Abstract
Alpha oscillations are associated with various psychiatric disorders, with many studies focusing on the prefrontal cortex, where transcranial alternating current stimulation (TACS) is applied in the alpha frequency band. This approach often involves selecting individualized alpha frequencies to resonate with their endogenous alpha oscillations. While strong alpha oscillations (8–13 Hz) are typically induced when the eyes are closed, they can also occur during the resting state with eyes open. However, it remains unclear whether these alpha oscillations share a common neural generation mechanism. Exploring which of these alpha oscillations is more suitable as a stable alpha peak frequency is a question of significant interest. Therefore, to systematically study this issue, we specifically collected resting-state electroencephalographic (EEG) data from the prefrontal region of 40 individuals, under both eyes-open and closed- eye conditions, with multiple follow-ups extending up to nine months. Through spectral analysis on each person’s entire dataset and averaging the results, we observed a significant positive correlation between the alpha-band power in the eyes-open and the eyes-closed states, in terms of both absolute power and relative power. Further analysis revealed that this correlation was primarily contributed by the periodic activity within the alpha band. Upon modelling the oscillatory components, we discovered distinct differences in the oscillatory characteristics-such as number of the alpha sub-oscillations between the eyes-open state and the eyes-closed state. Our study is the first to systematically explored the relationship between alpha oscillations in the prefrontal cortex in the eyes-open and eyes-closed states, identifying both shared part of the neural generation mechanism and some distinct neural mechanisms that are unique to each state.
Similar content being viewed by others
Introduction
The resting state is a common condition in everyday life for people1,2,3, experienced when awake with eyes open and when asleep with eyes closed are two common ways of experiencing this state, as well as these states are also standard experimental conditions in electroencephalogram (EEG) studies in neuroscience4,5,6,7. In the neural activities, alpha oscillations, which occur at approximately 8 to 13 Hz, are a key neurobiological feature observed during these states8,9,10,11. Studying alpha oscillations provides deeper insights into the brain’s functional mechanisms and cognitive processes. These oscillations are typically associated with relaxation, eyes-closed rest, and the resting state of wakefulness12. When people close their eyes and relax, the amplitude of alpha oscillations usually increases. Conversely, when the eyes are open, particularly during visual tasks, the amplitude of alpha oscillations tends to decrease. The reduction in alpha oscillations is indicative of the brain transitioning from a resting state to an active state, as it processes more external information. Alpha oscillations are most prominent in the occipital lobe of the brain but are also detected in other brain regions13,14. The interaction between the thalamus and the cortex is considered to be an important basis for the generation of alpha oscillations15,16. Inhibitory neuron activity within the cortex may play a key role in the formation and regulation of alpha oscillations17. Changes in the amplitude and phase of alpha oscillations are related to the attention distribution and concentration18,19,20,21,22. They also play a regulatory role in sensory processing, such as vision and hearing, and are involved in the memory encoding and retrieval processes11,23,24,25,26. Abnormalities in alpha oscillations may be a sign of mental disorders27such as depression28, attention deficit hyperactivity disorder13, schizophrenia29,30, Alzheimer’s disease31,32,33,34, autism35, and others. In-depth research into alpha oscillations will provide important theoretical and practical foundations for understanding the working principles of the brain and for the treatment of related diseases.
Previous research has primarily focused on comparing the EEG differences between eyes-open and eyes-closed states36,37,38, with alpha oscillations being significantly stronger during eyes-closed condition, also known as the Berger effect10. The alpha oscillations in the eyes-open state during resting state are often overlooked, despite their important role in different task-related states19,39. But a fundamental question that remains unclear at present is whether the alpha oscillations in the eyes-open and eyes-closed states during resting state share similar neural mechanisms. Many studies choose the frequency of a subject’s intrinsic alpha oscillations as the stimulation frequency for electrical or magnetic stimulation40, with the prefrontal cortex being an important target area for neural modulation. Therefore, exploring the connections between the characteristics of alpha oscillations across different states during resting state is of great importance.
Therefore, in this study, to address this question, we specifically collected resting-state EEG data from the prefrontal cortex of 40 participants (including both eyes-open and eyes-closed conditions). The data collection included multiple times, with the longest duration extending up to nine months. We conducted spectral analysis on entire dataset for each participant, beginning by comparing the relationship between the absolute power and relative power in the alpha frequency band. Further, we employed descriptive models to distinguish between oscillatory and non-oscillatory components of the spectrum, allowing us to compare the finer oscillatory characteristics of the alpha frequency band between the eyes-open and eyes-closed states.
Materials and methods
Participants and EEG
A total of 40 right-handed healthy individuals, consisting of 17 females and 23 males with an average age of 33.7 ± 7.2 years, participated in this study, all of whom provided informed consent. The study procedures adhered to the National Institutes of Health Guidelines and received ethical approve from the Shenzhen Institute of Advanced Science and Technology, Chinese Academy of Sciences (SIAT-IRB-231113-H0677). Each participant contributed at least two sets of resting-state EEG data within a nine-month period, resulting in a total of 182 resting-state EEG datasets. Resting-state EEG recordings were obtained under both eyes-open and eyes-closed conditions using a single-electrode setup (Fpz) with the Brain Pro system (China). The reference electrode was positioned at the earlobe, and online data were filtered using a bandpass filter with cutoff frequencies of 0.5 and 30 Hz. For further details, please refer to a previous study that utilized the same dataset41.
Power spectrum analysis
The power spectrum of the EEG response was estimated using the multitaper method42,43 (time-bandwidth product, 3; tapers, 5; Chronux toolbox (http://chronux.org/), implemented through custom software written in MATLAB. This method has been widely applied in various biomedical studies (e.g., biological science44,45,46, and public health47,48,49,50,51. To guarantee that all alpha peaks could be captured, the relative power is defined as Eq. 1.
Where power is the absolute power calculated using multi-taper method.
Descriptive model for dissecting aperiodic and periodic components
The aperiodic component of the power spectrum was extracted as a 1/f like function (Eq. 2), where a, b,c and d represent the model parameters to be estimated. The periodic component was defined as the residual obtained by subtracting the aperiodic component from the raw power spectrum. This method has been used in the characterization of gamma-band and alpha-band activities13,46,52,53.
where a, b and c are parameters that determine the slope of the aperiodic activity, while d is a parameter that represents the minimum value of the aperiodic activity. The parameters are estimated by least square method.
The Three Gaussian Model13 is the summation of a baseline (aperiodic component) and three Gaussian functions (periodic components) representing three component sources in the alpha band for all 40 subjects. It is described as follows (Eqs. 3–4):
where Si(f) is the i-th source function of the signal depending on the frequency f, Wi is the weight of each source, µi and σi are the peak frequency and bandwidth of the i-th source, respectively. The parameters are estimated by least square method.
Statistical analysis
A t-test was used to compare the sex differences in absolute and relative power in alpha band in the eyes-open and eyes-closed states respectively. Pearson correlation analysis was conducted to assess the relationship between absolute alpha power in the open and eyes-closed states, as well as between relative alpha power, aperiodic and periodic alpha power. Additionally, Pearson correlation analysis was used to measure the relationship between age and both absolute and relative power in alpha band in eyes-open and eyes-closed states respectively. Chi square test to test the difference of the number of alpha peaks (no alpha peak and one or more alpha peaks) between eyes-open and eyes-closed state.
Results
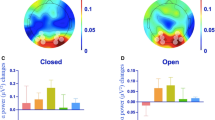
In this study, we utilized a previously published portable single-channel EEG dataset41, which captured the resting-state electroencephalogram (EEG) activity in the frontal lobe under both open and eyes-closed conditions. The dataset encompassed multiple follow-up EEG recordings from each participant, with each individual contributing at least twice, up to a maximum of 11 times (average 4.6 times ± 2.6), over a maximum interval of 9 months between the first and last recording. We first conducted spectral analysis on the EEG measurements for each participant, averaging the results to represent their neural activity during resting-state across different frequencies under both open and eyes-closed conditions. We calculated the relative power under open (Fig. 1A) and eyes-closed (Fig. 1B) conditions and observed a significant positive correlation between them (Fig. 1C, r = 0.50, p < 0.001). Similarly, we computed the absolute power under open (Fig. 1D) and eyes-closed (Fig. 1E) conditions and found a significant positive correlation (Fig. 1F, r = 0.50, p < 0.001). Neither the absolute nor the relative power was significantly correlated with age (Fig. 1G), and no strong gender differences were observed (Fig. 1H). Since relative power removes the effect of dimension, making it more comparable across individuals, we proceeded with the relative power for all subsequent analyses.
Relationship of alpha band activities between eyes-open and eyes-closed states. (A) Relative power spectra in eyes-open state for all subjects (n = 40). (B) Relative power spectra in eyes-closed state for all subjects (n = 40). (C) Scatter plot depict the relationship between relative alpha power in open and eyes-closed states. (D) Absolute power spectra in eyes-open state for all subjects (n = 40). (E) Absolute power spectra in eyes-closed state for all subjects (n = 40). (F) Scatter plot depict the relationship between absolute alpha power in open and eyes-closed states. (G) Scatter plot depict the relationship between age and both absolute and relative alpha power in open and eyes-closed states respectively. (H) Comparison between male and female regarding both absolute and relative alpha power in open and eyes-closed states respectively.
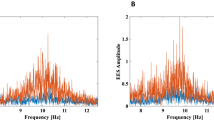
We further decomposed the periodic and aperiodic activities in the alpha band for each participant in both open and eyes-closed states. As illustrated in Fig. 2A-D, an example participant exhibited no prominent alpha peak in the eyes-open state (Fig. 2A), so the majority of its spectrum was dominated by the aperiodic activity (Fig. 2B). Conversely, in the eyes-closed state, a strong alpha peak was present (Fig. 2C), which constituted the periodic activity (Fig. 2D). Correlation analyses were then conducted on these different alpha-band power. We found that the alpha-band power in aperiodic activity were not correlated between open and eyes-closed states (Fig. 2E, r = -0.02, p = 0.89). In contrast, the alpha-band power in periodic activity showed a significant correlation between the two states (Fig. 2F, r = 0.476, p = 0.0019). This indicates that the observed correlation of power between open and eyes-closed states in the original spectrum mainly stems from the periodic activity in the alpha band rather than the aperiodic activity.
Relationship of periodic and aperiodic activities in alpha band between eyes-open and eyes-closed states. (A) Raw power spectrum (black circles) and its aperiodic activity (orange line) in eyes-open state in an example subject. (B) Periodic activity in eyes-open state was obtained by subtracting the aperiodic component in A from the raw power spectrum. (C) Raw power spectrum (black circles) and its aperiodic activity (orange line) in eyes-closed state in an example subject. (D) Periodic activity in eyes-closed state was obtained by subtracting the aperiodic component in C from the raw power spectrum. (E) Scatter plot depict the relationship between alpha-band power in aperiodic activity in eyes-open and eyes-closed states. (F) Scatter plot depict the relationship between alpha-band power in periodic activity in eyes-open and eyes-closed states.
Furthermore, we fitted the periodic activity using the sum of multiple Gaussian functions to determine the number of alpha oscillations, peak frequency, and bandwidth for each participant (Fig. 3A-C). Our analysis revealed obvious difference in the number of alpha sub-oscillations between eyes-open and eyes-closed states (p < 0.001). Approximately 85% of the participants (n = 34) did not show strong alpha oscillations in the eyes-open state, whereas less than 10% of the participants showed a lack of strong alpha oscillations in the eyes-closed state (Fig. 3D). This result indicates that, although there is a significant correlation in alpha power between eyes-open and eyes-closed states, there are still significant differences in other characteristics. This suggests that while there is a shared neural mechanism underlying alpha band activity across both states, distinct generating mechanisms are also at play. Further descriptive analysis of the 6 participants with strong alpha oscillations in the eyes-open state revealed that both the frequency and bandwidth of the alpha oscillations were inconsistent with those observed in the eyes-closed state (Fig. 3EF). Due to the limitation of the data size, we will not draw a conclusion here, but for reference only.
Comparison between oscillatory properties in alpha band. (A) Raw power spectrum (black circles) and its aperiodic activity (yellow line) and periodic activity (orange line) in eyes-open state in an example subject. (B) Raw power spectrum (black circles) and its aperiodic activity (yellow line) and periodic activity (orange line) in eyes-closed state in an example subject shown in (A). (C) Periodic activity (orange line) in eyes-open and eyes-closed states in an example subject shown in A and (B) (D) Distribution of alpha peak numbers in eyes-open (white) and eyes-closed (black) states. (E) Comparison of peak frequencies of periodic activities in alpha band in eyes-open and eyes-closed states. (F) Comparison of bandwidths of periodic activities in alpha band in eyes-open and eyes-closed states. .
Discussion
In this study, we conducted a detailed and systematic comparison of alpha band activity (including oscillatory and non-oscillatory activities, as well as fine oscillatory characteristics) between eyes-open and eyes-closed states. Our goal was to determine whether similar neural mechanisms are shared in the generation of alpha oscillations in these two states. We found a significant positive correlation in the original spectral power of the alpha band between eyes-open and eyes-closed states, primarily driven by the oscillatory components of the alpha band. This indicates that alpha oscillations share some common oscillatory mechanisms between eyes-open and eyes-closed states. However, when we further refined finer details of oscillatory characteristics (such as the number of alpha peaks), we observed significant inconsistencies across participants. This suggests that alpha oscillations also have independent oscillatory mechanisms in the eyes-open and eyes-closed states.
Previous studies have extensively compared the differences between eyes-open and eyes-closed states in electroencephalogram (EEG) recordings, with a greater focus on power comparisons36,37,38. It has been consistently observed that the alpha oscillations are significantly stronger during eyes-closed conditions compared to eyes-open conditions, a phenomenon known as the Berger effect37,54,55,56. Despite being less prevalent, alpha oscillations during the eyes-open resting state still exist with considerable strength in a subset of participants, yet they are often overlooked in research. Another point of difference from previous research is that our study primarily focuses on the prefrontal cortex. As it is well-known that alpha oscillations are most prominent in the occipital and parietal regions in EEG recordings, their characteristics in the prefrontal cortex are often neglected. Additionally, prior research has shown that alpha oscillations consist of multiple distinct sub-oscillations13,14,19, a phenomenon we also observed in the prefrontal cortex34. Different frequency sub-oscillations may have distinct origin mechanisms52,57,58, and more refined mechanisms require further exploration and validation through future experiments.
An important application of our research result is that it provides a more refined guidance for neuromodulation methods, especially in the personalized stimulation such as TACS or TMS. Different people have different individual alpha peak frequencies. Hence, many studies chose the frequency of the participants’ intrinsic alpha oscillations as the target frequency for electrical59,60,61,62or magnetic63,64,65 stimulation, but this choice can sometimes be arbitrary. Whether to base it on the alpha frequency during eyes-open or eyes-closed states may lead to different outcomes. We also noticed that some participants do not exhibit alpha oscillations in either the eyes-open or eyes-closed states. Although this proportion is relatively small, using alpha frequency as the stimulation frequency for electrical stimulation in the prefrontal cortex may not be an appropriate choice for these individuals. Furthermore, a considerable proportion of participants display multiple alpha sub-oscillations in the prefrontal cortex. Therefore, determining which frequency within the alpha frequency range to use for electrical stimulation, and understanding how different choices may affect the participants’ cognitive functions, are critical questions that warrant further investigation in future studies.
Data availability
The dataset and analysis codes are available upon reasonable request to the corresponding author.
References
Ezaki, T., Himeno, Y., Watanabe, T. & Masuda, N. Modelling state-transition dynamics in resting-state brain signals by the hidden Markov and Gaussian mixture models. Eur. J. Neurosci. 54, 5404–5416. https://doi.org/10.1111/EJN.15386 (2021).
Khanna, A., Pascual-Leone, A., Michel, C. M. & Farzan, F. Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav Rev. 49, 105–113. https://doi.org/10.1016/j.neubiorev.2014.12.010 (2015).
Smit, D. J. A., Linkenkaer-Hansen, K. & de Geus, E. J. C. Long-range temporal correlations in resting-state alpha oscillations predict human timing-error dynamics. J. Neurosci. 33, 11212–11220. https://doi.org/10.1523/JNEUROSCI.2816-12.2013 (2013).
Reichert, J. L., Kober, S. E., Neuper, C. & Wood, G. Resting-state sensorimotor rhythm (SMR) power predicts the ability to up-regulate SMR in an EEG-instrumental conditioning paradigm. Clin. Neurophysiol. https://doi.org/10.1016/j.clinph.2014.09.032 (2015).
Wang, Y., Duan, W., Dong, D., Ding, L. & Lei, X. A test-retest resting, and cognitive state EEG dataset during multiple subject-driven states. Sci. Data 2022;9. https://doi.org/10.1038/s41597-022-01607-9
Murias, M., Webb, S. J., Greenson, J. & Dawson, G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol. Psychiatry. 62, 270–273. https://doi.org/10.1016/j.biopsych.2006.11.012 (2007).
Woltering, S., Jung, J., Liu, Z. & Tannock, R. Resting state EEG oscillatory power differences in ADHD college students and their peers. Behav. Brain Funct. 8, 1–9. https://doi.org/10.1186/1744-9081-8-60 (2012).
Bollimunta, A., Mo, J., Schroeder, C. E. & Ding, M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J. Neurosci. 31, 4935–4943. https://doi.org/10.1523/JNEUROSCI.5580-10.2011 (2011).
Bollimunta, A., Chen, Y., Schroeder, C. E. & Ding, M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J. Neurosci. 28, 9976–9988. https://doi.org/10.1523/JNEUROSCI.2699-08.2008 (2008).
Bazanova, O. M. & Vernon, D. Interpreting EEG alpha activity. Neurosci. Biobehav Rev. 44, 94–110. https://doi.org/10.1016/j.neubiorev.2013.05.007 (2014).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195. https://doi.org/10.1016/S0165-0173(98)00056-3 (1999).
Berger, H. Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr Nervenkr. https://doi.org/10.1007/BF01797193 (1929).
Han, C. et al. Compensatory mechanism of attention-deficit / hyperactivity disorder recovery in resting state alpha rhythms. Front. Comput. Neurosci. 2022:1–10. https://doi.org/10.3389/fncom.2022.883065
Barzegaran, E., Vildavski, V. Y. & Knyazeva, M. G. Fine structure of posterior alpha rhythm in human EEG: frequency components, their cortical sources, and temporal behavior. Sci. Rep. 7, 1–12. https://doi.org/10.1038/s41598-017-08421-z (2017).
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X. & Kastner, S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 2012;337:753–6. (1979). https://doi.org/10.1126/science.1223082
Lopes da Silva, F. H., van Storm, W. & van Rotterdam, A. The sources and spread of the alpha rhythm of the cortex of dog. Electroencephalogr. Clin. Neurophysiol. (1977).
Jensen, O. & Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4, 2008. https://doi.org/10.3389/FNHUM.2010.00186/BIBTEX (2010).
Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617. https://doi.org/10.1016/j.tics.2012.10.007 (2012).
Jia, J., Fang, F. & Luo, H. Selective spatial attention involves two alpha-band components associated with distinct spatiotemporal and functional characteristics. Neuroimage. 199, 228–236. https://doi.org/10.1016/J.NEUROIMAGE.2019.05.079 (2019).
Hanslmayr, S., Gross, J., Klimesch, W. & Shapiro, K. L. The role of alpha oscillations in temporal attention. Brain Res. Rev. https://doi.org/10.1016/j.brainresrev.2011.04.002 (2011).
Bagherzadeh, Y., Baldauf, D., Pantazis, D. & Desimone, R. Alpha synchrony and the Neurofeedback Control of Spatial Attention. Neuron. 105, 577–587. .e5 (2020).
Gulbinaite, R., Van Viegen, T., Wieling, M., Cohen, M. X. & Vanrullen, R. Individual alpha peak frequency predicts 10 hz flicker effects on selective attention. J. Neurosci. 37, 10173–10184. https://doi.org/10.1523/JNEUROSCI.1163-17.2017 (2017).
Benchenane, K., Tiesinga, P. H. & Battaglia, F. P. Oscillations in the Prefrontal Cortex: A Gateway to Memory and Attention (Curr Opin Neurobiol, 2011).
Michels, L., Moazami-Goudarzi, M., Jeanmonod, D. & Sarnthein, J. EEG alpha distinguishes between cuneal and precuneal activation in working memory. Neuroimage. https://doi.org/10.1016/j.neuroimage.2007.12.048 (2008).
Escolano, C., Aguilar, M. & Minguez, J. EEG-based upper alpha neurofeedback training improves working memory performance. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, (2011). https://doi.org/10.1109/IEMBS.2011.6090651
Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 26, 319–340. https://doi.org/10.1016/S0167-8760(97)00773-3 (1997).
Gordon, E., Palmer, D. M. & Cooper, N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin. EEG Neurosci. 41, 178–183. https://doi.org/10.1177/155005941004100404 (2010).
Wang, B. et al. Characterizing major depressive disorder (MDD) using alpha-band activity in resting-state electroencephalogram (EEG) combined with MATRICS Consensus Cognitive Battery (MCCB). J. Affect. Disord. 355, 254–264. https://doi.org/10.1016/j.jad.2024.03.145 (2024).
Koshiyama, D. et al. Abnormal phase discontinuity of alpha- and theta-frequency oscillations in schizophrenia. Schizophr Res. 231, 73–81. https://doi.org/10.1016/J.SCHRES.2021.03.007 (2021).
Cao, Y. et al. Correlation between resting Theta Power and Cognitive performance in patients with Schizophrenia. Front. Hum. Neurosci. 16. https://doi.org/10.3389/fnhum.2022.853994 (2022).
Babiloni, C. et al. Reactivity of cortical alpha rhythms to eye opening in mild cognitive impairment and Alzheimer’s disease: an EEG study. J. Alzheimer’s Disease. 22, 1047–1064. https://doi.org/10.3233/JAD-2010-100798 (2010).
Wiesman, A. I. et al. Visuospatial alpha and gamma oscillations scale with the severity of cognitive dysfunction in patients on the Alzheimer’s disease spectrum. Alzheimers Res. Ther. 13. https://doi.org/10.1186/s13195-021-00881-w (2021).
Wiesman, A. I. et al. Visuospatial alpha and gamma oscillations scale with the severity of cognitive dysfunction in patients on the Alzheimer’s disease spectrum. Alzheimers Res. Ther. 13, 1–12. https://doi.org/10.1186/s13195-021-00881-w (2021).
Han, C. et al. Enhancement of the neural response during 40 hz auditory entrainment in closed-eye state in human prefrontal region. Cogn. Neurodyn. https://doi.org/10.1007/s11571-022-09834-x (2022).
Han, C. et al. Oscillatory biomarkers of autism : evidence from the innate visual fear evoking paradigm. Cogn. Neurodyn. 2022;6. https://doi.org/10.1007/s11571-022-09839-6
Barry, R. J., Clarke, A. R., Johnstone, S. J. & Brown, C. R. EEG differences in children between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 120, 1806–1811. https://doi.org/10.1016/j.clinph.2009.08.006 (2009).
Barry, R. J., Clarke, A. R., Johnstone, S. J., Magee, C. A. & Rushby, J. A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 118, 2765–2773. https://doi.org/10.1016/j.clinph.2007.07.028 (2007).
Barry, R. J. & De Blasio, F. M. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol. Psychol. 129, 293–304. https://doi.org/10.1016/J.BIOPSYCHO.2017.09.010 (2017).
Weisz, N., Müller, N., Jatzev, S. & Bertrand, O. Oscillatory alpha modulations in right auditory regions reflect the validity of acoustic cues in an auditory spatial attention task. Cereb. Cortex. https://doi.org/10.1093/cercor/bht113 (2014).
Grover, S., Fayzullina, R., Bullard, B. M., Levina, V. & Reinhart, R. M. G. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl Med. https://doi.org/10.1126/SCITRANSLMED.ABO (2044). 2023;15:eabo2044.
Lin, Y. et al. The neural oscillatory mechanism underlying human brain fingerprint recognition using a Portable EEG Acquisition device. Neuroimage 2024:120637. https://doi.org/10.1016/J.NEUROIMAGE.2024.120637
Bell, B., Percival, D. B. & Walden, A. T. Calculating Thomson’s Spectral Multitapers by Inverse Iteration (Journal of Computational and Graphical Statistics, 1993).
Percival, D. B. & Walden, A. T. Spectral Analysis for Physical Applications. (1993). https://doi.org/10.1017/cbo9780511622762
Han, C. et al. Neural mechanism of orientation selectivity for distinct gamma oscillations in cat V1. J. Vis. 20, 1116 (2020).
Han, C., Shapley, R. & Xing, D. Gamma rhythms in the visual cortex: functions and mechanisms. Cogn. Neurodyn. https://doi.org/10.1007/s11571-021-09767-x (2021).
Wang, B. et al. Superimposed gratings induce diverse response patterns of gamma oscillations in primary visual cortex. Sci. Rep. 2021:1–20 .
Cao, Y. et al. Oscillatory properties of class C notifiable infectious diseases in China from 2009 to 2021. Front. Public. Health. 10. https://doi.org/10.3389/fpubh.2022.903025 (2022).
Han, C., Li, M., Haihambo, N., Cao, Y. & Zhao, X. Enlightenment on oscillatory properties of 23 class B notifiable infectious diseases in the mainland of China from 2004 to 2020. PLoS One. 16, e0252803 (2021).
Han, C., Li, M., Haihambo, N. & Babuna, P. Mechanisms of recurrent outbreak of COVID-19 : a model- based study. Nonlinear Dyn. 106, 1169–1185. https://doi.org/10.1007/s11071-021-06371-w (2021).
Zhao, X. et al. Changes in temporal properties for epidemics of notifiable infectious diseases in China during the COVID-19 epidemic: population-based surveillance study. JMIR Public. Health Surveill. 8, 1–12. https://doi.org/10.2196/35343 (2022).
Zhao, X. et al. Periodic characteristics of Hepatitis Virus infections from 2013 to 2020 and their Association with Meteorological factors in Guangdong, China: Surveillance Study. JMIR Public. Health Surveill. 9, e45199. https://doi.org/10.2196/45199 (2023).
Han, C. et al. Multiple gamma rhythms carry distinct spatial frequency information in primary visual cortex. PLoS Biol. 2021:1–23. https://doi.org/10.1371/journal.pbio.3001466
Donoghue, T. et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665. https://doi.org/10.1038/s41593-020-00744-x (2020).
Liley, D. T. J. & Muthukumaraswamy, S. D. Evidence that alpha blocking is due to increases in system-level oscillatory damping not neuronal population desynchronisation. Neuroimage 2020;208. https://doi.org/10.1016/j.neuroimage.2019.116408
Kirschfeld, K. The physical basis of alpha waves in the electroencephalogram and the origin of the berger effect. Biol. Cybern. 92, 177–185. https://doi.org/10.1007/s00422-005-0547-1 (2005).
Bazanova, O. M., Nikolenko, E. D. & Barry, R. J. Reactivity of alpha rhythms to eyes opening (the Berger effect) during menstrual cycle phases. Int. J. Psychophysiol. 122, 56–64. https://doi.org/10.1016/j.ijpsycho.2017.05.001 (2017).
Han, C. et al. The generation and modulation of distinct Gamma oscillations with local, horizontal, and feedback connections in the primary visual cortex: a Model Study on large-scale networks. Neural Plast. 2021, 8874516 (2021).
Fernández-Ruiz, A. et al. (1979) Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 372(6537) 2021.
Riddle, J., Alexander, M. L., Schiller, C. E., Rubinow, D. R. & Frohlich, F. Reduction in Left Frontal Alpha oscillations by Transcranial Alternating Current Stimulation in Major Depressive disorder is context dependent in a Randomized Clinical Trial. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 7, 302–311. https://doi.org/10.1016/J.BPSC.2021.07.001 (2022).
Orendáčová, M. & Kvašňák, E. Effects of Transcranial Alternating Current Stimulation and Neurofeedback on Alpha (EEG) dynamics: a review. Front. Hum. Neurosci. 15, 628229. https://doi.org/10.3389/FNHUM.2021.628229/BIBTEX (2021).
Daughters, S. B., Yi, J. Y., Phillips, R. D., Carelli, R. M. & Fröhlich, F. Alpha-tACS effect on inhibitory control and feasibility of administration in community outpatient substance use treatment. Drug Alcohol Depend. 213, 108132. https://doi.org/10.1016/J.DRUGALCDEP.2020.108132 (2020).
Alexander, M. L. et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Translational Psychiatry 2019 9:1 2019;9:1–12. https://doi.org/10.1038/s41398-019-0439-0
Chota, S., Marque, P. & VanRullen, R. Occipital alpha-TMS causally modulates temporal order judgements: evidence for discrete temporal windows in vision. Neuroimage. 237 https://doi.org/10.1016/J.NEUROIMAGE.2021.118173 (2021).
Herring, J. D., Thut, G., Jensen, O. & Bergmann, T. O. Attention modulates TMS-Locked alpha oscillations in the visual cortex. J. Neurosci. 35, 14435–14447. https://doi.org/10.1523/JNEUROSCI.1833-15.2015 (2015).
Ding, Z., Wang, Y., Li, J. & Li, X. Closed-loop TMS-EEG reactivity with occipital alpha-phase synchronized. J. Neural Eng. 19 https://doi.org/10.1088/1741-2552/AC9432 (2022).
Acknowledgements
This work was funded by the Discipline Innovation and Talent Introduction Bases in Higher Education Institutions (B20087), and the related funding supported by Shenzhen Shuimu AI Technology Co., Ltd (E2Z1171001).
Author information
Authors and Affiliations
Contributions
CH and YL conceived and designed the study. YZ, JM, SH, YL, FW, WX, WC, FD, JS contributed to data collection, CH, ZZ and YZ contributed to the literature search, contributed to the data analysis, and the interpretation of results. All authors contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing interests
S.H., J.M., W.X., F.W., Y.L., and W.C. are employees of Shenzhen Shuimu AI Technology Co., Ltd. The authors declare that no other competing interests exist. The other authors declare no competing financial conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Zhang, Z., Du, F. et al. Shared oscillatory mechanisms of alpha-band activity in prefrontal regions in eyes open and closed state using a portable EEG acquisition device. Sci Rep 14, 26719 (2024). https://doi.org/10.1038/s41598-024-78173-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78173-0
Keywords
This article is cited by
-
Distinct oscillatory mechanisms in low and high alpha-band activities for screening and potential treatment of Schizophrenia
Translational Psychiatry (2025)