Abstract
Cardiac biomarkers are being increasingly applied in pediatric populations, including those with cardiogenic and non-cardiogenic myocardial injury disease. The upper reference limits of biomarkers are an important reference for disease diagnosis and health evaluation. However, the upper reference limits of pediatric cardiac biomarkers remains unknown. A total of 1,797 serum samples were collected prospectively from healthy respondents aged 0 to 18 years old. These samples were analyzed using Roche cobas e411 to determine the concentrations of hs-cTnT, CK-MB mass, and NT-proBNP. The upper reference limits and 90% confidence interval were calculated according to Clinical and Laboratory Standards Institute C28- A3 guidelines. The study successfully established the upper reference limits for hs-cTnT, CK-MB mass, and NT-proBNP in healthy children and adolescents aged 0 to 18. The hs-cTnT, CK-MB mass, and NT-proBNP levels were visibly higher in neonatal serum. With the increase in age, hs-cTnT, CK-MB mass, and NT-proBNP decline gradually. They decrease quickly in the first year after birth and decrease slowly after 1 year old. Gender differences were observed in the concentrations of hs-cTnT and CK-MB mass among healthy children and teenagers aged 10 to 18 and 13 to 18. Specifically, boys had relatively higher concentrations of hs-cTnT and CK-MB mass. However, no gender difference was found in NT-proBNP concentration. The concentrations of hs-cTnT, CK-MB mass, and NT-proBNP were simultaneously tested in healthy Chinese children for the first time. The specific upper reference limits of age and gender in pediatrics were established. The results provide comprehensive references for medical institutions specializing in children’s healthcare, which are conducive to preliminary screening, diagnosis, and treatment of pediatric cardiovascular diseases.
Similar content being viewed by others
Introduction
Cardiac biomarker refers to proteins and (or) enzymatic substances that are released to peripheral blood after myocardial damage and can be detected continuously. It has high sensitivity and specificity, and providing valuable guidance for the diagnosis, prognosis, and therapeutic evaluation of diseases1. Given immature myocardial functions and neurodevelopment, weak myocardial compensatory capacity, and high incidence of myocardial damage in China, detection of cardiac biomarkers such as high-sensitivity cardiac troponin T (hs-cTnT), creatine kinase isoenzyme (CK-MB) mass, and N-terminal fragment prohormone B-type natriuretic peptide (NT-proBNP) is of great significance to the diagnosis of cardiovascular disease (CVD) and secondary myocardial damage due to other causes in children2,3,4,5.
In 2021, the National Health Commission of China issued The Reference Intervals of Clinical Common Biochemical Detection Items in Children(WS/T 780–2021), which contains no upper reference limits of hs-cTnT, CK-MB mass, and NT-proBNP. As a result, most laboratories in China still use the upper reference limits for healthy adults in reagent manuals6. Given the particularity of the growth and development of children in different stages, the upper reference limit of adults is unsuitable for children and teenagers at different ages7. The pathogenesis and pathophysiological process of myocardial damage in China are different from those of adults. Hence, upper reference limits of cardiac biomarkers for children and teenagers at different ages need to be evaluated. Some studies have evaluated the hs-cTnT, CK-MB mass, and NT-proBNP of healthy children and teenagers. Bohn et al8. selected serum samples of 598 children and adolescents from the biobank of the Canadian Laboratory Initiative on Pediatric Reference Intervals and established reference intervals of hs-cTnT and NT-proBNP for healthy children and teenagers aged 0 to < 19. Karlen et al9. conducted a study on 158 newborns (including umbilical cord blood) in Stockholm Southern General Hospital and established the reference interval of hs-cTnT in healthy newborns. Lam et al10. collected blood samples from 484 healthy children in Canada and established the reference intervals of hs-cTnT and NT-proBNP for healthy children and adolescents aged 0 to < 19. Nir et al11. summarized four studies that used the same method to detect NT-proBNP concentration. According to statistical analysis, the reference interval of NT-proBNP for children and adolescents aged 0 to 18 was calculated. Hu et al12. provided reference intervals for hs-cTnT and CK-MB mass for healthy people aged 0 to 80 in Yibin. These research results provide evidence of the application of hs-cTnT, CK-MB mass, and NT-proBNP to children. However, these studies have some disadvantages, such as a small sample size and an imperfect age classification system.
In this study, concentrations of hs-cTnT, CK-MB mass, and NT-proBNP of healthy children and adolescents aged 0 to 18 were detected by using electrochemical luminescence immunoassay (ECLIA). The aim was to investigate the distributions of these three cardiac biomarkers levels. The upper reference limitsof hs-cTnT, CK-MB mass, and NT-proBNP concentrations of healthy children and teenagers aged 0 to 18 in Zhengzhou, China, were established.
Materials and methods
Subjects
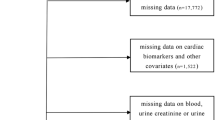
Healthy children and adolescents aged 0 to 18 who underwent health and medical examination for enrollment or were born in the obstetrics department of the Third Affiliated Hospital of Zhengzhou University between April 2021 and October 2023 were chosen as respondents. All respondents had normal physical examination and had no conditions such as hypoxemia hypoxemia, hypotension, renal injury, cardiac failure, cardiac disease, autoimmune disease, malignant tumor, inherited metabolic disorders, and so on. Unqualified objects were deleted through a questionnaire survey, physical examination, and medical history review. Finally, 1,797 healthy children were included, including 870 boys and 868 girls. The research process is shown in Fig. 1. The methods were carried out in this study according to the relevant guidelines and regulations. All donor candidates were given an anonymous code number. Identifying information was known only to the staff of the clinical laboratory of the Third Affiliated Hospital of Zhengzhou University. This study was approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University and followed the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants before conducting the study.
Laboratory analysis
According to Operation Specifications of National Clinical Laboratory(Version 4), samples (serum tubes) and transportation samples (scientific research channel) were collected according to Roche’s standard operating procedure. Children (< 3 years old) were fasted for at least 3 h, and children (> 3 years old) were fasted for 8 h before collection of 2 ml venous blood. Blood samples were condensed for 30 min under 25 ℃ and then centrifuged for 10 min at the rate of 3000 r/min. Unqualified samples, including hemolysis, jaundice, and opacifying, were deleted. hs-cTnT, CK-MB mass, and NT-proBNP concentrations in blood samples were detected immediately by using Roche cobas e 411 automatic electrochemical luminescence analyzer. (Serum samples that cannot be detected immediately were stored immediately in an ultra-low temperature refrigerator under − 80 ℃ and tested on the next day.) Laboratory quality control was carried out before each group of tests, and the control state was maintained at a good control state (CV < 6%). All test results were recorded and stored. Our laboratory participates in quality control projects of the Ministry of Health and Henan Laboratory every year, consistently achieving satisfactory results. Reagent batch numbers during detection are hs-cTnT (51134601, 51134602, 51134603), CK-MB mass (49712201, 49712202, 49712203), and NT-proBNP (52631901, 52631902, 52631903). Reagents from adjacent batches were compared and found to meet the consistency analysis standards outlined in EP-09c13.
Statistical analysis
Statistical analysis was conducted by using SPSS 24.0. Python language and matplotlib library were employed for plotting. For the continuous reference limits, all statistical analysis was performed using statsmodels library. Continuous reference limits were established14. Data were analyzed completely according to Clinical and Laboratory Standards Institute (CLSI) C28-A315. In this study, some of the ages rounded to the nearest whole number. According to Clinical and Laboratory Standards Institute (CLSI) C28-A3, this will not affect the derived reference limits. According to Dixon rules, outliers were determined and eliminated15,16. Samples were classified according to age and gender through a visual scatter diagram, and sample classification was verified by using the Harris–Boyd method17,18. According to the Harris–Boyd method16,17, the Z and Z* values were calculated and compared. If Z>Z*, then the upper reference limit shall be divided according to age or gender; otherwise, the upper reference limit is combined for statistical analysis. Scatter plots and line graphs were used to analyze data trends with age. Under this circumstance, the upper reference limit and 90% confidence interval of cardiac biomarkers were calculated using the nonparametric method according to the requirements of CLSI. The 99th percentile of hs-cTnT was calculated as the upper limit of reference. The upper limit of the reference interval of CK-MB mass and NT-proBNP used the 97.5th percentile.
Results
In this study, 1,964 blood samples were collected. After screening according to the exclusion criteria, 1,797 children met the inclusion criteria, including 900 boys (50.1%) and 897 girls (49.9%). The study subjects’ ages ranged from 0 to 18 years.
The pediatric upper reference limit and 90% confidence interval of hs-cTnT, CK-MB mass, and NT-proBNP were built and are summarized in Tables 1 and 2. The scatter diagrams that are plotted according to the final data analysis of hs-cTnT, CK-MB mass, and NT-proBNP are shown in Figs. 2, 3 and 4. Both the age partitioned and continuous 99th percentile upper reference limits of hs-cTnT are plotted in Fig. 5. Both the age partitioned and continuous 97.5th percentile upper reference limits of CK-MB mass and NT-proBNP are plotted in Figs. 6 and 7. The trend and gender differences of these parameters in terms of age and gender are displayed.
Results showed that with the increase in age, hs-cTnT, CK-MB mass, and NT-proBNP all present a decreasing trend. Moreover, they decrease quickly before 1 year old and then decrease slowly after 1 year old (Figs. 2, 3, 4, 5, 6 and 7). Table 1; Fig. 5 show that from 0 to 1 year old, the upper limit of reference of hs-cTnT decreased from 97.0 pg/ml to 22.5 pg/ml. The 97.5th percentile curve of CK-MB mass ranged from 14.04 ng/ml (0–28 d) to 7.13 ng/L (6 months to < 1 y; Table 2; Fig. 6). The 97.5th percentile curve of NT-proBNP ranged from 3876 pg/ml (0–28 days) to 577 pg/ml (29 days to < 1 y; Table 2; Fig. 7). These findings indicate that the concentrations of these three cardiac biomarkers are highest in newborns. No significant gender difference of NT-proBNP at all ages from 0 to 18 was found. Hence, the parameter values of boys and girls were integrated to build the common upper reference limit (Table 2; Fig. 7). Gender differences of hs-cTnT are found from ages 10 to 18, showing statistical significance. Specifically, the hs-cTnT concentration of boys is higher than that of girls (Table 1; Fig. 5). Gender differences of CK-MB mass concentration are found from ages 13 to 18, and the CK-MB mass concentration of boys is higher than that of girls (Table 2; Fig. 6).
.
Discussions
Cardiac biomarkers are indispensable for the diagnosis, prognosis, and monitoring of myocardial damage of patients with primary and secondary heart diseases. Common clinical cardiac biomarkers include troponins, creatine kinase isoenzyme, B-type brain natriuretic peptide (BNP), and NT-proBNP. For a special group of children, myocarditis and myocardial damage (microdamage) caused by bacterial and virus infections develop quickly and have poor prognosis, which can cause arrhythmia, dilated cardiomyopathy, cardiogenic shock, and even death, with a fatality rate as high as 50%1,19,20. Therefore, the detection of cardiac biomarker is crucial to early diagnosis, timely and appropriate treatment, monitoring, and prognosis improvement of myocardial damage in children. Myocardial damage in children lacks typical clinical symptoms. Thus, Niggemann pointed out that cardiac biomarkers may change before an electrocardiogram (ECG) to some extent under myocardial damage. Screening cardiac biomarkers can decrease misdiagnosis of myocardial damage and the rare acute coronary syndrome in children21. Nevertheless, the application of cardiac biomarkers in pediatrics is faced with unique challenges due to the lack of reference intervals. This issue is often ignored in studies that focus on adults22. For hs-cTnT, CK-MB mass, and NT-proBNP, only the upper limit of the reference has medical significance. Hence, concentrations of common cardiac biomarkers hs-cTnT, CK-MB mass, and NT-proBNP in children and adolescents aged 0–18 in China were studied and analyzed. Their specific upper reference limits of different ages and gender were established.
Cardiac troponin (cTn) is a major regulatory protein of myocardial contraction and is the primary marker to define myocardial damage23. In China, suggestions for diagnosing myocarditis in children include the use of increased cTn or CK-MB levels with dynamic fluctuations as major clinical diagnosis references24,25. Apple et al26. suggested establishing uniform standards for double judgment critical value by using cTn detection. The new generation of the high-sensitivity cardiac troponin T (hs-cTnT) test method conforms to guideline requirements. The 99th percentile detection imprecision (coefficient of variation, CV) of the upper limit of reference interval is ≤ 10%, and cardiac troponin T (cTnT) can be detected in more than 50% of the apparently healthy group27. Diversified cardiac troponin I (cTnI) detection systems exist, which are difficult to standardize. Different detection systems have different resistances to interference of atypical antibodies (e.g., heterophile antibody and rheumatoid factors), thus resulting in significant difference of detection results. Roche detects cTnT by using ECLIA, showing good standardization and consistency. This method has better long-term prognosis prediction accuracy than the previous method does28. Adult patients with acute chest pain often undergo the cTnT test to evaluate acute myocardial infarction (AMI) and the possibility of major cardiovascular adverse events when they seek emergency treatment. Although children have a low possibility of AMI, the cTnT test in children patients with chest pain is conducive to early diagnosis of myocardial damage when clinical suspicion exists or discovering anomalies through early ECG is difficult27. cTnT varies with age, gender, and race in apparently healthy groups. Moreover, laboratory detection systems are different. Although the International Federation of Clinical Chemistry and Laboratory Medicine has formulated upper reference limits that are applicable to most contemporary laboratories, they are limited within adult data, and the upper reference limits provided by manufacturers all come from European adults29. Therefore, it is necessary to establish specific upper reference limits for children and teenagers at different ages. This study found that hs-cTnT concentration is the highest in newborns and decreases rapidly before they reach 1 year old, which agrees with the latest research results8,10. This finding might be attributed to influences of physiological pressure and temporary anoxia during delivery as well as increased cardiomyocyte programmed apoptosis of infants after birth30,31. The hs-cTnT concentration of different groups decreases slowly with the increase in age, and it decreases to the reference range of adults after 1 year old (0–14 pg/ml). This finding is consistent with the observation of Lam et al10. Franzini et al32. calculated the 99th percentile upper limit of hs-cTnT concentration (8.1 pg/ml) in teenagers (10–14 years old). This finding agrees with the results for boys (8.9 pg/ml) and girls (8.6 pg/ml) aged 10–18 in this study. Bohn et al8. found through a study in Canada that the 99th percentile values of hs-cTnT in infants (0 to < 6 months and 6 to < 12 months) are 93 and 21 pg/ml, respectively. In this study, the 99th percentile values in the same age ranges (0–28 days, 29 days to < 6 months, and 6 to < 12 months) are 97.0, 60.6, and 22.5 pg/ml, respectively. This result might be caused by different age distribution and racial difference. Disputes remain over gender differences of hs-cTnT concentration between children and teenagers. Lam et al10. did not observe differences of hs-cTnT concentration between boys and girls aged 0–18. Bohn et al8. observed significant differences of hs-cTnT concentration between boys and girls aged 1–19, and the reference limit of boys was higher compared to girls (14 pg/ml vs. 11 pg/ml). This finding is consistent with results in adults. In this study, difference of hs-cTnT concentration is found only between boys and girls aged 10–18, which agrees with the observation results of Bohn et al. Overall, the hs-cTnT concentration of boys is higher compared to girls.
CK-MB is primarily found in in the myocardium and serves as an early indicator of myocardial damage. When using the immunosuppression method to detect CK-MB, adenylate cyclase and macro-CK cannot be neutralized by the anti-M antibody. Interferences from mitochondria CK may lead to a false increase in CK-MB activity33. Hence, pediatric departments should test CK-MB mass. CK-MB mass lacks specific clinical expressions in early mild viral myocarditis in children, and about 5% children patients do not demonstrate ECG changes34. The CK-MB mass appears earlier than cTn does in acute stage, and the sensitivity of CK-MB mass is higher compared to cTn. The specificity and observation window of cTn are better. Hence, CK-MB mass and cTn should be combined for detection, which can increase the diagnosis accuracy35. The present study observed that CK-MB mass concentration is highest in newborns and then decreases gradually with the increase in age. This condition might be related to hypoxia, imperfect myocardial development, and updating myocardium in newborns. Hu et al12. observed that the upper limits of reference of newborns (0–28 days) were 14.97 ng/ml in boys and 14.14 ng/ml in girls. This finding is basically consistent with the upper limit of reference (14.04 ng/ml) in newborn statistics. In the present study, gender difference of CK-MB mass concentration was observed only in the age group from 13 to 18. Specifically, the upper limit of reference was 3.36 ng/mL in boys and 2.71 ng/mL in girls. The gender difference in children is smaller than that in adults according to the upper limits of reference for adults (4.94 ng/mL in males and 2.88 ng/mL in females) on reagent specification of the Roche Group. This finding further supports the need to establish a upper reference limit of CK-MB mass concentration for pediatrics.
BNP and NT-proBNP are important links in the diagnosis and treatment of acute and chronic heart failures in children36BNP has a short biological half-life period (only 18–20 min) and has high requirements for sample collection and storage. Thus, the laboratory detection performance of NT-proBNP are better than those of BNP. At present, NT-proBNP is increasingly being used in clinics. Congenital heart disease (CHD) is the most common cause of death among infants with birth defects. Most children patients often have overloaded ventricular pressure, cyanosis, and pulmonary hypertension due to an abnormal cardiac structure. Such abnormal hemodynamics will cause the release of inflammatory factor and the activation of fibroblast and vascular endothelial cells, resulting in myocardial damage and myocardial hypertrophy21. Detection of cardiac biomarkers is beneficial to monitor such pathologic changes. NT-proBNP is usually used as a marker for preliminary screening of CHD in newborns37. During the COVID-19 pandemic, a new multisystem inflammatory syndrome among children was discovered, and its clinical symptoms were similar to those of severe sepsis38. According to the latest study, most European children patients with multisystem inflammatory syndrome related to COVID-19 had increased BNP upon admission to hospitals, and the increasing degree is related to whether intensive care support is needed or not39. Kawasaki disease (KD) is a common cause of acquired heart disease of children and often attacks children under the age of 5. A meta-analysis shows that NT-proBNP is a marker for KD diagnosis, and its general sensitivity and specificity could reach 0.89 and 0.72, respectively40. The upper limit of reference of NT-proBNP for adults provided in Roche’s specification is 125 pg/ml. This study found that the NT-proBNP concentration in all age groups of children was higher than that of adults, especially in newborns. The NT-proBNP concentration reaches the highest in newborns, decreasing quickly before 1 year old, which is consistent with other research reports using different analysis technologies11,41. This finding might be related to increased BNP in peripheral blood caused by physiological water loss, ventricular volume, and increased pressure in newborns42. Immature renal functions and limited clearance rate might also be one of the possible causes11. According to existing studies, NT-proBNP concentration decreases slightly with the increase in age until adulthood11,43. This finding is consistent with our observations. We observed a slight decrease in NT-proBNP concentration with increasing age during childhood and adolescence. Different age groups need different reference values of NT-proBNP. Albers et al44. analyzed NT-proBNP in 408 individuals aged 1–29 on the Roche platform and built different age groups. However, sample sizes of all age groups were smaller than the minimum sample size (120) that is recommended for building aupper reference limit. More respondents aged 0 to 18 (n= 1797) were included in this study, and the sample size of each age group was higher than 120. This approach might increase the robustness of the reference interval. On the Roche platform, Nir et al11. evaluated the NT-proBNP of 690 Canadian children and teenagers aged 0–18 and divided them into seven age groups: 0–2 days, 3–11 days, > 1 month to 1 year old, > 1 to 2 years old, > 2 to 6 years old, > 6 to 14 years old, and > 14 to 18 years old. According to our analysis, the upper limit of reference of NT-proBNP in each age group was lower than that in Canada, reported suggesting the necessity to establish race-specific upper reference limit for NT-proBNP. Dispute over gender difference of NT-proBNP in the adolescence stage is ongoing. Although some studies demonstrated that the NT-proBNP of adolescent females is higher with the increase in age44, the relations among BNP, sex hormone, and adolescence remain unknown. The results of this study do not support the need for specific gender interpretations, which is consistent with some previous studies10,11,45.
In this study, upper reference limits of hs-cTnT, CK-MB mass, and NT-proBNP of the pediatric department in Zhengzhou, Henan, were established. The research data provide valuable references for clinical evaluation of pediatric diseases in China. This work is a prospective study. Respondents were screened according to strict exclusion criteria to ensure that they belonged to healthy reference groups conforming to the inclusion criteria of CLSI.
Our investigation had limitations that should be noted. First, it is a single-center study with a limited sample size. Cooperation with clinical laboratories of relevant departments should be strengthened, and the recruitment range should be expanded to carry out multi-center and large-sample study and expand sample size verification. Second, the patient collective that our data is based on was not defined echocardiographically but clinically as patients without compromised cardiovascular function. Severe heart diseases, therefore, are unlikely but may not be excluded completely.
Conclusions
It is crucial for individuals to enhance their understanding of the important clinical significance of cardiac biomarker detection in the diagnosis and prognosis of various pediatric diseases. The upper reference limits of cardiac biomarkers of pediatric departments exhibit significant variations based on age, gender, and races. Specific upper reference limits for different groups need to be built. On the basis of the same analysis platform, this study reported specific upper reference limits of hs-cTnT, CK-MB mass, and NT-proBNP for healthy children and teenagers of different genders from ages 0 to 18 in China. These findings provide valuable data for the specific interpretation of pediatric cardiac biomarkers, especially in the newborn stage. Moreover, the research results serve as a foundation for future large-scale establishment of pediatric reference intervals.
Data availability
The datasets used during the current study are available from the corresponding author on a reasonable request.
Abbreviations
- hs-cTnT:
-
High-sensitivity cardiac troponin T
- CK-MB:
-
Creatine kinase MB isoform
- NT-proBNP:
-
N-terminal fragment prohormone B-type natriuretic peptide
- cTnT:
-
Cardiac troponin T
- CI:
-
Confidence intervals
- CV:
-
Coefficient of variation
- ECLIA:
-
Electrochemical luminescence immunoassay
- CLSI:
-
Clinical and Laboratory Standards Institute
- CVD:
-
Cardiovascular disease
- ECG:
-
electrocardiogram
- AMI:
-
Acute myocardial infarction
- CHD:
-
Congenital heart disease
- KD:
-
Kawasaki disease
References
Bohn, M. K. et al. Cardiac biomarkers in pediatrics: an undervalued resource. Clin. Chem. 67, 947–958. https://doi.org/10.1093/clinchem/hvab063 (2021).
Elgendy, M. M. et al. Urinary N-terminal pro-brain natriuretic peptide in newborn infants with cardiac and pulmonary diseases. Am. J. Perinatol. 41, 53–59. https://doi.org/10.1055/s-0041-1740213 (2024).
Rodriguez-Gonzalez, M., Perez-Reviriego, A. A., Castellano-Martinez, A. & Cascales-Poyatos, H. M. N-terminal probrain natriuretic peptide as biomarker for diagnosis of Kawasaki disease. Biomark. Med. 13, 307–323. https://doi.org/10.2217/bmm-2018-0324 (2019).
Li, T. G. A review on the clinical application of high-sensitivity cardiac troponin T in neonatal diseases. Chin. J. Contemp. Pediatr. 21, 936–941. https://doi.org/10.7499/j.issn.1008-8830.2019.09.018 (2019).
Alehan, F. et al. Elevated CK-MB mass and plasma brain-type natriuretic peptide concentrations following convulsive seizures in children and adolescents: possible evidence of subtle cardiac dysfunction. Epilepsia. 50, 755–760. https://doi.org/10.1111/j.1528-1167.2008.01793.x (2009).
Zhang, Y., Wang, W., He, F., Wang, Z. & Zhong, K. Sources and distribution decision on reference values of myocardial injury markers in China: results from 150 laboratories. Chin. J. Cardiol. 42, 193–196 (2014).
Shaw, J. L., Marvasti, B., Colantonio, T., Adeli, K. & D. & Pediatric reference intervals: challenges and recent initiatives. Crit. Rev. Clin. Lab. Sci. 50, 37–50. https://doi.org/10.3109/10408363.2013.786673 (2013).
Bohn, M. K., Higgins, V., Kavsak, P., Hoffman, B. & Adeli, K. High-sensitivity generation 5 cardiac troponin T sex- and age-specific 99th percentiles in the CALIPER cohort of healthy children and adolescents. Clin. Chem. 65, 589–591. https://doi.org/10.1373/clinchem.2018.299156 (2019).
Karlén, J., Karlsson, M., Eliasson, H., Bonamy, A. E. & Halvorsen, C. P. Cardiac troponin T in healthy full-term infants. Pediatr. Cardiol. 40, 1645–1654. https://doi.org/10.1007/s00246-019-02199-9 (2019).
Lam, E. et al. Normative values of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in children and adolescents: a study from the CALIPER cohort. J. Appl. Lab. Med. 6, 344–353. https://doi.org/10.1093/jalm/jfaa090 (2021).
Nir, A. et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr. Cardiol. 30, 3–8. https://doi.org/10.1007/s00246-008-9258-4 (2009).
Man, S. et al. Establishment of reference intervals of ten commonly used clinical chemistry analytes: a real-world study in China. Biomark. Med. 15, 797–806. https://doi.org/10.2217/bmm-2021-0233 (2021).
Greg Miller, W., Greenberg, N., Budd, J. & Delatour, V. The evolving role of commutability in metrological traceability. Clin. Chim. Acta. 514, 84–89. https://doi.org/10.1016/j.cca.2020.12.021 (2021).
Smith, J., Karlaftis, V., Hearps, S., Chiriano, A. & Monagle, P. Age partitioned and continuous upper reference limits for Ortho VITROS high sensitivity troponin I in a healthy paediatric cohort. Clin. Chem. Lab. Med. (CCLM). 60, 1449–1454. https://doi.org/10.1515/cclm-2022-0433 (2022).
Doyle, K. & Bunch, D. R. Reference intervals: past, present, and future. Crit. Rev. Clin. Lab. Sci. 60, 466–482. https://doi.org/10.1080/10408363.2023.2196746 (2023).
Yang, D., Su, Z. & Zhao, M. Big data and reference intervals. Clin. Chim. Acta. 527, 23–32. https://doi.org/10.1016/j.cca.2022.01.001 (2022).
Harris, E. K. & Boyd, J. C. On dividing reference data into subgroups to produce separate reference ranges. Clin. Chem. 36, 265–270 (1990).
Horn, P. S. & Pesce, A. J. Reference intervals: an update. Clin. Chim. Acta. 334, 5–23. https://doi.org/10.1016/s0009-8981(03)00133-5 (2003).
Clerico, A. et al. The 99th percentile of reference population for cTnI and cTnT assay: methodology, pathophysiology and clinical implications. Clin. Chem. Lab. Med. 55, 1634–1651. https://doi.org/10.1515/cclm-2016-0933 (2017).
Sachdeva, S., Song, X., Dham, N., Heath, D. M. & DeBiasi, R. L. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am. J. Cardiol. 115, 499–504. https://doi.org/10.1016/j.amjcard.2014.11.029 (2015).
Sugimoto, M. et al. Cardiac biomarkers in children with congenital heart disease. World J. Pediatrics: WJP. 11, 309–315. https://doi.org/10.1007/s12519-015-0039-x (2015).
Niggemann, B. How to diagnose psychogenic and functional breathing disorders in children and adolescents. Pediatr. Allergy Immunol. 21, 895–899. https://doi.org/10.1111/j.1399-3038.2010.01060.x (2010).
Adamcová, M. Troponins in children and neonates. Acta Paediatr. 92, 1373–1375. https://doi.org/10.1080/08035250310007637 (2003).
Thygesen, K. et al. Fourth universal definition of myocardial infarction (2018). Kardiologia Polska. 76, 1383–1415. https://doi.org/10.5603/kp.2018.0203 (2018).
Putschoegl, A., Auerbach, S. & Diagnosis Evaluation, and treatment of myocarditis in children. Pediatr. Clin. North Am. 67, 855–874. https://doi.org/10.1016/j.pcl.2020.06.013 (2020).
Apple, F. S. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin. Chem. 55, 1303–1306. https://doi.org/10.1373/clinchem.2009.128363 (2009).
Clerico, A., Aimo, A. & Cantinotti, M. High-sensitivity cardiac troponins in pediatric population. Clin. Chem. Lab. Med. 60, 18–32. https://doi.org/10.1515/cclm-2021-0976 (2022).
Perrone, M. A. et al. Cardiac troponins: are there any differences between T and I? J. Cardiovasc. Med. 22, 797–805. https://doi.org/10.2459/jcm.0000000000001155 (2021).
Wu, A. H. B. et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the academy of the American association for clinical chemistry and the task force on clinical applications of cardiac bio-markers of the international federation of clinical chemistry and laboratory medicine. Clin. Chem. 64, 645–655. https://doi.org/10.1373/clinchem.2017.277186 (2018).
Correale, M. et al. Troponin in newborns and pediatric patients. Cardiovasc. Hematol. Agents Med. Chem. 7, 270–278. https://doi.org/10.2174/187152509789541927 (2009).
Cruz, M. A. et al. Cardiac troponin T and cardiac dysfunction in extremely low-birth-weight infants. Pediatr. Cardiol. 27, 396–401. https://doi.org/10.1007/s00246-005-0942-3 (2006).
Franzini, M. et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: a multicenter study in Italy. Clin. Chim. Acta. 438, 376–381. https://doi.org/10.1016/j.cca.2014.09.010 (2015).
Hoshino, T. et al. Clinical evaluation of a new creatine kinase MB activity reagent abrogating the effect of mitochondrial creatine kinase. Clin. Lab. 59, 307–316. https://doi.org/10.7754/clin.lab.2012.120516 (2013).
Shauer, A. et al. Acute viral myocarditis: current concepts in diagnosis and treatment. Isr. Med. Association J. 15, 180–185 (2013).
Chen, J. & Deng, Y. Diagnostic performance of serum CK-MB, TNF-α and Hs-CRP in children with viral myocarditis. Open. Life Sci. 14, 38–42. https://doi.org/10.1515/biol-2019-0005 (2019).
Lin, C. W. et al. Role of the NT-proBNP level in the diagnosis of pediatric heart failure and investigation of novel combined diagnostic criteria. Experimental Therapeutic Med. 6, 995–999. https://doi.org/10.3892/etm.2013.1250 (2013).
Cantinotti, M., Giovannini, S., Murzi, B. & Clerico, A. Diagnostic, prognostic and therapeutic relevance of B-type natriuretic hormone and related peptides in children with congenital heart diseases. Clin. Chem. Lab. Med. 49, 567–580. https://doi.org/10.1515/cclm.2011.106 (2011).
Mamishi, S. et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol. Infect. 148, e196. https://doi.org/10.1017/s095026882000196x (2020).
Valverde, I. et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 143, 21–32. https://doi.org/10.1161/circulationaha.120.050065 (2021).
Alexoudi, I., Kanakis, M., Kapsimali, V. & Vaiopoulos, G. Kawasaki disease: current aspects on aetiopathogenesis and therapeutic management. Autoimmun. rev. 10, 544–547. https://doi.org/10.1016/j.autrev.2011.04.005 (2011).
Albers, S., Mir, T. S., Haddad, M. & Läer, S. N-Terminal pro-brain natriuretic peptide: normal ranges in the pediatric population including method comparison and interlaboratory variability. Clin. Chem. Lab. Med. 44, 80–85. https://doi.org/10.1515/cclm.2006.016 (2006).
Nir, A. & Nasser, N. Clinical value of NT-ProBNP and BNP in pediatric cardiology. J. Card. Fail. 11, 76–80. https://doi.org/10.1016/j.cardfail.2005.04.009 (2005).
Palm, J. et al. Continuous, complete and comparable NT-proBNP reference ranges in healthy children. Clin. Chem. Lab. Med. 58, 1509–1516. https://doi.org/10.1515/cclm-2019-1185 (2020).
Mu, S. et al. NT-proBNP reference intervals in healthy U.S. children, adolescents, and adults. J. Appl. Lab. Med. 8, 700–712. https://doi.org/10.1093/jalm/jfad024 (2023).
Koerbin, G. et al. NTproBNP concentrations in healthy children. Clin. Biochem. 45, 1158–1160. https://doi.org/10.1016/j.clinbiochem.2012.05.008 (2012).
Acknowledgements
We deeply thank all the families for their participation in the present study. We also acknowledge members of the clinical laboratory for their generous help.
Funding
This work was supported by research grants from the Medical Science and Technology Joint Construction Project of Henan Province (LHGJ20200467).
Author information
Authors and Affiliations
Contributions
E.Y., L.Z. and K.D. contributed to the conception and design the research; J.X. and X.S. performed experiments; M.F., and X.Z. contributed to data acquisition; Y. D and J.L. analyzed the data; K.D. drafed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, K., Xing, J., Dai, Y. et al. Age- and sex-specific upper reference limits for cardiac biomarkers in Chinese children and adolescents: a prospective study. Sci Rep 14, 28295 (2024). https://doi.org/10.1038/s41598-024-77153-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77153-8