Abstract
Background: Tetralogy of Fallot (TOF) and hypertrophic cardiomyopathy (HCM) are common types of congenital heart disease with unique pathophysiologic features. Mutations in LBX1, a key regulator of muscle precursor cell migration, may disrupt these critical developmental processes, resulting in severe developmental abnormalities. Case presentation: This case reports on a 4-year-old girl diagnosed with both TOF and HCM. Genetic analysis revealed iUPI-F (uniparental disomy) on the entire chromosome 10, with a c.808G > A (p. Glu270Lys) pure mutation in the LBX1. Conclusion: This patient exhibited both TOF and HCM with mutations in the LBX1 gene, suggesting a potentially novel genetic link. This case study emphasizes the need for further studies on the function of the LBX1 gene in cardiac development and its potential impact on TOF and HCM.
Similar content being viewed by others
Introduction
Congenital heart defects (CHDs) are one of the most prevalent forms of birth defects, affecting approximately 8 out of every 1,000 babies worldwide. They represent a significant cause of neonatal and infant morbidity and mortality1. Among the various CHDs, tetralogy of Fallot (TOF) and hypertrophic cardiomyopathy (HCM) are particularly noteworthy due to their distinctive pathophysiological characteristics and complex genetic etiology, which give rise to a range of phenotypic expressions1. TOF has four classic clinical manifestations: pulmonary stenosis, ventricular septal defect, right ventricular hypertrophy, and aortic ride-through, which pumps hypoxic blood into the body to achieve left-to-right shunting of arterial blood, resulting in cyanosis and other complications. Genetic factors are a significant contributor to the development of TOF, with approximately 20% of cases linked to pathogenic copy number variants or chromosomal abnormalities. These include mutations in crucial developmental genes such as NOTCH1 and FLT41. Recent genome-wide analyses have identified rare and very rare genetic variants that contribute to the disease, suggesting pathways involved in cardiac development, vascular signaling, and cellular differentiation. Conversely, HCM is an inherited cardiomyopathy caused mainly by mutations in myonectin, including MYBPC3 and MYH7. These mutations account for approximately 40–60% of diagnosed cases, depending on the study population2,3. While recent review articles have emphasized the differences in the prevalence of mutations in different groups, with MYBPC3 and MYH7 being the most common mutations, other genes also play a role in a smaller percentage of cases4. In contrast to TOF, where structural defects are the main clinical manifestation, HCM involves both structural and functional disturbances that may lead to heart failure, arrhythmias, and sudden cardiac death, particularly in young people and athletes.
The genetic intricacy of TOF and HCM exemplifies the challenges inherent in diagnosing and managing these conditions. Despite extensive research, a considerable number of cases of HCM and TOF remain unexplained by known genetic mutations. This suggests that other, as yet unidentified, genetic, epigenetic, or environmental factors may contribute to the pathogenesis of these diseases.
The LBX1 gene is a key regulator of muscle precursor cell migration and has been implicated in a variety of congenital developmental anomalies and complications such as congenital central hypoventilation syndrome5. However, its function in cardiac development remains uncertain.This gene influences the migration and differentiation of associated muscle precursor cells and the formation of specific neural circuits. Mutations in this gene disrupt these critical developmental processes, leading to severe developmental abnormalities6. The disruption of these pathways may result in the structural and functional abnormalities observed in TOF and HCM.
Case presentation
The patient was a 4-year-7-month-old girl, G2P2, born by caesarean section to a mother who had a normal pregnancy of 36 weeks, with a normal maternal condition, a birth weight of 2.4 kg, and no asphyxia. A heart murmur was detected on physical examination after birth, and the child began to have shortness of breath that increased progressively with age, became more pronounced with activity, and was squatting, with bruising on the lips and lips that worsened with crying. It is aggravated by crying. In addition, the patient was prone to colds and her exercise tolerance was lower than that of her peers. However, there was no cardiac edema in both lower extremities. Previous examinations diagnosed her with tetralogy of Fallot (TOF), and she was recently hospitalized for deterioration of her cardiac function.
Upon admission, a cardiac murmur was identified in the precordial region, accompanied by symptoms of hypertension. The blood pressure readings were as follows: left upper extremity, 138/107 mmHg; left lower extremity, 165/128 mmHg; right upper extremity, 163/119 mmHg; and right lower extremity, 170/106 mmHg. Color Doppler echocardiography and cardiovascular CT were suggestive of TOF. Echocardiography revealed an enlarged right ventricle, an abnormally large myocardial bundle in the right ventricular cavity, and marked thickening of the right ventricular anterior wall and interventricular septum. The electrocardiogram demonstrated sinus tachycardia, right ventricular hypertrophy, and bi-atrial enlargement, in addition to an incomplete right bundle branch block.
On the 12th day after admission, the patient underwent correction of tetralogy of Fallot under extracorporeal circulation. Intraoperatively, it was found that the heart was abnormally enlarged (LV+++ LA+++ RV++++ RA+++), and the anterior wall of the right ventricle, ventricular septum, and posterior wall of the left ventricle were significantly thickened and stiff.
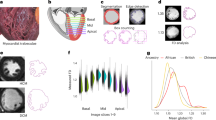
A pathological examination revealed mild swelling and degeneration of muscle fibers, local collagen deposition, and hyaline degeneration with myxoid degeneration (Fig. 1). These findings, in conjunction with myocardial hypertrophy and disorder, are postulated to be characteristics of hypertrophic cardiomyopathy (HCM), which is distinct from the traditional tetralogy of Fallot and is highly suspected to be genetically related. Following the consent of the child’s parents, Mygeno Medical Laboratory conducted Trio whole exome sequencing (Fig. 2), which revealed that the child exhibited iUPI-F (incomplete uniparental isodisomy - father). This indicates that the patient inherited both copies of chromosomes 10 from his father. This disease may result in the manifestation of recessive genetic disorders. Sanger sequencing confirmed that a homozygous mutation occurred at site 808 of the LBX1 gene. The father was a heterozygote, while the mother did not carry the mutation (Fig. 3).
The HE-stained histopathologic section of myocardium reveals mild swelling and degeneration of myofibers with localized collagen deposition, hyaline degeneration, and mucus-like degeneration.(A) Arrowheads indicate swollen cardiomyocytes (200x). (B) Arrowheads indicate swollen cardiomyocytes (400x). (C) Arrowheads indicate areas of mucus degeneration (200x). (D) Arrowheads indicate areas of mucus degeneration (400x).
LBX1 gene mutations are associated with congenital central hypoventilation syndrome type 3 (CCHS3), which affects the respiratory and cardiovascular systems2. The patient’s high blood pressure symptoms may be related to this mutation. Protein structure analysis using SWISS-MODEL and PyMOL tools7,8 showed that the mutation changes the 270th amino acid from glutamic acid (E) to lysine (K), thus changing the charge of the protein and possibly affecting its interaction with other molecules (Fig. 4). The charge distribution of wild-type and mutant LBX1 proteins showed significant changes in surface charge (Fig. 5). Although the pathogenicity of genetic test results is uncertain, mutations in the LBX1 gene may affect the function of the protein in muscle and neuronal development. This variant was classified as a variant of uncertain significance (VUS) according to the American College of Medical Genetics and Genomics (ACMG) 2015 standards and guidelines5,9.
Discussion
The LBX1 gene is essential for the development of muscle and nerve tissue. Mutations in this gene may lead to abnormal development in the body6,10. The pure (p.Glu270Lys) mutation found in patients may disrupt the normal function of LBX1 and lead to developmental abnormalities. It has the potential to disrupt the interactions between this protein and DNA, as well as other regulatory proteins that are essential for normal developmental processes. Potential maps of wild-type and mutant LBX1 proteins also reveal changes in surface charge distribution. Wild-type LBX1 proteins maintain a balanced electrical potential, which is essential for interaction with negatively charged DNA and other proteins involved in transcriptional regulation. The correct folding and stability of the protein support its normal function. In contrast, the mutation in p.Glu270Lys changes the negatively charged glutamate to a positively charged lysine, which alters the local charge distribution and overall electrostatic potential of the encoded protein. This alteration may impede the protein’s interactions with DNA and other proteins, thereby disrupting the regulatory mechanisms essential for normal heart and muscle development.
A comprehensive search of the PubMed database did not reveal any prior reports directly linking LBX1 to TOF and HCM, suggesting this case may be the first to identify such an association. Although LBX1 is primarily associated with skeletal muscle development, it remains unclear whether its mutations could also lead to abnormal myocardial growth, contributing to HCM. Prior research has identified genes such as MYOM2 and FLNC as being associated with both TOF and HCM, thereby supporting the hypothesis that mutations in muscle development genes may lead to heart defects11,12. The LBX1 mutation found in this patient indicates it may play a role in the etiology of HCM by disrupting the normal myocardial developmental process. In this case, the co-occurrence of TOF and HCM may be a compound effect of mutations in the LBX1 gene13,14. Mutations in the LBX1 gene may disrupt the normal development of the myocardium, leading to structural abnormalities of the heart and abnormal thickening of the myocardium. In turn, the cardiac structural defects of TOF and the hypertension caused by CCHS3 put additional stress on the heart5. Recent studies have indicated that paroxysmal hypertension in CCHS3 may contribute to the progression of cardiovascular diseases such as HCM by leading to increased afterload and altered myocardial strain patterns, which further complicate the hypertrophic response and potentially exacerbate myocardial fibrosis15. This cross-sectional study indicates that the management of blood pressure fluctuations in patients with CCHS3 is crucial for the mitigation of additional stress on the already damaged myocardium in HCM. However, there are limitations to this approach, including the difficulty of distinguishing between genetic factors and the secondary effects of autonomic dysregulation observed in diseases such as CCHS3.
The scarcity of LBX1 mutations in cardiac pathology underscores a knowledge gap regarding the broader role of mutations in this gene. The evidence presented in this case study lends support to the hypothesis that LBX1 mutations may have a cumulative effect when occurring in conjunction with other structural and genetic abnormalities. For example, in TOF, neural crest cell migration and disruption of cardiac developmental pathways are known causative factors, and the role of the LBX1 gene in muscle precursor migration may intersect with these processes. Nevertheless, the absence of functional validation studies connecting the LBX1 gene directly to cardiomyocytes constrains the certainty of its role in HCM. Consequently, further experimental work is imperative to validate these associations. The extant literature also underscores that while genes such as MYBPC3 and MYH7 are well established in the pathogenesis of HCM, the discovery of other causative genes, such as LBX1, has added to the complexity of the genetic landscape of these cardiomyopathies2. Furthermore, a significant limitation of the existing studies is that they primarily focus on myonectin, neglecting genes involved in broader muscle and neurodevelopment, such as the LBX1 gene. These genes could potentially provide new insights into the mechanisms of cardiac hypertrophy.
In addition, the presence of iUPI-F (incomplete uniparental isodisomy - father.
) complicates the genetic picture as it may lead to purity of recessive mutations in the gene, thereby amplifying the pathogenic effects of LBX1 mutations. the LBX1 gene is also involved in the development of neural circuits that regulate autonomic function, and disruptions in these pathways may lead to dysregulation of blood pressure control, which may result in paroxysmal hypertension6. This interaction between autonomic dysregulation and structural cardiac defects may result in the exacerbation of HCM hypertrophy, underscoring the necessity for a comprehensive cardiovascular and autonomic assessment in these patients.
Conclusion
The identification of mutations in the LBX1 gene in an individual with both tetralogy of Fallot (TOF) and hypertrophic cardiomyopathy (HCM) offers novel insights into the genetic underpinnings of these intricate congenital heart defects and indicates a potential involvement in the etiology of TOF and HCM, particularly in tetralogy of Fallot-related disorders. This case emphasizes the need for further studies to explore the role of LBX1 in cardiac development and its potential as an influencing factor for TOF and HCM. Comprehensive genetic screening, including LBX1, should be considered for patients with these conditions to improve diagnostic accuracy and patient outcomes. Further research should concentrate on corroborating these preliminary findings through larger cohort studies and functional analyses, with the objective of elucidating the precise role of the LBX1 gene in cardiac and neurodevelopmental processes. A deeper comprehension of the pathways influenced by mutations in the LBX1 gene could facilitate the development of targeted interventions and provide new avenues for hope for patients with complex congenital heart disease.
Patients and methods
The study was conducted in accordance with the Declaration of Helsinki guidelines. The case was a surgical one, and the patient had signed an informed consent form for the surgical procedure as well as the pathological examination. All methods were performed following the relevant guidelines and regulations. And all protocols were approved by a named institutional(Management Committee of Children’s Hospital of Chongqing Medical University). Prior to the commencement of genetic testing and the publication of research on the patient’s disease, the patient’s legal guardian provided informed consent.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Manshaei, R. et al. Genes and pathways implicated in tetralogy of Fallot revealed by Ultra-rare Variant Burden Analysis in 231 genome sequences. Front. Genet. 11, 957. https://doi.org/10.3389/fgene.2020.00957 (2020). PMID: 33110418; PMCID: PMC7522597.
Velicki, L. et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 20, 516. https://doi.org/10.1186/s12872-020-01807-4 (2020).
Maron, B. J. & Maron, M. S. Hypertrophic cardiomyopathy. Lancet 381(9862): 242 – 55. 10.1016/S0140-6736(12)60397-3 (epub 2012 Aug 6) (2013).
Luis, R. et al. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 45(30), 2727–2734 (2024).
Hsu, K. H. & Chang, C. I. A rare association of tetralogy of Fallot and hypertrophic cardiomyopathy. Eur. J. Cardiothorac. Surg. 41(6) (2012).
Hernandez-Miranda, L. R. et al. Mutation in LBX1/Lbx1 precludes transcription factor cooperativity and causes congenital hypoventilation in humans and mice. Proc. Natl. Acad. Sci. U S A. 115 (51), 13021–13026. https://doi.org/10.1073/pnas.1813520115 (2018). Epub 2018 Nov 28. PMID: 30487221; PMCID: PMC6304989.
Benn, P. Uniparental disomy: Origin, frequency, and clinical significance. Prenat. Diagn. 41(5), 564–572. 10.1002/pd.5837 (epub 2021 Mar 5) (2021).
Biasini, M. et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 1 42 (W1), W252–W258 (2014).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. https://doi.org/10.1038/gim.2015.30 (2015).
Xu, L. et al. A functional SNP in the promoter of LBX1 is associated with the development of adolescent idiopathic scoliosis through involvement in the myogenesis of paraspinal muscles. Front. Cell. Dev. Biol. 9, 777890. https://doi.org/10.3389/fcell.2021.777890 (2021). PMID: 34917617; PMCID: PMC8670502.
Lewin, M. B., Towbin, J. A. & Thapar, M. K. The rare association of tetralogy of Fallot with hypertrophic cardiomyopathy. Report of 2 neonatal patients. Tex. Heart Inst. J. (1997).
Auxerre-Plantié, E. et al. Identification of MYOM2 as a candidate gene in hypertrophic cardiomyopathy and tetralogy of Fallot, and its functional evaluation in the Drosophila heart. Dis. Model. Mech. 13 (12), dmm045377. https://doi.org/10.1242/dmm.045377 (2020). PMID: 33033063; PMCID: PMC7758640.
Carvalho, A. M. et al. Tetralogy of Fallot and hypertrophic cardiomyopathy: A rare association. Arq. Bras. Cardiol. 80 (2), 217. https://doi.org/10.1590/s0066-782x2003000200010 (2003). 214-6. English, Portuguese.
Karl, T. R. & Stocker, C. Tetralogy of Fallot and its variants. Pediatr. Crit. Care Med. 17(8 Suppl 1), S330-6 https://doi.org/10.1097/PCC.0000000000000831 (2016).
Rodrigues, J. C. et al. Hypertensive heart disease versus hypertrophic cardiomyopathy: multi-parametric CMR predictors beyond end-diastolic wall thickness ≥ 15mm. J. Cardiovasc. Magn. Reson. 18 (Suppl 1). https://doi.org/10.1186/1532-429X-18-S1-P264 (2016).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (NSFC) (NSFC 81370432) funded by the Chongqing Natural Science Foundation Upper-level Project (CSTB2023NSCQ-MSX0579) funded by the Chongqing Yuzhong District High-level Talent Program Project 2024.
Author information
Authors and Affiliations
Contributions
P.W.C. is the primary author of the article. P.W.C., G.Z.W. and drafted the manuscript and performed the literature search. Y.Z.S. conducted genome-wide sequencing analysis. Y.A supervised this work. All authors participated in manuscript revision, agreed to submit the manuscript, and approved the final version of the manuscript. All clinical authors had full access to the clinical data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, P., Wang, G., Song, Y. et al. Novel association of LBX1 mutation with tetralogy of Fallot and hypertrophic cardiomyopathy: implications for cardiac development. Sci Rep 14, 26179 (2024). https://doi.org/10.1038/s41598-024-77187-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77187-y