfaces of naïve mice showed no plaques on E. faecalis lawn agar plates.
Temporal population dynamics of orally administered E. faecalis cells and E. faecalis bacteriophages in the gastrointestinal tracts of mice
To investigate the in vivo adherence and colonization ability of E. faecalis cells and E. faecalis bacteriophages in the gastrointestinal tract after oral administration, time-dependent alterations of both population sizes in the fecal samples were examined at 2, 4, 6, 8, 12, 24, and 48 h after administration. To this end, three types of 4 × 1010 PFU total bacteriophages and 2 × 109 CFU E. faecalis cells were individually administered once to the mice, and their feces were continually harvested. E. faecalis cells in the feces, the amounts of which were indicated by PCR band intensity of DDL (D-alanine–D-alanine ligase) gene, increased up to 4 h after administration, gradually decreased to 12 h, and then disappeared. It means that the administered bacteria washed out from the intestine after 12 h based on the PCR detection method (Fig. 2a; F6,14 = 98.58, p < 0.001). The relative level of E. faecalis tyrosine decarboxylase gene, which converts L-DOPA to dopamine in the gastrointestinal tract, also exhibited a pattern similar to that of E. faecalis cells (Fig. 2b; F6,14 = 26.18, p < 0.001). In contrast, the three types of bacteriophages remained high at 8–12 h in the feces and completely disappeared at 48 h based on the plaque assay (Fig. 2c; F6,14 = 3.790, p = 0.0187).
Time-courses of the relative amounts of DDL gene copies indicating E. faecalis cell numbers (a), bacterial TDC (tyrosine decarboxylase) gene copies (b), and three types of bacteriophages (4 × 1010 PFU total, plaque assay for (c)). n = 3 per group. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage. All experiments were independently conducted at least three times, and the results were analyzed by one-way ANOVA test. The results are indicated as mean ± standard error, and the statistical significance was defined as a p-value of *p < 0.05, **p < 0.01, and ***p < 0.001.
Long-term administration of E. faecalis inhibits the pharmacological effect of L-DOPA and co-administration with E. faecalis bacteriophages avoids the effect in MPTP-induced PD mouse model
Based on the in vitro activity of E. faecalis, which decarboxylates L-DOPA to dopamine, it was demonstrated that colonization of E. faecalis cells in the gastrointestinal tract could inhibit the pharmacological effects of L-DOPA on PD behavior in an MPTP-induced PD mouse model. The entire schedule of animal experiments is shown in Fig. 3a. At first, it was observed that MPTP treatment for 5 days significantly induced behavioral impairments such as locomotor dysfunction and sensorimotor coordination deficits, assessed by cylinder and rotarod performance, and oral administration of L-DOPA exhibited therapeutic effects on MPTP-induced PD behaviors (‘LD effect’ in Fig. 3b; F2,21 = 28.84, p < 0.001 and Fig. 3c; F2,21 = 24.19, p < 0.001). However, co-administration of E. faecalis with L-DOPA daily for 13 days eliminated the pharmacological effects of L-DOPA, as shown in the ‘EF effect’ in Fig. 3b (F2,21 = 23.30, p < 0.001) and Fig. 3c (F2,21 = 30.18, p < 0.001). Further, administration of E. faecalis to naïve mice for the same period did not cause any behavioral change, as observed in the EF only groups. Subsequently, we examined whether co-administration of the three kinds of E. faecalis bacteriophages could modulate the inhibitory effect of E. faecalis cells on LD-induced extinction of PD behaviors in the MPTP-PD mouse model. As shown in ‘PhaG effect’ in Fig. 3b (F2,21 = 0.9545, p = 0.4011) and Fig. 3c (F2,21 = 4.071, p = 0.0320), co-administration of phages with E. faecalis cells daily for 13 days attenuated ‘EF effect’ by E. faecalis in the intestine of L-DOPA -treated PD mouse model, resulting in the recurrence of PD behaviors even though the effects were not statistically significant. Administration of the same dose of phages for the same period did not cause any change in the behavior of naïve mice, as shown in the PhaG only groups. The body weights slightly decreased immediately after the i.p. injection of MPTP on Day 1 in all MPTP-treated groups and then showed an increasing rate similar to those of naïve mice during the entire study period. Further, no significant difference was observed in the body weights of treatment groups of L-DOPA, E. faecalis cells, and E. faecalis bacteriophages (Fig. 3d).
Schematic drawing of the experimental schedule (a), the effects of long-term administration of E. faecalis cells and/or E. faecalis bacteriophages on the therapeutic effect of L-DOPA, indicated by PD behaviors using cylinder (b) and rotarod tests (c) in MPTP-induced PD mouse model, and changes in the body weights of several main groups (d). Behavioral tests were performed during the last 2 days of the entire 12-day experimental period following the initial MPTP injection. n = 8 per group. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage; d: day; w: week. ***p < 0.001 vs. each Vehicle group, ###p < 0.001 vs. MPTP or MPTP + L-DOPA + EF group. All results are indicated by mean ± standard error analyzed by one-way ANOVA or two-way ANOVA (for body weight) tests. The mice (6-week-old) were forcefully administered L-DOPA for 12 days and E. faecalis cells and E. faecalis bacteriophages for 13 days. The cylinder and rotarod tests for locomotor function were performed during the last 2 days of the entire 12-day-experimental period after the first MPTP injection on Day 1. i.p.: intraperitoneal; p.o.: per oral; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; CFU: colony forming unit; PFU: plaque forming unit.
Next, we measured the relative amounts of E. faecalis cells and their bacteriophages in feces harvested after the behavioral tests at the end of the experimental schedule. As verified in Fig. 3b and c, the use of solvents as vehicles for MPTP, L-DOPA, EF, and/or PhaG did not significantly affect Parkinson’s behaviors in our mouse model. Therefore, for the RT-PCR and plaque assays on feces, the LC-MS/MS assay on blood, and the histological analysis on whole brains, samples were obtained from mouse groups including NOR(naïve), MPTP, MPTP + L-DOPA, MPTP + L-DOPA + EF, and MPTP + L-DOPA + EF + PhaG, without vehicle control groups, in a separate animal study.
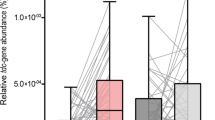
PCRs of E. faecalis-specific DDL gene and the bacterial TDC gene were performed using fecal DNA in each group as the template, and their band intensities were compared with those of 16 S rDNAs of all bacteria (Fig. 4). In groups treated only with E. faecalis cells, such as the MPTP + L-DOPA + EF group, there was a marked increase in E. faecalis-specific DDL copy number, and this increase was significantly ameliorated by simultaneous treatment with E. faecalis bacteriophages in the MPTP + L-DOPA + EF + PhaG group (Fig. 4a; F4,10 = 8.718, p = 0.0027). However, no PCR band was detected in the non-treated groups, such as the non-treated NOR (naïve), MPTP, and MPTP + L-DOPA groups. In case of bacterial TDC genes, the changing pattern of PCR bands among groups was quite similar to that of E. faecalis-specific DDL gene, except for a more remarkable increase in E. faecalis-specific DDL gene copy numbers in MPTP + L-DOPA + EF + PhaG group and basal levels detected in the non-treated groups (Fig. 4b; F4,10 = 29.20, p < 0.001). At the mRNA expression level of bacterial TDC genes, the changing pattern of RT-PCR bands was similar to that of the gene copy number itself; however, the TDC mRNA level in MPTP + L-DOPA + EF + PhaG group almost restored to that of the non-treated groups (Fig. 4c; F4,10 = 19.00, p < 0.001). In case of E. faecalis bacteriophages in feces, plaques were not detected in NOR, MPTP, MPTP + L-DOPA, and MPTP + L-DOPA + EF groups. However, as expected, a significant number of plaques was observed on the agar plates with E. faecalis lawn only in the feces of MPTP + L-DOPA + EF + PhaG group (Fig. 5a,b; F4,10 = 5.490, p = 0.0133).
The relative amounts of DDL (D-alanine–D-alanine ligase) gene copies indicating E. faecalis cell numbers (a), bacterial TDC gene copies (b), and its mRNA transcript (c) in the feces of MPTP-induced PD mice treated with L-DOPA for 12 days. Primers targeting 16 S rDNA gene for all bacteria were used as internal controls for sample bias and total bacteria load in PCR analysis. n = 3 per group. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage; ns: non-significance. $$p < 0.05 and $$$p < 0.001 vs. NOR, MPTP, or MPTP + L-DOPA group; &p < 0.05 vs. MPTP + L-DOPA + EF group. All results are mean ± standard error analyzed by one-way ANOVA and confirmed by Tukey post hoc test.
Representative images of E. faecalis bacteriophage plaques on agar plates (a), and the bar graph indicating the plaque numbers (b) in the feces from MPTP-induced PD mice treated with L-DOPA for 12 days, and E. faecalis and/or E. faecalis bacteriophages for 13 days. n = 3 per group. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage. *p < 0.05 vs. NOR, MPTP, or MPTP + L-DOPA, or MPTP + L-DOPA + EF group. All results are expressed as mean ± standard error analyzed by one-way ANOVA and confirmed by Tukey post hoc test.
Immunohistochemistry of tyrosine hydroxylase in the substantia nigra (SN) and choline acetyltransferase in the striatum confirms depletion and supplementation of dopamine in the brain, respectively
To verify the loss of dopaminergic neurons resulting in dopamine depletion in the brain following i.p. injection of MPTP, the brains in each group were harvested after behavioral evaluation on Day 12, 30-µm thick frozen brain sections were obtained, and immunohistochemical staining of tyrosine hydroxylase (TH) in the substantia nigra pars compacta (SNpc) and choline acetyltransferase (ChAT) in the striatum was performed (Fig. 6a,b). Subchronic i.p. treatment with MPTP for 5 days significantly decreased the number of TH-immunopositive dopaminergic neurons in SN, a midbrain dopaminergic nucleus, in all MPTP-treated groups such as the MPTP, MPTP + L-DOPA, MPTP + L-DOPA + EF, and MPTP + L-DOPA + EF + PhaG groups compared to naïve mouse brains in the NOR group. No significant difference was observed among the MPTP-treated groups (Fig. 6c; F4,10 = 89.72, p < 0.001).
Representative stained images of tyrosine hydroxylase (TH)-immunopositive cells, substantia nigra pars compacta (SNpc, a), and choline acetyltransferase (ChAT)-immunopositive cells in the striatum (b) of L-DOPA-, E. faecalis-, and E. faecalis bacteriophage-treated mouse models with MPTP-induced Parkinson’s disease. Scale bar: 200 μm and 500 μm. The numbers of TH- and ChAT-immunopositive neurons were counted in the SNpc and striatum regions, as indicated by the dotted red lines in the NOR group images of (a) and (b). These counts are quantified in the bar graphs of (c) and (d), respectively. The images of SNpc and striatum are captured from the right side of the brain. n = 3 per group. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage; ns: non-significance. ***p < 0.001 vs. NOR group; ###p < 0.05 vs. MPTP group; $$p < 0.01 vs. MPTP + L-DOPA group; &p < 0.05 vs. MPTP + L-DOPA + EF group. All results are expressed as mean ± standard error analyzed by one-way ANOVA and confirmed by Tukey post hoc test.
Subsequently, under the condition of depletion of dopaminergic neurons in the SNpc by MPTP injection, the neurofunctional effects of dopamine supplemented in the form of L-DOPA from outside were investigated by measuring the immunoreactivity of ChAT in the striatum of L-DOPA -treated PD mouse groups such as the MPTP + L-DOPA, MPTP + L-DOPA + EF, and MPTP + L-DOPA + EF + PhaG groups. The selective deficiency of dopaminergic innervation in the midbrain by MPTP treatment resulted in a significant reduction in cholinergic interneurons, as indicated by ChAT immunoreactivity in the striatum, as compared to that in non-treated naïve mice (Fig. 6d; F4,10 = 27.15, p < 0.001). However, oral and daily administration of 200 mg/kg/day L-DOPA for 12 days significantly restored the number of ChAT-immunopositive cells in the brains of MPTP + L-DOPA group. Consistent with the behavioral results, administration of E. faecalis cells with L-DOPA eliminated the pharmacological effect of L-DOPA treatment and co-administration of E. faecalis bacteriophages with E. faecalis cells significantly blocked the effect of E. faecalis cells in MPTP + L-DOPA-treated mice.
Blood and brain concentrations of L-DOPA and dopamine represent the modulating effects of E. faecalis and its bacteriophages on intestinal L-DOPA metabolism in MPTP-induced PD mouse model
To investigate whether the administration of E. faecalis cells and/or E. faecalis bacteriophages affects L-DOPA metabolism in the intestine and the resultant amounts of L-DOPA and dopamine absorbed across the intestinal lining into the bloodstream, serum concentrations of L-DOPA and dopamine were measured in each group using enzyme-linked immunosorbent assay (ELISA) kits. The blood concentration of L-DOPA in all L-DOPA -treated mice, such as the MPTP + L-DOPA, MPTP + L-DOPA + EF, and MPTP + L-DOPA + EF + PhaG groups, was significantly higher than that in the non-treated groups such as NOR and MPTP groups (Fig. 7a; F4,30 = 16.62, p < 0.001). No significant difference was observed between the L-DOPA-treated groups. However, dopamine concentration significantly increased in the MPTP + L-DOPA + EF group as compared to that in the MPTP + L-DOPA group. This increase was almost completely inhibited by treatment with E. facealis bacteriophages in the MPTP + L-DOPA + EF + PhaG group (Fig. 7b; F4,30 = 9.157, p < 0.001). In the MPTP + L-DOPA group, the increase in the blood concentration of dopamine after daily administration of L-DOPA for 12 days was not as high as that of L-DOPA.
The concentrations of L-DOPA in plasma (a), dopamine in plasma (b) and whole brain tissues (c), and standard HPLC chromatograms of dopamine and L-DOPA (d) in MPTP-induced PD mice forcefully given L-DOPA for 12 days and E. faecalis and/or E. faecalis bacteriophages for 13 days. The concentrations of L-DOPA or dopamine in plasma (n = 7) and brain tissues (n = 5) were determined by ELISA and LC-MS/MS analysis, respectively. L-DOPA: levodopa; EF: E. faecalis; PhaG: bacteriophage; ns: non-significance. ***p < 0.001 vs. NOR group; ##p < 0.05 and ###p vs. MPTP group; $$p < 0.01 vs. MPTP + L-DOPA group; &p < 0.05 vs. MPTP + L-DOPA + EF group. All results are expressed as mean ± standard error, analyzed by one-way ANOVA and confirmed by Tukey post hoc test.
The levels of L-DOPA and dopamine in the whole brain of each group were also measured using liquid chromatography in combination with tandem MS (LC-MS/MS) (Fig. 7d). Unfortunately, L-DOPA was not detected in any group. However, as shown in Fig. 7c, MPTP treatment significantly reduced dopamine concentrations in the mouse brain of MPTP group, and this reduction was significantly restored by exogenous L-DOPA supplementation in the MPTP + L-DOPA group. In the MPTP + L-DOPA + EF group, the pharmacological effect of L-DOPA was completely inhibited by long-term administration of E. faecalis cells, and this inhibitory effect was eliminated by co-administration of E. faecalis bacteriophages with E. faecalis cells, of which changes in the patterns were consistent with that of behavioral outcomes in each group (Fig. 7c; F4,20 = 25.65, p < 0.001).
Discussion
Unlike viruses that affect human and animal cells, bacteriophages are a unique group of viruses that specifically target bacteria and archaea. They infect these microorganisms, replicate within them, and eventually destroy them. Due to their selectivity and efficacy in killing target bacteria, bacteriophages have been used as alternative therapies for a variety of bacterial infections, primarily in the Soviet Union and Eastern Europe19. Recently, bacteriophage therapy has gained increasing attention as a promising approach for modulating intestinal microbiome, which is closely linked to chronic conditions ranging from obesity to brain disorders and aging20. Despite its potential as a negative modulator of microbiota, the practical application of bacteriophages in pharmaceuticals has been hindered by challenges such as stabilizing the preparations, public safety concerns, and the potential development of bacterial resistance21. Nonetheless, many animal and clinical studies have not reported any serious side effects associated with bacteriophage therapy for pathogenic bacteria22,23. In a single-arm, non-comparative clinical trial of bacteriophage therapy, in which a preparation of three Myoviridae bacteriophages was administered intravenously to treat severe Staphylococcus aureus infection, no significant adverse reaction or in vivo phage resistance were observed24.
This highlights the potential of bacteriophage therapy as a model for addressing the ectopic metabolism of orally administered drugs. This metabolism involves chemical processes such as reduction, hydrolysis, and carboxylation of ingested drugs, catalyzed by enzymes in human intestinal microbiota, which can either neutralize or render them toxic in the intestinal tract6. Eliminating specific intestinal bacteria that modify these drugs can be more immediate and effective than targeting intestinal pathobionts or pathogens associated with diseases of unclear etiology, such as Clostridium difficile, which causes diarrhea and colitis25. However, this approach only focuses on the interactions between ingested drugs and specific intestinal bacteria, bypassing the more complex interactions with human intestinal epithelial cells or mucosa in the gastrointestinal tract.
In this study, L-DOPA, the primary medication for managing motor function in PD, and its unwanted conversion (decarboxylation) to dopamine by E. faecalis, a commensal bacterium in the human intestine, were selected to demonstrate the potential of bacteriophages as modulators of intestinal microbiota. However, unlike in humans, bacterial TDC enzymes, including the one from E. faecalis that carboxylates L-DOPA as well as its primary substrate, tyrosine, were not detected in the feces and mucus layers in the gastrointestinal tract in naïve C57BL/6N mice using 16 S rDNA gene PCR. Consequently, to establish E. faecalis in the gastrointestinal tract, cultured E. faecalis cells were repeatedly administered into the mouse stomach via an oral zoned needle.
However, measurements of E. faecalis-specific DDL gene copies, which indicate the number of E. faecalis cells in fecal samples collected over time, showed that a single administration of bacterial cells did not persist in the gastrointestinal tract for more than a day, disappearing within 12 h of oral administration. This suggests that most orally administered bacterial cells rapidly transit through the gastrointestinal tract and expelled with undigested food. In many probiotic studies, ensuring the adherence and colonization of probiotic strains such as Lactobacillus, Bifidobacterium and Enterococcus is critical for achieving long-term health benefits26,27. For example, fluorescence-labeled Lactobacillus rhamnosus (109 CFU) administered once orally to BALB/c mice was detected in significant numbers in the mucus epithelial barrier of the duodenum, jejunum, ileum, and colon for up to 7 days, despite most bacteria likely being expelled in feces after intragastric administration28. However, for gut microbiome-associated drug metabolism, factors such as the population size of target bacteria that modify drugs, the contact time between ingested drugs and drug-metabolizing gut bacteria, and the reaction conditions in the intestine are crucial. Thus, the abundance of drug-metabolizing bacteria in the intestinal lumen is more critical than the small number of bacteria that adhere to the outer mucus layer of the intestinal epithelial cells and persist for a short period.
Other studies have used a variety of antibiotics to negatively regulate intestinal bacterial strains or taxonomic groups, especially harmful pathobionts that adversely affect human health29. However, as noted, antibiotic-induced removal of specific gut microbiota is often unsatisfactory, as it does not precisely target individual species or strains and can result in significant side-effects. For example, excessive antibiotic use can lead to psychiatric disorders such as anxiety and cognitive impairment, as well as severe colitis30,31,32. Consequently, there is a pressing need for effective methods to accurately and quickly remove intestinal bacteria known to cause diseases and dysfunction. Despite this, bacteriophage therapy has mainly been used to treat bacterial infections in the gastrointestinal tract and other organs24,25,26. Although the results of these studies have often been suboptimal, many findings strongly indicate that bacteriophage therapy is a promising and safe alternative to antibiotics33,34,35.
In the present study, to identify the conversion of ingested L-DOPA to dopamine by the mouse enzyme aromatic amino acid decarboxylase (AADC) in the brain tissues or by the E. faecalis TDC enzyme in the intestine, we measured the concentrations of L-DOPA and dopamine in both blood and brain tissues of mice using an ELISA kit and LC/MS, respectively. Unfortunately, dopamine was detected only in the brain tissue, and L-DOPA was not detected, whereas both L-DOPA and dopamine were detected in the blood. This discrepancy may be due to the longer and more complex pretreatment process required for the pretreatment of brain tissue samples for LC/MS analysis compared to blood samples for ELISA. It is well-known that L-DOPA is chemically unstable even when ingested with the AADC inhibitor, carbidopa, unless refrigerated; its stability significantly declines after 48 h at room temperature in solution form36.
In the brain, dopamine levels in all groups were closely associated with behavioral outcomes, indicating that the variations in motor function due to administration of L-DOPA, E. faecalis, and/or E. faecalis phages in the MPTP-induced PD mouse model were reflected in similar fluctuations in brain dopamine levels. However, blood levels of L-DOPA and dopamine did not align with the observed behavioral changes in the mouse model of PD. As shown in Fig. 7a and b, comparing the blood concentrations of L-DOPA and dopamine in the MPTP + L-DOPA group suggests that only a small portion (less than 2%) of L-DOPA is converted to dopamine in the intestinal tract or blood without E. faecalis, indicating that dopamine conversion by AADC in these areas is minimal and negligible. However, comparing the blood concentrations of L-DOPA and dopamine in the MPTP + L-DOPA and MPTP + L-DOPA + EF groups confirmed the undesirable conversion of L-DOPA to dopamine by E. faecalis TDCs and the blocking of this conversion by phages in the gastrointestinal tract.
E. faecalis and its bacteriophages were administered orally, meaning their potential impact on L-DOPA levels, would be limited to the gastrointestinal tract. They would not influence L-DOPA’s bioavailability once it had entered the bloodstream. Therefore, the blood level of L-DOPA in the MPTP + L-DOPA + EF and MPTP + L-DOPA + EF + PhaG groups is a key indicator of the bioavailability of L-DOPA in relation to the dose of bacteriophages administered. In this study, we measured L-DOPA levels only in blood and brain tissues, without assessing levels within the gastrointestinal tract. However, on the last day of the experiment (Day 12), we measured L-DOPA levels in feces using ELISA and observed a significant reduction in the MPTP + L-DOPA + EF mouse group compared to the MPTP + L-DOPA mouse group. Nonetheless, there was no significant increase in L-DOPA in the MPTP + L-DOPA group compared to the MPTP group, nor in the MPTP + L-DOPA + EF + PhaG group compared to the MPTP + L-DOPA + EF group (data not shown).
We did not measure the time-course of L-DOPA levels in blood following a single treatment with E. faecalis and its bacteriophages, leaving the pharmacokinetic relationship between the administered phages and blood L-DOPA levels remains unexamined. Our focus was on determining whether the effective concentrations of dopamine in blood and brain could be modulated by the oral administration of E. faecalis and its bacteriophages. Blood samples for measuring L-DOPA and dopamine levels were collected only after completing two behavioral tests on the last day of the experiment (Day 12). This indicates that L-DOPA concentration shown in Fig. 7a was measured approximately four hours after oral administration, as the behavioral tests began two hours post-L-DOPA administration and lasted for about one to two hours. It is well established that L-DOPA is a short-acting drug, reaching peak plasma concentration between 0.5 h after oral administration and rapidly metabolized in the body37. Therefore, the blood L-DOPA concentration at the time of sampling in this study could be significantly lower compared to the initial dose treated (200 mg/kg of body weight daily for 12 days).
The MPTP-induced impairment of motor function, its recovery through L-DOPA treatment, and the subsequent fluctuations in motor function following the administration of E. faecalis and/or E. faecalis phages are ultimately attributable to the dopamine levels in the brain, regardless of whether the dopamine is supplied externally or produced by dopaminergic neurons in the midbrain. From this perspective, it is meaningful that the changes in motor behaviors observed in each group in this study corresponded with changes in brain dopamine levels. Additionally, the neuropharmacological effects of dopamine, supplied exogenously as L-DOPA, were validated through immunohistochemical analysis of striatal cholinergic neurons in striatum38,39,40. Research has shown that the interplay between dopamine and acetylcholine at the synapses in the striatum is crucial for the synchronized functioning of motor and cognitive abilities. Activity of striatal cholinergic interneurons is reduced by selective dopaminergic depletion in the midbrain, leading to decreased acetylcholine availability or an imbalance between cholinergic and dopaminergic systems in the striatum41,42. Therefore, we investigated changes in cholinergic tone in the striatum of each group by performing immunostaining for ChAT. The results strongly correlated with the behavioral data and were consistent with previous findings38,39,40.
This study demonstrated the significant impact of orally administered E. faecalis cells and E. faecalis bacteriophages on the effectiveness of L-DOPA, a common treatment for motor function impairment in PD, using a mouse model. The observed effects included improvements in PD-related behavior, modulation of striatal cholinergic activity, and changes in dopamine levels in both the brain and blood. However, further research is needed to optimize the experimental conditions. This includes establishing longer-lasting colonization of E. faecalis through fecal microbiota transplantation (FMT) in germ-free mice, developing an excipient that can sustain phage infection and lytic activity, and determining the ideal doses and treatment durations for oral administration of E. faecalis bacteriophages. Additionally, the effect will need to be confirmed in other experimental models of PD.
Conclusions
In summary, our results demonstrated that bacteriophages can accurately target and effectively remove specific intestinal microbes. This highlights their invaluable potential as negative regulators of the intestinal microbiome that cause or exacerbate diseases or ectopically metabolize orally administered drugs for treatment. However, for the commercialization of bacteriophages as comprehensive tools for gut microbiota modulation, further studies are needed. These should include developing genetically engineered phages to prevent entry into the lysogenic cycle and alter host specificity, as well as creating optimal formulations for the intestinal tract.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TDC:
-
Tyrosine decarboxylase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PFU:
-
Plaque-forming unit
- CFU:
-
Colony-forming unit
- L-DOPA:
-
l-3,4-Dihydroxyphenylalanine (levodopa)
- PD:
-
Parkinson’s disease
- PCR:
-
Polymerase chain reaction
- PhaG:
-
Bacteriophage
- BHI:
-
Brain heart infusion
- PBS:
-
Phosphate-buffered saline
- TH:
-
Tyrosine hydroxylase
- ChAT:
-
Choline acetyltransferase
- DDL:
-
D-alanine-D-alanine ligase
- EDTA:
-
Ethylene-diamine-tetraacetic acid
- HPLC:
-
High performance liquid chromatography
- MS:
-
Mass spectrometry
- UPLC:
-
Ultra performance liquid chromatography
- PVDF:
-
Polyvinylidene difluoride
- MRM:
-
Multiple reaction monitoring
- CFU:
-
Colony-forming unit
- SN:
-
Substantia nigra
- SNpc:
-
Substantia nigra pars compacta
- ELISA:
-
Enzyme-linked immunosorbent assay
- AADC:
-
Aromatic amino acid decarboxylase
- FMT:
-
Fecal microbiota transplantation
References
Tropini, C., Earle, K. A., Huang, K. C. & Sonnenburg, J. L. The gut microbiome: Connecting spatial organization to function. Cell Host Microbe 21, 433–442 (2017).
Velasquez-Manoff, M. The peacekeepers amid the trillions of microbes that live in the intestines, scientists have found a few species that seem to play a key role in keeping us healthy. Nature 518, S4-11 (2015).
Andrioaie, I. M. et al. The role of the gut microbiome in psychiatric disorders. Microorganisms 10, 2436 (2022).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Gebrayel, P. et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 20, 1–20 (2022).
Spanogiannopoulos, P., Bess, E. N., Carmody, R. N. & Turnbaugh, P. J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 14, 273–287 (2016).
Rekdal, V. M., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J. & Balskus, E. P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364, eaau6323 (2019).
Haiser, H. J., Seim, K. L., Balskus, E. P. & Turnbaugh, P. J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes 5, 233–238 (2014).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Zhong, Z., Ye, M. & Yan, F. A review of studies on gut microbiota and levodopa metabolism. Front. Neurol. 14, 1046910 (2023).
Francino, M. P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 6, 1–11 (2016).
Zhang, Y. et al. Association between microbial tyrosine decarboxylase gene and levodopa responsiveness in patients with Parkinson disease. Neurology 99, e2443–e2453 (2022).
van Kessel, S. P. et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 10, 310 (2019).
Tatton, N. A. & Kish, S. J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77, 1037–1048 (1997).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 1989).
Yeom, M. et al. Oral administration of Lactobacillus casei variety rhamnosus partially alleviates TMA-induced atopic dermatitis in mice through improving intestinal microbiota. J. Appl. Microbiol. 119, 560–570 (2015).
Arabestani, M. R., Nasaj, M. & Mousavi, S. M. Correlation between infective factors and antibiotic resistance in Enterococci clinical isolates in west of Iran. Chonnam Med. J. 53, 56–63 (2017).
van Kessel, S. P., Auvinen, P., Scheperjans, F. & El Aidy, S. Gut bacterial tyrosine decarboxylase associates with clinical variables in a longitudinal cohort study of Parkinsons disease. NPJ Parkinsons Dis. 7, 115 (2021).
Kutateladze, M. & Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 38, 426–430 (2008).
Paule, A., Frezza, D. & Edeas, M. Microbiota and phage therapy: Future challenges in medicine. Med. Sci. 6, 86 (2018).
Principi, N., Silvestri, E. & Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 10, 1–9 (2019).
Speck, P. & Smithyman, A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 363, 1–5 (2015).
Ooi, M. L. et al. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to staphylococcus aureus. JAMA Otolaryngol-Head Neck Surg. 145, 723–729 (2019).
Petrovic Fabijan, A. et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 5, 465–472 (2020).
Phothichaisri, W. et al. Characterization of bacteriophages infecting clinical isolates of clostridium difficile. Front. Microbiol. 9, 1–13 (2018).
Zmora, N. et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174, 1388-1405.e21 (2018).
Han, S. et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell Infect. Microbiol. 11, 1–12 (2021).
Li, C. et al. Adhesion and colonization of the probiotic Lactobacillus rhamnosus labeled by Dsred2 in mouse gut. Curr. Microbiol. 76, 896–903 (2019).
Bao, H.-D. et al. Alterations in the diversity and composition of mice gut microbiota by lytic or temperate gut phage treatment. Appl. Microbiol. Biotechnol. 102, 10219–10230 (2018).
Ceylani, T., Jakubowska-Doğru, E., Gurbanov, R., Teker, H. T. & Gozen, A. G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 4, e00644 (2018).
Jang, H. M., Lee, H. J., Jang, S. E., Han, M. J. & Kim, D. H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 11, 1386–1397 (2018).
Lee, K. E., Kim, J. K. & Kim, D. H. Orally administered antibiotics vancomycin and ampicillin cause cognitive impairment with gut dysbiosis in mice with transient global forebrain ischemia. Front. Microbiol. 11, 1–14 (2020).
Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 39, 2000–2025 (2019).
Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733 (2019).
Schooley, R. T. et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61, e00954 (2017).
Pappert, E. J. et al. Levodopa stability in solution: Time course, environmental effects, and practical recommendations for clinical use. Mov. Disord. 11, 24–26 (1996).
Contin, M. & Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 257(Suppl 2), S253–S261 (2010).
Nordberg, A., Nyberg, P. & Windblad, B. Topographic distribution of choline acetyltransferase activity and muscarinic and nicotinic receptors in Parkinson brains. Neurochem Pathol. 3, 223–236 (1985).
Mattila, P. M. et al. Choline acetytransferase activity and striatal dopamine receptors in Parkinson’s disease in relation to cognitive impairment. Acta Neuropathol. 102, 160–166 (2001).
Bugiani, O. et al. Loss of striatal neurons in Parkinson’s disease: A cytometric study. Eur Neurol. 19, 339–344 (1980).
McKinley, J. W. et al. Dopamine deficiency reduces striatal cholinergic interneuron function in models of Parkinson’s disease. Neuron 103, 1056-1072.e6 (2019).
Won, L., Ding, Y., Singh, P. & Kang, U. J. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. J. Neurosci. 34, 3090–3094 (2014).
Acknowledgements
We extend our gratitude to Dr. Song Her, who serves as a senior researcher at the Bio-Imaging Research Group (Seoul Centre, Korea Basic Science Institute, Seoul). Dr. Her provided invaluable guidance regarding histology of mouse brain and contributed to the development of related experimental designs.
Funding
This study was supported by Basic Research Laboratory grants from the National Research Foundation of Korea (RS-2024-00409969). The disclosed funders were independent of the study design, data collection and analysis, interpretation, decision to publish, and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J-PH, SS, YK, M-cS, J-gS, H-JP, K-HJ, and D-HH designed the study concept and protocol, and collected the samples. J-PH, YK, and BL worked on the histological slides. SHC and JH performed the LC-MS/MS operating in multiple reaction monitoring (MRM) and provided the related images. J-PH and MY performed the biochemical and PCR assays. J-PH, JH and D-HH write original draft. J-PH and D-HH edit and revise drafts. The final draft of the manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, JP., Shin, S., Chung, S.H. et al. Bacteriophages targeting Enterococcus faecalis enhance the therapeutic efficacy of levodopa in an MPTP-induced Parkinson’s disease mouse model with E. faecalis gut colonization. Sci Rep 14, 26146 (2024). https://doi.org/10.1038/s41598-024-77038-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77038-w