Abstract
Background: The use of immune checkpoint inhibitors (ICIs) as neoadjuvant therapy is a promising novel approach in resectable non-small-cell lung cancer (NSCLC). This study aimed to investigate the prognostic value of PD-L1 in patients with NSCLC receiving neoadjuvant immune checkpoint inhibitor plus chemotherapy (CT). Materials and methods: Several databases (PubMed, Embase, and cochrane central register of controlled trials [CENTRAL]) were systematically searched. Randomized controlled trials (RCTs) investigating pathological and survival outcomes with neoadjuvant ICI + CT versus CT alone in NSCLC were analyzed. Results: Overall, eight RCTs (n = 3,404) were included. The analyses showed neoadjuvant ICI + CT significantly improved complete pathological response (pCR) and event-free survival (EFS) in either tumor PD-L1 < 1%, ≥ 1%, 1-49%, or ≥ 50% population (both p < 0.0001) compared with neoadjuvant CT alone. The overall survival (OS) data are not yet mature among all included RCTs, and only three RCTs presented OS data by PD-L1 status of patients. The pooled OS favored neoadjuvant ICI + CT in the PD-L1 ≥ 1% population (hazard ratio [HR], 0.45; 95% CI, 0.31–0.65; p < 0.0001), but not in the PD-L1 < 1% population (HR, 0.89; 95% CI, 0.66–1.19; p = 0.43). Conclusions: Compared with neoadjuvant CT alone, neoadjuvant ICI + CT significantly enhanced pCR and EFS for patients with resectable NSCLC regardless of the expression of PD-L1. It seems that only patients with PD-L1 positive tumors may achieve a better OS, but it’s currently inconclusive due to immature data, so future research with long-term follow-up is still needed.
Similar content being viewed by others
Introduction
Lung cancer is the most common malignant tumor and the leading cancer killer worldwide, which seriously threatens the health of humans1. Long term smoking, environmental pollution, occupational exposure, and family history are high-risk factors for the onset of lung cancer2. Due to atypical early symptoms, most patients are diagnosed with advanced or metastatic diseases, and only a small number of patients have the opportunity for direct surgery3. Non-small-cell lung cancer (NSCLC) is the most common pathological type of lung cancer, and surgery is the main treatment for early-stage NSCLC, but only a quarter of patients have resectable diseases at the time of diagnosis4,5. To improve the resection rate and survival for resectable patients, neoadjuvant CT followed by surgery with or without adjuvant therapy is a commonly used treatment mode in clinical practice. However, approximately 30–55% of patients will still experience recurrence after surgery6. Therefore, further exploration of more effective neoadjuvant strategies is urgently needed.
With the development of immunotherapy, immune checkpoint inhibitors (ICIs) play a huge role in the treatment of various solid tumors, including lung cancer7. A large number of clinical studies have confirmed that ICI-based regimens can improve the survival of patients in either neoadjuvant, adjuvant, or metastatic setting for lung cancer8,9,10. Evidence has shown that the efficacy of immunotherapy is not ideal for NSCLC patients who had driver gene mutations (such as epidermal growth factor receptor [EGFR] and anaplastic lymphoma kinase [ALK] mutations), so immunotherapy was not recommended for these patients11,12. Except patients with driver gene mutations, other patients are generally sensitive to ICI therapies. Thus, exploring prognostic factors for predicting outcomes for patients treated with ICI-based regimens is currently a great interest. Among various biomarkers, PD-L1 is a widely recognized prognostic predictor for ICI treatments13. Many clinical trials found that patients with high expression of PD-L1 had better efficacy than those with low PD-L1 expression14,15, but there were also studies reporting that the expression of PD-L1 was not related to prognosis of NSCLC16. A study by Goulart et al. found that the correlation between PD-L1 expression (PD-L1 tumor proportion score < 1%, 1-49%, and ≥ 50%) and patient prognosis was unstable in patients with metastatic NSCLC17. They found that when evaluating subgroups through PD-L1 expression, the correlation ranged from weak to moderate.
Until now, it has been unclear whether PD-L1 can predict long-term outcomes for NSCLC patients treated with neoadjuvant ICI + CT. The aim of this study is to assess the predictive value of PD-L1 expression in long-term outcomes for patients with resectable NSCLC treated with neoadjuvant ICI combined with CT.
Methods
Screening of literatures
We searched several databases including PubMed, Embase, and cochrane central register of controlled trials [CENTRAL] for relevant clinical trials from inception to March 2024. The keywords for the search strategy are as follows: “Immunotherapy or immune checkpoint inhibitor or pembrolizumab or nivolumab OR cemiplimab OR camrelizumab OR sinilimab OR toripalimab OR tislelizumab OR spartalizumab OR pidilizumab OR atezolizumab or avelumab or tremolumab or durvalumab or OR sugemalimab” and “non small cell lung cancer OR NSCLC OR lung adenocarcinoma OR adenocarcinoma of the lung OR lung squamous cell carcinoma OR squamous cell carcinoma of the lung”. We also manually searched relevant references to identify other relevant studies. Only published articles of RCTs reporting experimental data related to neoadjuvant ICI + chemotherapy (CT) versus neoadjuvant CT alone in patients with resectable NSCLC were included. Non-publication literatures, and papers published in languages other than English were not eligible for inclusion. Besides, we excluded clinical trials investigating the use of radiation therapy, molecular targeted therapy, or immunotherapy monotherapy in neoadjuvant setting. Studies that only included NSCLC patients with EGFR or ALK mutations were also excluded. Two independent reviewers (HJF and LPH) screened the literatures based on inclusion and exclusion criteria. Firstly, by reading the titles and abstracts, literatures that were identified as not relevant or published repeatedly were excluded. Secondly, further screening was conducted by reading the abstracts and full texts to exclude literatures that did not meet the inclusion criteria or met the exclusion criteria. Finally, reviewers extracted data from the final included literatures, including study name, author details, publication year, tumor stage, sample size, age, gender, PD-L1 status of patients, treatment regimens, and study outcomes. In the process of literature screening, disagreements were resolved by a third reviewer (MDC).
Data extraction
The available pooled outcomes in this analysis were complete pathological response (pCR), event-free survival (EFS) and overall survival (OS). Other outcomes such as surgical rate, major pathological response (MPR), and R0/R1 resection rates were not available when evaluating subgroups by PD-L1 expression. Two independent reviewers (LP and WHL) extracted data on study name, author details, publication year, tumor stage, sample size, age, gender, PD-L1 status of patients, and neoadjuvant treatment regimen. Clinical outcomes including pCR, EFS, and OS were extracted in detail for further analysis.
Quality assessment
Two independent reviewers (LXJ and HSX) assessed the risk of bias of the included RCTs using the Cochrane risk of bias tool18, which includes seven items: randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other biases. Based on the above seven items, the judgments on the risk of bias were classified into three levels: “high risk,” “unclear risk,” and “low risk”. We used funnel plots to evaluate publication bias for the included studies. Disagreements were resolved by a third reviewer (MDC).
Statistical analysis
We conducted meta-analysis of included RCTs using the statistical software of Review Manager 5.4. The outcomes of EFS and OS were pooled as hazard ratios (HRs) with 95% confidence interval (CI), while outcome of complete pathological response was pooled as risk ratio (RR) with its 95% CI. Heterogeneity between studies was evaluated based on the I-squared (I2) test when conduting meta-analysis. When I2 > 50%, the heterogeneity was assessed as high, and then a random-effects model was applied; Otherwise, a fixed-effects model was chosen. If P-value is less than 0.05, it is considered statistically significant.
Results
Search results
Through initial search, 2062 articles were returned. After further screening and removal of duplicate studies, eight RCTs (AEGEAN19, CheckMate-81620, KEYNOTE-67121, NADIM II22, Neotorch23, RATIONALE-31524, TD-FOREKNOW25, CheckMate 77T26) with a total of 3,404 patients met the inclusion criteria. The prisma diagram of the screening process is shown in Fig. 1. Across RCTs, all patients were diagnosed with NSCLC and received neoadjuvant ICI + CT in the study group and neoadjuvant CT alone in the control group. These studies were published between 2022 and 2024. Among these eight RCTs, five programmed cell death 1 (PD-1) antibodies (nivolumab, pembrolizumab, toripalimab, camrelizumab, and tislelizumab) and one PD-L1 antibody (durvalumab) were included. The basic characteristics of the included studies and the PD-L1 expression status of patients are shown in Table 1.
Outcome of pCR
Across the eight RCTs, five studies (AEGEAN19, CheckMate-81620, NADIM II22, RATIONALE-31524, and TD-FOREKNOW25) evaluated the pCR data based on PD-L1 expression of patients. The analysis showed that the pooled pCR favored neoadjuvant ICI + CT over neoadjuvant CT in either tumor PD-L1 < 1% (RR, 4.32; 95% CI, 2.52–7.42; p < 0.0001; I2 = 0%), ≥ 1% (RR, 8.78; 95% CI, 4.88–15.78; p < 0.0001; I2 = 0%), 1-49% (RR, 4.33; 95% CI, 2.05–9.17; p < 0.0001; I2 = 21%), or ≥ 50% (RR, 6.85; 95% CI, 3.21–14.62; p < 0.0001; I2 = 0%) population (Fig. 2).
Outcome of EFS
The EFS data for the PD-L1 subgroups can be extracted from six studies (AEGEAN19, CheckMate-81620, KEYNOTE-67121, NADIM II22, Neotorch23, and CheckMate 77T26). The analysis showed that neoadjuvant ICI + CT was associated with significantly improved EFS compared with neoadjuvant CT in either PD-L1 < 1%(HR, 0.68; 95% CI, 0.56–0.83; p < 0.0001; I2 = 0%), ≥ 1% (HR, 0.44; 95% CI, 0.35–0.56; p < 0.0001; I2 = 16%), 1-49% (HR, 0.56; 95% CI, 0.45–0.70; 0.35–0.56; p < 0.0001; I2 = 43%), or ≥ 50% (HR, 0.40; 95% CI, 0.30–0.51; p < 0.0001; I2 = 24%) population (Fig. 3).
Outcome of OS
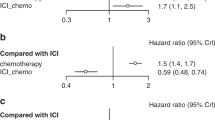
The OS data of included trials was immature. There were only three studies (CheckMate-81620, KEYNOTE-67121, and NADIM II22) presented OS data for the PD-L1 subgroups (The OS data by PD-L1 status of the CheckMate-816 and KEYNOTE-671 studies are available in the reports of the European Society of Medical Oncology [ESMO] Congress 2023–2024 Abstracts27,28). The analysis showed that neoadjuvant ICI + CT was associated with significantly improved OS compared with neoadjuvant CT in the PD-L1 ≥ 1% (HR, 0.45; 95% CI, 0.31–0.65; p < 0.0001; I2 = 52%) population. However, there was no significant difference (HR, 0.89; 95% CI, 0.66–1.19; p = 0.43; I2 = 0%) in OS between the two groups in patients with tumor PD-L1 < 1% (Fig. 4) .
Quality of the included RCTs
As shown in Fig. 5, the quality of each included RCT was assessed as high, suggesting there was a low risk of bias in this analysis. The funnel plots for EFS and OS were symmetrical, suggesting no publication bias (Fig. 6).
Discussion
For more than a decade, immunotherapy has been applied to the treatment of various cancers, including lung cancer, and ICI-based regimens have completely changed the treatment pattern of NSCLC29,30. Thus, exploring sensitive predictive factors for efficacy of ICI treatments is very important for clinical physicians to choose the best beneficial population and appropriate ICI strategies. Although the predictive value of PD-L1 may vary in different cancers or different ICI strategies, PD-L1, as the most important predictive factor for ICI-based therapies, has always been of great concern.
To our knowledge, this is the first meta-analysis of RCTs to comprehensively and systematically evaluate the correlation between PD-L1 expression and its prognostic value in patients with NSCLC undergoing preoperative neoadjuvant immunochemotherapy. Before our study, a research by Deng et al. found that patients with PD-L1 expression ≥ 1% who received neoadjuvant immunotherapy before surgery for NSCLC was associated with a higher rate of MPR and pCR compared with those with PD-L1 expression < 1%31. In our study, we found that regardless of PD-L1 expression, pCR and EFS of neoadjuvant immunochemotherapy were superior to those of neoadjuvant CT alone. We also observed a phenomenon that patients with higher PD-L1 expression had lower HR values for EFS, indicating that patients with higher PD-L1 expression might have a lower risk of disease progression. These results were consistent with those by Banna et al., who reported that NSCLC patients who had PD-L1 negative tumors were at higher risk of relapse than those with low or high PD-L1 tumors when treated with neoadjuvant chemo-immunotherapy32. Furthermore, in terms of OS, we found that only patients with PD-L1 positive tumors achieved significant OS benefit, whereas in PD-L1 negative patients, no better OS was observed. Despite the immaturity of OS data, these results may indicate that PD-L1 can be used as an valuable prognostic factor for predictiving outcomes for patients with resectable NSCLC treated with preoperative immunochemotherapy. Due to the valuable predictive value of PD-L1, our results recommend PD-L1 detection in clinical practice before choosing ICI immunotherapy so as to better predict outcomes of patients.
It is worth noting that the results of our analysis were similar to those of studies on metastatic NSCLC. In the KEYNOTE-189 trial33, pembrolizumab plus chemotherapy was associated with significantly improved OS and progression free survival (PFS) compared with chemotherapy alone in either PD-L1 < 1%, ≥ 1%, 1-49%, or ≥ 50% subgroup, and the higher PD-L1 expression, the longer median OS seems to achieve. In the Impower150 trial34, atezolizumab in combination with chemotherapy and bevacizumab resulting in significantly longer PFS and OS in metastatic non-squamous NSCLC, regardless of PD-L1 expression. In addition, similar results were also observed in the studies of IMpower13035, IMpower13236, and KEYNOTE-40737. Among these trials, we found a commonality that when immunotherapy combined with chemotherapy, the efficacy of their combination therapy is likely to have a synergistic effect, and patients with high PD-L1 expression would have a greater trend of OS benefit.
Another concern is that evidence has shown that the expression and prognostic value of PD-L1 in primary lung and metastatic lesions are highly inconsistent. A recent research reported that the expression of PD-L1 varies at different biopsy sites, and PD-L1 has different predictive value for the benefits of ICIs in NSCLC38. The researchers found a significant correlation between PD-L1 and biopsy site (p = 0.004). PD-L1 expression was high in adrenal, liver, and lymph node metastases, while PD-L1 expression was low in bone and brain metastases. Higher PD-L1 levels in primary lung lesions and distant metastatic specimens were associated with higher tumor response, longer PFS, and OS. However, PD-L1 in lymph node specimens was not correlated with response or survival rate. Another study reported that PD-L1 negative was more common in primary lung lesion compared to metastatic samples. The distribution of PD-L1 expression (PD-L1 expression was high in lymph nodes but was predominantly negative in bones) and the predictive ability of PD-L1 expression for tumor response to immunotherapy varies by organ39. We believe that this is a great concern that physicians should pay close attention to. When selecting tumor specimens for PD-L1 detection in clinical practice, it would be better to choose the same tissue specimens with high prognostic value (such as lung rather than bone specimens). In our study, because the included patients were all newly diagnosed NSCLC without distant metastasis, the samples tested for PD-L1 were relatively consistent, avoiding research bias caused by PD-L1 testing on different tumor specimens.
Our study has the following main limitations. First, only published RCTs were included in this analysis. Other valuable sources, such as grey literatures and clinical trials on-course were excluded, which may lead to a possibility of selective bias. Besides, our study focused on a comprehensive meta-analysis of outcomes for patients but lacked a systematic literature review process. Second, the number of included RCTs is relatively small, and some RCTs were studies with small sample sizes, which lead to a heterogeneity when analyzing outcomes by PD-L1 subgroups. Third, among the six included ICIs, five were PD-1 antibodies, while only one was PD-L1 antibody. Thus, in-depth analysis cannot be conducted to distinguish whether the prognostic value of PD-L1 expression varies among different types of ICI drugs. Fourth, due to limited data, besides the outcomes of pCR, EFS, and OS, other outcomes such as surgical rate, major pathological response, and R0/R1 resection rates were not feasible when analyzing by PD-L1 subgroups. Finally, the OS data of included studies was not mature, and only three studies provided OS data of PD-L1 subgroups, which have had a great impact on the analysis of OS outcome, so further investigations with longer follow-up time are needed.
Conclusions
This meta-analysis suggested that regardless of PD-L1 expression, neoadjuvant ICI + CT resulted in better pCR and EFS in resectable NSCLC than neoadjuvant CT alone. In terms of OS, it seems that patients with PD-L1 negative tumors had no more OS benefit, and only patients with PD-L1 positive tumors might achieve a significant transformation from EFS benefit to OS benefit; however, the analysis of OS results is currently inconclusive due to immature data. Our research findings support PD-L1 was a valuable biomarker for predicting outcomes of NSCLC patients in this setting. Due to the immature OS data, further in-depth research is necessary.
Data availability
Data is provided within the manuscript.
Abbreviations
- ICI:
-
Immune checkpoint inhibitor
- PD-L1:
-
Programmed cell death 1 ligand 1
- PD-1:
-
Programmed cell death 1
- NSCLC:
-
Non-small-cell lung cancer
- pCR:
-
Complete pathological response
- EFS:
-
Event-free survival
- OS:
-
Overall survival
- RCT:
-
Randomized controlled trial
- EGFR:
-
Epidermal growth factor receptor
- ALK:
-
Anaplastic lymphoma kinase
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. https://doi.org/10.3322/caac.21834 (2024).
Hong, Q. Y. et al. Prevention and management of lung cancer in China. Cancer. 121 (Suppl 17), 3080–3088. https://doi.org/10.1002/cncr.29584 (2015).
Hirsch, F. R. et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 389 (10066), 299–311. https://doi.org/10.1016/S0140-6736(16)30958-8 (2017).
Duma, N., Santana-Davila, R. & Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 94 (8), 1623–1640. https://doi.org/10.1016/j.mayocp.2019.01.013 (2019).
Rudin, C. M., Brambilla, E., Faivre-Finn, C. & Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers. 7 (1), 3. https://doi.org/10.1038/s41572-020-00235-0 (2021). Published 2021 Jan 14.
Kelsey, C. R. et al. Local recurrence after surgery for early stage lung cancer: An 11-year experience with 975 patients. Cancer. 115 (22), 5218–5227. https://doi.org/10.1002/cncr.24625 (2009).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249. https://doi.org/10.1146/annurev-pathol-042020-042741 (2021).
Mountzios, G. et al. Immune-checkpoint inhibition for resectable non-small-cell lung cancer - Opportunities and challenges. Nat. Rev. Clin. Oncol. 20 (10), 664–677. https://doi.org/10.1038/s41571-023-00794-7 (2023).
Pirker, R. Immune checkpoint inhibitors as adjuvant therapy in patients with completely resected nonsmall cell lung cancer. Curr. Opin. Oncol. 36 (1), 24–28. https://doi.org/10.1097/CCO.0000000000001003 (2024).
Reck, M., Remon, J. & Hellmann, M. D. First-line immunotherapy for non-small-cell lung cancer [published correction appears in J Clin Oncol. ;40(11):1265]. J. Clin. Oncol. 40(6), 586–597 (2022). https://doi.org/10.1200/JCO.21.01497
Attili, I. et al. Immune checkpoint inhibitors in EGFR-mutant non-small cell lung cancer: A systematic review. Cancer Treat. Rev. 119, 102602. https://doi.org/10.1016/j.ctrv.2023.102602 (2023).
Mazieres, J. et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 30 (8), 1321–1328. https://doi.org/10.1093/annonc/mdz167 (2019).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14 (4), 847–856. https://doi.org/10.1158/1535-7163.MCT-14-0983 (2015).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl. J. Med. 373 (17), 1627–1639. https://doi.org/10.1056/NEJMoa1507643 (2015).
Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 387 (10027), 1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7 (2016).
Carbone, D. P. et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376 (25), 2415–2426. https://doi.org/10.1056/NEJMoa1613493 (2017).
Goulart, B. H. L. et al. Correlations of response rate and progression-free survival with overall survival in immunotherapy trials for metastatic non-small-cell lung cancer: An FDA pooled analysis. Lancet Oncol. 25 (4), 455–462. https://doi.org/10.1016/S1470-2045(24)00040-8 (2024).
Higgins, J.P. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011) (published 2011 Oct 18).
Heymach, J. V. et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 389 (18), 1672–1684. https://doi.org/10.1056/NEJMoa2304875 (2023).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386 (21), 1973–1985. https://doi.org/10.1056/NEJMoa2202170 (2022).
Wakelee, H. et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 389 (6), 491–503. https://doi.org/10.1056/NEJMoa2302983 (2023).
Provencio, M. et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 389 (6), 504–513. https://doi.org/10.1056/NEJMoa2215530 (2023).
Lu, S. et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: The Neotorch Randomized Clinical Trial. JAMA. 331 (3), 201–211. https://doi.org/10.1001/jama.2023.24735 (2024).
Yue, D. et al. VP1-2024: RATIONALE-315: Event-free survival (EFS) and overall survival (OS) of neoadjuvant tislelizumab (TIS) plus chemotherapy (CT) with adjuvant TIS in resectable non-small cell lung cancer (NSCLC). Ann. Oncol. https://doi.org/10.1016/j.annonc.2024.01.005 (2024).
Lei, J. et al. Neoadjuvant camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for Chinese patients with resectable stage IIIA or IIIB (T3N2) non-small cell lung cancer: The TD-FOREKNOW randomized clinical trial. JAMA Oncol. 9 (10), 1348–1355. https://doi.org/10.1001/jamaoncol.2023.2751 (2023).
Cascone, T. et al. Checkmate 77T: A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J. Clin. Oncol. 38 https://doi.org/10.1200/jco.2020.38.15_suppl.tps9076 (2024).
Provencio Pulla, M. et al. LBA57 neoadjuvant nivolumab (N) + chemotherapy (C) in the phase III CheckMate 816 study: 3-y results by tumor PD-L1 expression. Ann. Oncol. 34(supplement 2), S1298-S1299. ISSN 0923-7534 (2023). https://doi.org/10.1016/j.annonc.2023.10.053. https://www.sciencedirect.com/science/article/pii/S0923753423041972.
Garassino, M. C. et al. 1210P Neoadjuvant pembrolizumab (pembro) or placebo (pbo) plus chemotherapy and adjuvant pembro or pbo for early stage NSCLC: Subgroup analyses of the phase III KEYNOTE-671 study. Ann. Oncol. 35(Supplement 2), S778-S779. ISSN 0923–7534. https://doi.org/10.1016/j.annonc.2024.08.1269 (2024). https://www.sciencedirect.com/science/article/pii/S0923753424027881.
Lahiri, A. et al. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer. 22 (1), 40. https://doi.org/10.1186/s12943-023-01740-y (2023) (published 2023 Feb 21).
Mamdani, H., Matosevic, S., Khalid, A. B., Durm, G. & Jalal, S. I. Immunotherapy in lung cancer: Current landscape and future directions. Front. Immunol. 13, 823618. https://doi.org/10.3389/fimmu.2022.823618 (2022) (published 2022 Feb 9).
Deng, H. et al. PD-L1 expression and tumor mutation burden as pathological response biomarkers of neoadjuvant immunotherapy for early-stage non-small cell lung cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 170, 103582. https://doi.org/10.1016/j.critrevonc.2022.103582 (2022).
Banna, G.L., Hassan, M.A., Signori, A. et al. Neoadjuvant chemo-immunotherapy for early-stage non-small cell lung cancer: A systematic review and meta-analysis. JAMA Netw. Open. 7(4), e246837 (2024) (published 2024 Apr 1) . https://doi.org/10.1001/jamanetworkopen.2024.6837.
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl. J. Med. 378 (22), 2078–2092. https://doi.org/10.1056/NEJMoa1801005 (2018).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl. J. Med. 378 (24), 2288–2301. https://doi.org/10.1056/NEJMoa1716948 (2018).
West, H. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (7), 924–937. https://doi.org/10.1016/S1470-2045(19)30167-6 (2019).
Nishio, M. et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. J. Thorac. Oncol. 16 (4), 653–664. https://doi.org/10.1016/j.jtho.2020.11.025 (2021).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379 (21), 2040–2051. https://doi.org/10.1056/NEJMoa1810865 (2018).
Hong, L. et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J. Thorac. Oncol. 15 (9), 1449–1459. https://doi.org/10.1016/j.jtho.2020.04.026 (2020).
Schoenfeld, A. J. et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann. Oncol. 31 (5), 599–608. https://doi.org/10.1016/j.annonc.2020.01.065 (2020).
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
Study design and writing: MDC and HJF; Data collection and selection: LP and WHL; Statistical analysis: LPH and HJF; Risk of bias assessment: HSX and XLJ; All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mo, DC., Huang, JF., Lin, P. et al. The role of PD-L1 in patients with non-small cell lung cancer receiving neoadjuvant immune checkpoint inhibitor plus chemotherapy: a meta-analysis. Sci Rep 14, 26200 (2024). https://doi.org/10.1038/s41598-024-78159-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78159-y