Abstract
The substitution of microbial fertilizer for chemical fertilizer can not only improve soil fertility but also effectively enhance rice quality. To investigate the effect of different amounts of combined application of chemical fertilizer and microbial fertilizer on the amylose content of rice, this study adopts theoretical calculations to compare the preprocessed Raman spectroscopy information of rice with reduced fertilization and establishes a recognition model for the amylose content of rice, which is used to detect the amylose content in rice. Based on the amylose spectral values measured by Raman spectroscopy and the known starch structure and functional groups, the Raman peaks are mainly distributed in the range of 400 cm− 1 to 1400 cm− 1. The Raman characteristic peaks at 483 cm− 1, 869 cm− 1, 933 cm− 1, 1082 cm− 1, 1126 cm− 1, 1335 cm− 1, 1385 cm− 1, and 1455 cm− 1 exhibit strong vibration modes, which are consistent with its main nutrient component of amylose. By comparing the measured amylose content in the regions treated with microbial fertilizer combined with different amounts of reduced fertilization and the spectral intensity values of amylose measured by Raman spectroscopy, the results show that the treatment of combining conventional amounts of microbial fertilizer with different amounts of reduced chemical fertilizer exhibits a decreasing trend in the amylose content of rice.
Similar content being viewed by others
Introduction
Traditional fertilization methods often lead to waste and pollution issues. Scientific and rational regulation of reduced fertilization for rice can not only improve soil fertility but also effectively enhance rice quality. Achieving higher rice yields necessitates proper fertilizer application at each growth stage1. Zhou studied the impact of fertilization methods on nutrient losses in rice fields, focusing on analyzing the dynamic changes in nutrient concentrations during fertilization. The results indicated that split-application of ear-fertilizer significantly increases rice yield2. In terms of soil nutrient analysis, Kuzman et al. employed an adaptive neuro-fuzzy inference system to predict fertilizers and analyze soil macronutrients, offering insights into optimizing crop yields3. Zhang et al., on the other hand, assessed rice yields based on water demand, evaporation, and effective precipitation during the rice growth period, providing effective irrigation analysis for different rice-growing regions in Northeast China4. Zhao uses the (back propagation network, BP) to analyze the nutrient content of crops, predict the nutrient content of crops at different growth stages, and use it for fertilization guidance5. After physical processing or microbial treatment, straw can be returned to the field as microbial fertilizer, increasing soil organic carbon content and releasing nutrients like nitrogen, phosphorus, and potassium to meet crop nutrient demands while reducing chemical fertilizer application. This strategy of straw return combined with reduced fertilization ensures crop yields while enhancing soil organic matter and nutrient content6,7,8.
Compared with the application of chemical fertilizers alone, the combined application of organic fertilizers can significantly reduce the chalkiness and amylose content of rice, while improving the gel consistency, appearance, and cooking quality9. This improvement is mainly attributed to the sustained effect of organic fertilizers, which enhances the photosynthetic efficiency of rice leaves in the middle and late growth stages, promotes the accumulation of photosynthetic products, thereby ensuring the full filling and grain plumpness of rice, and optimizing the processing, appearance, and quality of rice10. In addition, compared with conventional chemical fertilizer treatments, the use of different types of organic fertilizers as base fertilizers to replace chemical fertilizers generally reduces the protein content of rice and increases the gel consistency. However, it is worth noting that different organic fertilizer treatments have varying effects on improving various indicators of rice quality, and not all indicators reach a significantly improved level. This indicates that the effect of organic fertilizers replacing chemical fertilizers is type-dependent and can promote the improvement of rice quality to varying degrees11.
Scholars mentioned above have conducted research on crop nutrient requirements from an agronomic perspective. Zhang Qing et al. found that combining biological microbial fertilizers with different chemical fertilizers can increase lettuce yield and quality while comprehensively improving soil nutrient conditions12. Other scholars have studied various fertilization treatments, such as Deng et al.13, who discovered that microbial fertilizer application increases rice dry matter, filling percentage, and 1000-grain weight. Vu Van Long’s experiments in the Mekong River Delta region of Vietnam showed that reducing nitrogen fertilizer by 50% and combining it with 5 to 10 tons/hectare of biochar significantly increased soil available nitrogen and phosphorus content in intensive rice cultivation systems, thereby promoting rice growth and yield14. This innovative agricultural management approach not only has the potential to reduce dependence on nitrogen fertilizers but also helps alleviate soil degradation, significantly enhancing the sustainability of rice production.
Spectroscopy technology, with its unique advantages, has found widespread applications in science and technology. From a spectroscopic perspective, Liu Hongmei established a near-infrared analysis model, whose results showed no significant difference from those obtained through chemical analysis15. In recent years, Guo YB used a combination of infrared and Raman spectroscopy to detect interactions and structural changes between proteins and starches, experimentally proving that Raman spectroscopy can distinguish between new and old rice through starch detection16.
To advance rice quality inspection, this study employs theoretical calculations to compare the preprocessed Raman spectral information of rice under reduced fertilization conditions. By establishing a recognition model for rice amylose content, we aim to quickly, efficiently, non-pollutingly, and cost-effectively detect amylose content in rice, providing a basis for rice cultivation techniques to enhance yields and quality. This experiment investigates changes in rice amylose content under microbial fertilizer and various ratios of combined chemical fertilizer application, offering theoretical support for sustainable rice production, rational fertilization, and reduced fertilizer losses.
Samples and experiments
Rice samples
The test selected the Tongjing 612 japonica rice samples grown and produced in Yongsheng Village, Taonan City, Jilin Province, in 2023. This variety not only matches the local accumulated temperature in its growth cycle but also possesses universal characteristics. Before the experiment, rice with good appearance and uniform shape was selected and stored at room temperature for 48 h before spectral experiments. This was done to ensure that all samples and spectral instruments were under isothermal and isohumidity conditions, thereby reducing the impact of the external environment on the test results. A total of 100 rice grains were selected as samples, with full and symmetrical grains, moderate size, and free from pests, diseases, mold, or mechanical damage.The rice grains were dehulled three times using a household portable polished rice machine, and the polished rice was ground using an agate grinder. A 100-mesh sieve was used to screen out the rice powder as the sample. Test sample: amylose (Shanghai Aladdin Biochemical Technology Co., Ltd.), CAS: 9005-82-7. The rated power of the household portable polished rice machine is 200 ~ 240 V, with dimensions of 345 * 253 * 342 mm; agate grinder specifications: 40 –210 mm.
Experimental equipment
The TriVistaTMM555CRS micro-area three-stage Raman spectroscopy detector produced by Princeton Instruments was selected as the experimental instrument. It was equipped with an OLYMPUS 10x magnifying objective lens and a CCD detector, with a liquid nitrogen cooling temperature of -120 °C. For this study, a 532 nm laser was selected as the excitation light source, with the laser model being LE-LS-532-100. The laser linewidth was 0.1 nm, and the laser power range was 0-500 mW. The reflectivity of the laboratory walls was less than 3%, and the dust concentration was 3 × 10^5 particles per cubic meter (≥ 0.5 μm/m3).
Experimental design
The experiment selects Jinzi brand compound fertilizer from Jiangsu Huachang Chemical Co., Ltd., and Dihuantong brand organic soil conditioner and multifunctional microbial fertilizer from Beijing Jiabowen Biotechnology Co., Ltd. Fertilizer specifications: (1) Chemical fertilizer: N-P2O5-K2O ratio is 20-10-12, with a total nutrient content of ≥ 42%; (2) Organic fertilizer: effective viable count ≥ 500 million/gram, food-grade organic matter ≥ 55%, chelating trace elements CaO ≥ 8%, MgO ≥ 1%, and total content of trace elements (iron, manganese, boron, zinc) ≥ 0.2%, with a pH value controlled between 6.5 and 8.0.
In the experiment, the amount of microbial fertilizer was fixed (3 kg as base fertilizer and 1 kg as topdressing), while a gradual reduction strategy was implemented for chemical fertilizer. The initial amount of base fertilizer was 1.2 kg, and the topdressing started from 0.7 kg, with a 10% reduction in each subsequent application. By systematically collecting and analyzing data on changes in crop spectral characteristics, the impact of this reduced fertilizer application scheme on crop growth was verified. Field management followed local farmer’s conventional practices, and base and topdressing fertilizers were applied to each experimental plot according to this strategy, specifically applied on June 5th for base fertilizer and transplantation, with additional fertilization in mid-June. See Table 1 for detailed data on the reduced chemical fertilizer experiment. The experiment was designed with two factors: microbial fertilizer combined with chemical fertilizer and different fertilization amounts. A total of 4 fertilization methods were set up, namely: conventional dosage of microbial fertilizer + 90% reduction in chemical fertilizer (T1), conventional dosage of microbial fertilizer + 80% reduction in chemical fertilizer (T2), conventional dosage of microbial fertilizer + 70% reduction in chemical fertilizer (T3), and conventional dosage of microbial fertilizer + 60% reduction in chemical fertilizer (T4).
Spectral data acquisition
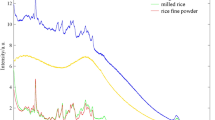
The original Raman spectra of rice from the same origin but with different treatment and fertilization amounts were collected. During spectral detection, the sample position was changed by uniformly fine-tuning the displacement platform to change the measurement points. After eliminating invalid spectra, 100 pieces of data were saved for each group.Although the nutritional components of the rice are basically the same, their respective contents differ. It can be seen that rice with different fertilizer application amounts exhibits different peak intensities, but the peak positions are basically the same. The original Raman spectra of rice with different fertilization methods are shown in Fig. 1.
Theoretical calculation of amylose
Starch is the main component of rice, accounting for about 75-85% of its total material content17. Starch is a polysaccharide composed of multiple α-D-glucose molecules, with the molecular formula (C6H10O5)n. Based on its structure, starch can be divided into amylose and amylopectin. The basic unit of amylose is composed of a pyran six-membered ring with an oxygen heteroatom, and it is a polysaccharide chain where D-glucopyranosyl units are linked by α-(1,4) glycosidic bonds. The structural formula of amylose is shown in Fig. 2. Through the analysis of the structural formula, it can be seen that the chemical bonds and functional groups contained in amylose include C-O-C, C-C-C, C-OH, CH, CH2, etc.
The density functional theory was used to optimize the structure of rice amylose, and the theoretical Raman spectrum was derived and compared with the experimental spectrum. The molecular structure was optimized using the B3LYP/3–21 g and B3LYP/6-311 + G(d) basis sets, respectively. The optimized structure was calculated, and the obtained spectral data were frequency-corrected with correction factors of 0.965 and 0.977, respectively, to plot the Raman spectrum of the molecule. By comparison, it was found that the corrected data of the 6-311 + G(d) basis set showed no imaginary frequency in the calculation results, as shown in Fig. 3. This indicates that a stable structure was obtained, and the specific vibration frequency assignments were identified.
Based on the known Raman peak information of starch structure and functional groups, it was observed that the Raman peaks were mainly distributed in the range of 400 cm− 1 to 1400 cm− 1, as shown in Table 2. The peak at 476 cm− 1 is formed by the ring skeleton vibration and torsion vibration of starch, caused by the breathing vibration of the skeleton in the pyran ring and the stretching vibration of C-O-C and C-C-O in the pyran ring, representing the degree of polymerization of polysaccharides and being a characteristic peak of starch18. The peak at 517 cm− 1 characterizes O-H wagging vibration, the peak at 570 cm− 1 characterizes O-H wagging vibration, the peak at 610 cm− 1 characterizes O-H and C-H wagging vibration, the peak at 720 cm− 1 characterizes O-H wagging vibration, O-C-C symmetric stretching vibration, and C-C-C symmetric stretching vibration, and the peak at 869 cm− 1 characterizes O-H wagging, C-C-O, and C-O-C stretching vibration. The peak at 1084 cm− 1 is the ester C-O-C symmetric stretching, alcohol C-C-O asymmetric stretching deformation, or amylose C-O-H bending vibration. The peak at 1126 cm− 1 corresponds to C=C stretching vibration, the peak at 1260 cm− 1 is O-H wagging and C-H wagging vibration, the peak at 1335 cm− 1 is ester or cyclic oxide C-H wagging vibration19, the peak at 1376 cm− 1 is C-H wagging vibration and C-C stretching vibration, the peak at 1395 cm− 1 is C-H wagging vibration, and the weak absorption peak near 1455 cm− 1 corresponds to the bending vibration of CH2.
Spectral data acquisition and preprocessing
Preprocessing of spectral data
In the process of Raman detection, fluorescence interference and the noise interference from the instrument itself are inevitable. If appropriate data processing methods are not adopted, the analysis results will be greatly affected. In order to improve the discrimination accuracy of the model, reduce the interference of noise signals, and reduce data differences to achieve the purpose of spectral identification, the Raman spectra are processed by smoothing (Savitzky-Golay Smoothing, SG) and multiple scattering correction (Multiple Scattering Correction, MSC).
To deal with the noise, stray light, and other interferences embedded in the spectra, SG and MSC are used in this experiment to preprocess the original spectra. Figure 4a shows the Raman spectrum of rice after smoothing by SG, and Fig. 4b shows the Raman spectrum of rice after MSC. SG smoothing can smooth the spectral curve20, and the average spectrum after preprocessing is similar to the original average spectrum; The MSC method eliminates spectral interference information caused by light scattering, obtaining a more concentrated reflection spectrum that is more utilizable than the original spectral information21.
Comparing Fig. 4a and b, it can be seen that the effect of SG is relatively small, and the entire curve is relatively smooth, which can effectively reduce spectral noise interference, display richer information, and improve the signal-to-noise ratio. In order to retain the original spectral information while reducing noise, the spectrum after SG preprocessing is therefore used for subsequent analysis.
Analysis and discussion
After preprocessing and feature extraction, we obtained the characteristic information of the Raman spectra of rice. It can be observed that while the peak intensities of rice differ, the peak positions are largely consistent. By referring to the known Raman peaks associated with starch structure and functional groups, we can establish a correspondence between them. Since different chemical compositions, contents, and structures produce distinct vibrational spectroscopic information, the Raman spectra of rice exhibit prominent characteristic peaks at 476 cm− 1, 577 cm− 1, 613 cm− 1, 869 cm− 1, 1084 cm− 1, 1126 cm− 1, 1260 cm− 1, 1335 cm− 1, and 1455 cm− 1. Each peak represents a unique chemical bond and vibration mode, as depicted in Fig. 5. Comparing the fitting results in Fig. 4 against actual measurements and theoretical calculations, we find a high degree of agreement between the calculated spectral ratios and the Raman-measured spectral feature peaks.
To eliminate background noise caused by fluorescence signals, baseline correction and noise reduction were applied to the Raman spectra of rice based on Fig. 4, and normalization was performed using the maximum peak intensity to mitigate the impact of external factors such as incident power on the measurement results.
In this study, Gaussian functions were employed to perform peak fitting for samples with different amounts of chemical fertilizer application, as shown in Fig. 6. The upper curve represents the preprocessed spectrum, while the lower curve depicts the fitted and separated spectrum. After fitting, it was observed that the peak positions remained largely unchanged, with the main peaks appearing at 476 cm− 1, 577 cm− 1, 613 cm− 1, 869 cm− 1, 1084 cm− 1, 1126 cm− 1, 1260 cm− 1, 1335 cm− 1, and 1455 cm− 1. The peak intensities, full widths at half maximum (FWHM), and peak areas of these Raman peaks were further extracted and summarized in Tables 3, 4 and 5, respectively.
Through detailed analysis of the peak information in the Raman spectra, we observed a consistent downward trend in the peak intensities, FWHM values, and peak areas at different peak positions as the amount of chemical fertilizer application decreased, specifically manifested as T1 > T2 > T3 > T4. Notably, the changes in Raman peaks located at 476 cm− 1, 577 cm− 1, 869 cm− 1, 1126 cm− 1, 1260 cm− 1, and 1335 cm− 1 were particularly pronounced, and this trend is detailed in Tables 3, 4 and 5. Especially for the Raman peaks near 1126 cm− 1 and 1260 cm− 1, the decreases in peak intensities, FWHM values, and peak areas were particularly significant after reducing the amount of chemical fertilizer.
As seen in Tables 3 and 5, as the amount of chemical fertilizer application decreased (T1, T2, T3, T4), both the peak intensities and peak areas of the Raman spectra showed a gradual decrease. Among them, the changes at 577 cm− 1, 869 cm− 1, 1126 cm− 1, and 1260 cm− 1 were the most significant. This could be attributed to the reduced absorption and utilization of nutrients by rice during growth due to the decrease in chemical fertilizer content, which in turn led to a decrease in starch content in the rice22.
In addition, it can also be observed from Table 4 that apart from the Raman peaks at 577 cm− 1, 869 cm− 1, 1126 cm− 1, and 1260 cm− 1, the Full Width at Half Maximum (FWHM) values of the Raman peaks at 476 cm− 1 and 1335 cm− 1 also decrease with the reduction in chemical fertilizer application. This may be attributed to changes in the nutritional conditions of rice plants, which in turn affect the purity of starch in rice. Notably, the peak intensity, FWHM value, and peak area value of the Raman peaks near 577 cm− 1 and 1126 cm− 1 exhibit particularly significant decreases after reducing the amount of chemical fertilizer.
By combining the measured amylose content values from different reduced fertilizer application treatment areas with the amylose spectral intensity values obtained from Raman spectroscopy, we analyzed and concluded that the treatment involving conventional microbial fertilizer usage combined with different levels of reduced chemical fertilizer application leads to a decreasing trend in the amylose content of rice. This conclusion is consistent with previous research findings23,24, which indicate that the amylose content of rice decreases with the reduction in chemical fertilizer application.
Conclusion
In this study, rice samples subjected to reduced fertilizer application treatments (T1, T2, T3, T4) under straw returning mode were used to measure their Raman spectra of amylose, which were then compared and analyzed with DFT theoretical calculations. The results revealed that Raman peaks located near 476 cm− 1, 577 cm− 1, 869 cm− 1, 1126 cm− 1, 1260 cm− 1, and 1335 cm− 1 could serve as characteristic peaks for component identification. Additionally, the peak values, full width at half maximum (FWHM), and peak areas of other peaks were correlated with variable fertilizer application, suggesting their potential as correlation coefficients for quality assessment. This comparative analysis illuminated the patterns of molecular vibrations and successfully identified the corresponding amylose components in the spectra. Based on these findings, the scientific application of microbial fertilizer combined with reduced chemical fertilizer application can not only significantly increase the amylose content in rice but also provides a basis for macroeconomic regulation of subsequent reduced fertilizer application, effectively mitigating agricultural non-point source pollution. Furthermore, it lays a theoretical foundation for differentiating rice of various qualities.
Data availability
No datasets were generated or analysed during the current study.
References
Majumdar, P. et al. Demand prediction of rice growth stage-wise irrigation water requirement and fertilizer using Bayesian genetic algorithm and random forest for yield enhancement. Paddy Water Environ. 21(2), 275–293 (2023).
Jiang-Ming, Z. Improving fertilization practices to reduce the potential of nutrient loss from rice paddy fields. Paddy Water Environ. 21(1), 115–126 (2023).
Kuzman, B. et al. Estimation of optimal fertilizers for optimal crop yield by adaptive neuro fuzzy logic. Rhizosphere 18, 100358 (2021).
Zhang, L., Tan, F., Li, S. & Huo, Z. Potential dynamic of irrigation water requirement for rice across Northeast China. Theor. Appl. Climatol. 142(3), 1283–1293 (2020).
Zhao, Q., Fan, J., Ning, S. & Li, W. Prediction and guidance of fertilizer requirement in different growth stages of crops based on artificial neural network. Innov. Comput. 791, 1651–1655 (2022).
Wang, X. et al. Meta-analysis of changes in soil organic carbon content and its influencing factors in Chinese farmland under straw returning conditions. J. China Agric. Univ. 25(8), 12–24 (2020).
Li, L. et al. Effects of different amounts of straw returning to the field on rice yield and soil fertility. J. Anhui Agric. Sci. 48(10), 43–45 (2020).
Rushan, C. et al. Potassium nutrient resource of straw from major grain crops in China and its potential for substituting potassium fertilizer by straw returning. J. Plant Nutr. Fertilizers 26(2), 201–211 (2020).
Li, X. et al. Effects of organic fertilizers on rice yield and quality and nitrogen use efficiency. J. Hunan Agric. Univ. (Nat. Scie.) 36, 3 (2010).
Xu, L. et al. Effects of different organic fertilizers on the quality of dry direct-seeded rice. Acta Agric. Boreali-Sinica 37(1), 137–146 (2022).
Liu, H. et al. Effects of different types of organic fertilizers on rice yield and rice quality. Jiangsu J. Agric. Sci. 40(4), 645–651 (2024).
Zhang, Q. et al. Effects of combined application of bio-microbial fertilizer and chemical fertilizer on lettuce growth and soil environment. Chin. J. Eco-Agric. 31(5), 725–741 (2023).
Deng, W., Qing, X. G. & Yang, Y. Effects of applying organic fertilizer on rice lodging resistance and yield. Agric. Sci. Technol. 11(2), 98–101 (2010).
Gao, J. et al. Long-term combined application of organic and inorganic fertilizers on rice yield and soil available nutrients. Acta Pedol. Sinica 51(2), 314–324 (2014).
Liu, H. et al. Establishment of a near-infrared detection model for amylose content in rice. J. Hunan Agric. Univ. (Nat. Sci.) 45(2), 189–193 (2019).
Guo, Y. B. et al. Infrared and Raman spectroscopic characterization of structural changes in albumin, globulin, glutelin, and prolamin during rice aging. J. Agric. Food Chem. 61(1), 185–192 (2013).
Fan, M. et al. Research progress in the structure of rice amylopectin. Chin. J. Rice Sci. 147(02), 124–132 (2017).
Liu, Y. et al. Application of Raman spectroscopy in the characterization of starch crystalline structure. Food Sci. Technol. 40(1), 315–318 (2015).
Lou, X. et al. Preparation and characterization of wheat amylose-monoglyceride complexes. Food Sci. Technol. 45(11), 243–249. https://doi.org/10.13684/j.cnki.spkj.2020.11.039 (2020).
Wang, Y. Research on the Mechanism and System of Rice Origin Identification Based on Raman Spectroscopy (Heilongjiang Bayi Agricultural University, 2022).
Ji, J., Li, P., Jin, X., Ma, H. & Li, M. Quantitative detection of tomato seedling robustness during spring transplanting based on spectroscopy. Spectrosc. Spectr. Anal. 42(6), 1741–1748 (2022).
Fei, L. et al. Grain chalkiness is reduced by coordinating the biosynthesis of protein and starch in fragrant rice (Oryza sativa L.) grain under nitrogen fertilization. Field Crops Res. 302: 109098 (2023).
Huang, L. et al. Accurate detection of phenthoate pesticide residues in corn seeds using surface-enhanced Raman spectroscopy. Spectrosc. Spectr. Anal. 38(9), 2782–2787 (2018).
Yang, Z. et al. Effects of nitrogen fertilizer application timing and rate on rice grain quality. J. Huazhong Agric. Univ. 5, 429–500 (2002).
Funding
This work was supported by the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS510, 20230101187JC, JJKH20241646KJ).
Author information
Authors and Affiliations
Contributions
Concept and design: Jointly responsible by Yong Tan and Changming Li. Data collection: Primarily completed by Zhong Lv and Changming Li. Data analysis and interpretation: Conducted jointly by Changming Li, Chunyu Liu, and Xun Gao. Manuscript writing: The first draft was completed by Changming Li, with subsequent revisions handled by Chunyu Liu and Xun Gao. Final review: Conducted by Yong Tan. Funding and Material Acquisition: Jointly responsible by Yong Tan and Chunyu Liu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Tan, Y., Liu, C. et al. A study on the changes in rice composition under reduced fertilization conditions using Raman spectroscopy technology. Sci Rep 14, 27030 (2024). https://doi.org/10.1038/s41598-024-77492-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77492-6