Abstract
Positive results from cancer screenings, like a cancer diagnosis, can increase the risk of cardiovascular disease (CVD) mortality due to heightened psychological stress. However, positive screening results may also serve as a teachable moment to encourage the adoption of a healthier lifestyle. Consequently, the overall association between positive screenings and CVD mortality risk remains unclear. Based on PLCO data, the risk of CVD mortality associated with the number and types of positive screens was evaluated using Cox regression models among 149,258 eligible participants enrolled between 1993 and 2001. Additional analyses explored these associations stratified by prior CVD history. Exploratory analyses were also conducted to investigate whether positive screens were linked to the potential adoption of a healthier lifestyle. After a median follow-up of 19 years, significantly decreased risk of CVD mortality was observed for individuals with occasional positive screening (≤ 2 positive screens) [adjusted hazard ratio (HR, 95%CIs): 0.931 (0.897–0.968), P < 0.001] compared to the control arm. This effect was particularly notable for flexible sigmoidoscopy [0.842 (0.802–0.884), P < 0.001] and transvaginal ultrasound [0.855 (0.776–0.942), P = 0.002]. However, when the number of positive screens increased to more than two, the reduced risk of CVD mortality became non-significant [0.977 (0.941–1.014), P = 0.220]. Subgroup analyses revealed a greater reduction in CVD mortality risk among participants without a history of CVD [0.917 (0.864–0.973)] compared to those with a history of CVD [0.944 (0.898–0.993)]. Sensitivity analyses excluding screening-detected cancers showed similar association in the overall population [0.933 (0.897–0.970)], as well as in both subgroups with [0.945 (0.898–0.995)] and without previous CVD [0.919 (0.865–0.976)]. Exploratory analyses indicated a significantly higher proportion of any body mass index (BMI) reduction among those with a baseline BMI ≥ 25 kg/m2 who had positive screens compared to the control arm, particularly for individuals with occasional positive screens (48.07% vs. 47.04%, P value = 0.037). Occasional positive cancer screening are associated with reduced risk of CVD mortality, regardless of prior CVD history, cancer diagnosis, and other competitive risks.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) and cancer are the two leading causes of death or premature mortality worldwide1,2,3. It is estimated that CVD will account for 23.6 million deaths by 20304while the number of new cancer cases globally is projected to rise to 20.35 million by 20245. Shared risk factors such as aging, smoking, alcohol drinking, obesity, and diabetes, along with similar pathogenesis and progression related to chronic inflammation, contribute to the increasing global health issue of multimorbidity involving both cancer and CVD6,7,8.
It is well-established that a cancer diagnosis elevates the risk of CVD mortality6,7,8. Beyond the cardiotoxic effects of cancer treatments, the psychological stress and trauma associated with a cancer diagnosis also contribute to an increased risk of CVD mortality, independent of the disease or its treatment9,10,11. Similarly, a positive result from a cancer screening test—akin to a cancer diagnosis—can also heighten the risk of CVD mortality due to the associated psychological stress12,13,14,15. This concern is particularly relevant in the context of multiple screening tests. Immediate or long-term psychological stress after a positive screening can elevate the risk of CVD through various mechanisms, including activation of the hypothalamic-pituitary-adrenal axis, upregulation of the sympathetic nervous system and downregulation of the parasympathetic nervous system, endothelial injury, and immune system dysregulation16,17. Additionally, residents with positive results from low-dose CT screening for lung cancer or mammography screening for breast cancer are more likely to have coronary artery calcification, which is a marker of atherosclerotic CVD18,19,20. Meanwhile, several studies suggest that positive screens probably represent a teachable moment to encourage the adoption of a healthy lifestyle21,22,23,24. For example, smokers with abnormal lung CT findings from screening were more likely to quit smoking, and individuals with severe endoscopic findings are more likely to adhere to healthy lifestyles, including smoking cessation, alcohol abstinence, body weight control, regular physical activity, and a healthy diet21,22,23,25. In this context, positive screening results might also be associated with a decreased risk of CVD mortality, especially when such results do not lead to a definitive cancer diagnosis. However, to date, no studies have investigated the overall relationship between positive screenings and CVD mortality risk.
Therefore, using data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, we aimed to: (1) explore the overall association between positive screenings and the risk of CVD mortality; (2) investigate whether the risk of CVD mortality varies with the number and types of positive screens, particularly among participants without a history of CVD; and (3) perform sensitivity analyses to account for potential confounding effects of true positive screens (those resulting in a cancer diagnosis) and to address competitive risks of death other than CVD mortality. Finally, exploratory analyses were conducted to investigate whether positive screens has a potential effect on healthy lifestyle changes.
Method
Subjects design and selection of participants
The design of the PLCO Cancer Screening Trial has been described in detail elsewhere26. Briefly, the PLCO trial was a multicenter, two-arm, randomized trial that enrolled 154,887 participants aged 55 to 74 years between November 1993 and July 2001. Participants were randomly assigned to either the screening arm or the control arm in a 1:1 ratio. Those in the control arm received usual medical care, while participants in the screening arm underwent multiple rounds of screening for prostate, lung, colorectal, and ovarian cancers, as outlined in the study protocol. Specifically, the screening arm included 4 rounds of annual chest X-rays (XRY) for lung cancer, 2 rounds of flexible sigmoidoscopy (FSG) for colorectal cancer, 6 rounds of annual prostate-specific antigen (PSA) tests and 4 rounds of annual digital rectal examinations (DRE) for prostate cancer (for males), 6 rounds of annual cancer antigen 125 (CA-125) tests and 4 rounds of annual transvaginal ultrasounds (TVU) for ovarian cancer (for females).
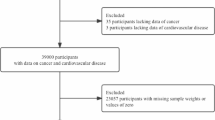
For this study, a total of 149,258 eligible participants (75,250 in the screening arm and 74,008 in the control arm) were included in the preliminary analyses, following the exclusion of 5,629 participants who were ineligible due to missing baseline questionnaire data after informed consent. To investigate the impact of positive screening on CVD mortality, residents in the screening group were reclassified into: the ‘No screen’ group, the ‘Negative screening’ group, and the ‘Positive screening’ group. Since the PLCO screening trial involves up to four rounds of screening for four different cancers using six different methods, non-compliance is observed with each screening method for every type of cancer. Given the complexity of non-compliance scenarios when various screening protocols are combined, a detailed list of every possible non-compliance scenario is not provided. However, the most critical non-compliance scenario, where residents assigned to the screening group did not receive any screening examinations, is defined as the ‘No screen’ group. The ‘Negative screening’ group is defined as those who had all tests result in negative. The ‘Positive screening’ group is defined as those who had at least one positive result in any of the tests. Within the ‘Positive screening’ group, individuals were further classified based on the number of positive results into: the ‘Occasional positive screening (≤ 2 positive screens)’ and the ‘Frequent positive screening (> 2 positive screens)’. For the sensitive analyses, 6,003 participants with any true positive screens for four index screening cancers were further excluded. As a result, 143,255 participants were included in the sensitivity analysis (see Supplementary Fig. 1).
Positive screen and diagnostic evaluation
According to the PLCO protocol, the criteria for a positive screen were as follows26,27,28,29,30,31: (1) A positive XRY was defined by the presence of any nodule, mass, hilar or mediastinal lymph node enlargement, major atelectasis, lobar collapse, infiltrate, consolidation, alveolar opacity, or pleural mass in the lung. (2) A positive CA-125 test was defined as CA-125 levels exceeding 35 U/ml. (3) A positive TVU was indicated by any ovary or cyst with a volume greater than 10 cc, any solid area or papillary projection extending into the lumen of a cystic ovarian tumor of any size, or any mixed (solid or cystic) component within the tumor. (4) A positive PSA test was defined as PSA levels exceeding 4 ng/ml. (5) A positive DRE was characterized by the presence of any nodule, induration, asymmetry, or loss of anatomical landmarks. (6) A positive FSG was defined by any mucosal abnormality, rectal nodule, rectal and/or colonic mass, or polyp. Participants with any positive screening results were encouraged to undergo further diagnostic evaluations.
Outcome assessment
The primary endpoints of this study were cardiovascular disease (CVD) mortality, including deaths from ischemic heart disease, cerebrovascular disease, and other circulatory conditions. To assess these endpoints, data on all deaths from any cause were collected and verified through several main sources27,28,29,30,31: (1) the administration of the annual study update (ASU) questionnaire, (2) reports from relatives, friends, or physicians, and (3) periodic linkage to the National Death Index (NDI) Plus searches. When deaths were reported, PLCO staff made efforts to obtain death certificates from state vital statistics bureaus and gathered comprehensive mortality data for each case. The leading cause of death was coded according to the 9th edition of the International Classification of Diseases (ICD-9) and recorded following the guidelines established by the National Center for Health Statistics. For cases where the underlying causes on the death certificate were not clear or accurately recorded, the causes of death were reviewed by an end-point adjudication team as part of the Death Review Process (DRP). Follow-up data on deaths were updated through December 2018. Therefore, the primary outcomes in this study were censored at the date of death, loss to follow-up, or the end of the follow-up period, whichever occurred first.
Statistical analysis
Kaplan-Meier (K-M) curves were initially employed to estimate the cumulative risk of CVD mortality over long-term follow-up in both the screening and control arms. Log-rank tests were used to compare differences in primary endpoints between the two groups.
Cox regression models were then used to identify potential independent factors associated with the overall risk of CVD mortality, beyond the effects of positive screening results. Given the different screening tests and examinations for males and females, sex-specific Cox regression models were used to independently identify influential factors related to CVD mortality for each gender. Potential confounding factors, including age, smoking, BMI, aspirin use, ibuprofen use, diabetes, benign diseases history, screening history, family history, history of CVD, oral contraception use (for females only), and hormone replacement therapy (HRT) (for females only), were further adjusted in Cox regression models to investigate the independent association between positive screenings and CVD mortality, with results expressed as adjusted hazard ratios (HR) and 95% confidence intervals (CI). Additional analyses examined whether the risk of CVD mortality varied based on the number of positive screens and the types of cancer screenings.
Given the differing risks of CVD mortality between participants with and without a history of CVD (including hypertension, heart attack, and stroke), subgroup analyses were performed to explore the associations between positive screenings and CVD mortality within these subgroups. Furthermore, to differentiate between the effects of true positive screens (resulting in a cancer diagnosis) and false positive screens (without a cancer diagnosis), sensitivity analyses were conducted by excluding true positive screens to assess the association between false positive screens and CVD mortality. To address the potential impact of competing risks, additional sensitivity analyses were conducted to examine the association between positive screens and CVD mortality while excluding deaths from causes other than CVD.
To explore whether positive screens might lead to healthier behaviors, chi-square tests compared smoking cessation rates among baseline heavy smokers (≥ 30 pack-years) between the screening and control arms, using data from the Supplemental Questionnaire collected between 2006 and 2008. Chi-square tests were also used to compare the proportion of participants with a baseline BMI of 25 or higher who experienced any reduction in BMI between the screening and control arms.
All statistical analyses were performed using SPSS software (version 25.0) and R software (version 4.2.3), with P-values ≤ 0.05 considered statistically significant.
Result
Association between baseline characteristics and risk of CVD mortality
After a median follow-up of 19 years, a total of 9,005 CVD deaths were recorded in the screening arm, compared to 8,788 CVD deaths in the control arm. Since there was no significant difference in the overall risk of CVD mortality between the screening and control arms (P > 0.05), participants from both arms were combined to explore the association between baseline characteristics and CVD mortality risk.
As shown in Supplementary Table 1, participants who died from CVD were generally older at recruitment, more likely to be smokers, had a higher BMI, used ibuprofen less frequently, and had a greater history of diabetes, benign diseases, and family history of cancer compared to those who did not die from CVD (all P-values < 0.05). Subgroup analyses by sex revealed similar associations between baseline characteristics and CVD mortality risk for both males and females (Supplementary Table 1).
Association between positive screenings and risk of CVD mortality
As shown in Fig. 1A, the Kaplan-Meier (K-M) curves indicated no significant difference in CVD mortality risk between the screening and control arms (P = 0.129). However, when participants in the screening arm were reclassified into three groups based on screening results (no screen, stable negative screen, and positive screens, Fig. 1B) or four groups based on compliance and the number of positive screens (no screen, stable negative screen, occasional positive screening, and frequent positive screening, Fig. 1C), significant differences in the risk of CVD mortality were observed between these subgroups (all P values < 0.001).
Kaplan-Meier curves of CVD mortality between different arms and subgroups. Note: (A), Overall risk of CVD mortality by different arms. (B), Subgroup risk of CVD mortality by different arms, compliance and positive screen. (C), Subgroup risk of CVD mortality by different arms, compliance and number of positive screens. Group: 0, control arm; 1, screening arm. Subgroup1: 0, control arm; 1, no screen; 2, negative screening; 3, positive screening. Subgroup2: 0, control arm; 1, no screen; 2, negative screening; 3, occasional positive screening (≤ 2 positive screens); 4, frequent positive screening (> 2 positive screens).
As shown in Table 1, adjusting for potential confounding factors and reclassifying participants in the screening arm by the number of positive screenings, a significantly decreased risk of CVD mortality was observed for occasional positive screening [HR (95% CI): 0.931 (0.897–0.968), P < 0.001], particularly for FSG [HR (95% CI): 0.842 (0.802–0.884), P < 0.001] and TVU [HR (95% CI): 0.855 (0.776–0.942), P = 0.002]. However, when the number of positive screens increased to more than two, the reduced risk of CVD mortality became non-significant [0.977 (0.941–1.014), P = 0.220]. Furthermore, participants who were assigned to the screening group but did not receive any screenings had a significantly increased risk of CVD mortality, with an HR (95% CI) of 1.436 (1.338–1.540).
Subgroup analyses by previous CVD
As shown in Supplementary Table 1, Table 2, and Table 3, the subgroup analyses included 91,379 participants without previous CVD and 57,879 participants with previous CVD. Figure 2 illustrates that the K-M curves for cumulative risk of CVD mortality in different arms and subgroups were consistent with the primary analyses. Participants with occasional positive screening showed a lower risk of CVD mortality compared to those with frequent positive screening, regardless of previous CVD (Fig. 2C and F), both P < 0.001).
Kaplan-Meier curves of CVD mortality between different arms, subgroups and previous CVD. Note: (A)/(B)/(C): without previous CVD; (D)/(E)/(F): with previous CVD. (A)/(D), Overall risk of CVD mortality by different arms. (B)/(E), Subgroup risk of CVD mortality by different arms, compliance and positive screen. (C)/(F), Subgroup risk of CVD mortality by different arms, compliance and number of positive screens. Group: 0, control arm; 1, screening arm. Subgroup1: 0, control arm; 1, no screen; 2, negative screening; 3, positive screening. Subgroup2: 0, control arm; 1, no screen; 2, negative screening; 3, occasional positive screening (≤ 2 positive screens); 4, frequent positive screening (> 2 positive screens).
As shown in Table 2 and Table 3, after adjusting for potential confounders, the decreased risk of CVD mortality associated with occasional positive screening remained significant for both participants with and without previous CVD. Notably, the decreased risk was more pronounced among participants without a history of previous CVD, with HR (95% CI) of 0.917 (0.864–0.973) compared to 0.944 (0.898–0.993) for those with previous CVD. Additionally, a consistent decreased risk of CVD mortality was observed for occasional positive FSG and TVU among participants with previous CVD [HR (95%CI): 0.831 (0.779–0.886) and 0.863 (0.756–0.986)] and without previous CVD [HR (95%CI): 0.862 (0.801–0.927) and 0.853 (0.739–0.983)]. However, increased risks of CVD mortality were observed for one and two positive X-rays [HR (95%CI): 1.128 (1.059-1.200)] and PSA [HR (95%CI): 1.231 (1.030–1.472)] in participants with previous CVD.
Sensitivity analyses by excluding screening-detected cancers and non-CVD mortality
As shown in Table 4, after excluding screening-detected cancers, the inverse associations between occasional positive screenings and the risk of CVD mortality remained consistent with those observed in the primary analyses. Specifically, for the entire population, the adjusted hazard ratio (HR) was 0.933 (95% CI: 0.897–0.970). In subgroup analyses, the HRs were 0.919 (95% CI: 0.865–0.976) for participants without previous CVD and 0.945 (95% CI: 0.898–0.995) for those with previous CVD. Additionally, a more pronounced decreased risk of CVD mortality was observed for both FSG and TVU, while increased risks were still noted for X-ray and PSA in participants with previous CVD [HR (95% CI): 1.132 (1.061–1.207) and 1.684 (1.331–2.132), respectively]. Sensitivity analyses excluding both screening-detected cancers and deaths from causes other than CVD mortality showed associations similar to those in the primary analyses (Supplementary Table 2).
Potential adoption of healthy behaviors after positive screen
As shown in Table 5, compared to the control arm, the overall smoking cessation rate among baseline heavy smokers in the screening arm with positive screens was slightly higher (9.80% vs. 9.65%), though this difference was not statistically significant. The difference in smoking cessation rates was more pronounced between participants with ≤ 2 positive screens and the control arm (10.04% vs. 9.65%), but still non-significant. Additionally, the proportion of participants with a baseline BMI ≥ 25 kg/m² who experienced any reduction in BMI was significantly higher in the screening arm with positive screens compared to the control arm (47.83% vs. 47.04%, P = 0.013). This effect was particularly notable among participants with ≤ 2 positive screens (48.07% vs. 47.04%, P = 0.037).
Discussion
To our knowledge, this is the first study to examine the association between multiple positive screenings and the risk of CVD mortality. Similar to cancer diagnosis, positive screenings can increase psychological stress or traumatic experiences, which may elevate the risk of CVD mortality. The more positive screenings a participant experiences, the greater the expected psychological stress. However, a positive screen is not equivalent to a cancer diagnosis, as only a small percentage of positive screenings result in a cancer diagnosis. Moreover, following a positive screen, participants may actively or passively adopt health-promoting behaviors, which could partially counterbalance the increased CVD mortality risk associated with psychological stress21,22,23,24,25. This study also suggest that occasional positive screenings are linked to a decreased risk of CVD mortality, with this effect being more pronounced in participants without a history of previous CVD compared to those with prior CVD. Sensitivity analyses excluding the impact of screening-detected cancers and non-CVD mortality confirmed the consistency of these results. These findings provide valuable insights into the combined effects of cancer screening and the broader strategies for preventing and managing the multimorbidity of cancer and CVD.
Several factors could explain our findings. Foremost, the shared risk factors between cancer and cardiovascular disease (CVD), such as social inequality, smoking, and alcohol consumption, offer a compelling explanation32,33. These factors contribute to the multimorbidity of cancer and CVD, predisposing individuals to both conditions6,34. Additionally, population aging exacerbates this multimorbidity due to common underlying mechanisms between cancer and CVD35, including chronic low-grade inflammation, metabolic dysregulation, and stem cell exhaustion6,8,36. These insights emphasize the importance of comprehensive interventions that address both shared risk factors and common pathophysiological mechanisms to mitigate the risk of multimorbidity37. Furthermore, occasional positive screenings may reflect temporary, non-specific elevations in test results, whereas frequent positive screenings could suggest more persistent and significant health issues. This distinction helps explain why occasional positive screenings are associated with a decreased risk of CVD mortality.
However, not all types of occasional positive screenings were consistently associated with a decreased risk of CVD mortality. This decreased risk was notably observed only for occasional positive screenings from FSG and TVU. In contrast, chest X-ray and PSA tests were consistently linked with an increased risk of CVD mortality, though these increased risks were primarily observed in participants with a history of previous CVD. The decreased risk of CVD mortality associated with positive FSG could be attributed to the removal of colorectal adenomas, which have been linked to elevated coronary artery calcium scores38,39. Similarly, TVU can detect ovarian-related disorders such as polycystic ovary syndrome, which are associated with an increased risk of CVD40,41. Thus, identifying and addressing these conditions might indirectly reduce the risk of CVD mortality. Conversely, chest X-rays remain an important diagnostic tool for some cardiovascular conditions, such as left ventricular systolic dysfunction42. One or two positive chest X-rays might indicate a high risk of CVD mortality or the progression of existing CVD. Further research is needed to determine whether the increased risk of CVD mortality associated with positive chest X-rays is confined to individuals with previous CVD. Additionally, PSA is not specific to prostate conditions; elevated serum PSA levels have been reported in cases of acute myocardial infarction and other cardiovascular diseases43,44,45. The varying associations between different positive screenings and CVD mortality underscore the broad implications beyond the positive screen itself, including psychological stress, potential motivation for health-promoting behaviors, and non-specific clinical indications.
Regarding the potential for health-promoting motivation following positive screens, we observed a significantly higher proportion of BMI reduction among participants with a baseline BMI ≥ 25 kg/m² in the screening arm with positive screens compared to the control arm. This effect was particularly notable for participants with ≤ 2 positive screens. Similar trends were observed in smoking cessation rates among baseline heavy smokers. Although the percentage changes in BMI reduction and smoking cessation between baseline and follow-up were relatively small, they are noteworthy given the long follow-up period of up to 15 years. Short-term changes in healthy behaviors following a positive screen might be more pronounced than long-term changes, but further data are needed to confirm this hypothesis. Future research should explore whether positive screens indeed lead to greater attention to health.
Another significant finding is that participants scheduled for multiple screenings but who did not undergo any of the screenings had a markedly increased risk of CVD mortality. Factors such as poor physical health preventing participation, low functional health literacy (the ability to understand and use basic health-related information), lack of concern for a healthy lifestyle, and skepticism about the effectiveness of screening could collectively contribute to this increased risk. Previous studies have shown that poorer health literacy is linked to reduced medication adherence46,47, and higher mortality48. This suggests that limited health literacy is a fundamental barrier to engaging in cancer screening, following screening procedures, and adopting a healthy lifestyle49. To address this issue, efforts should focus on improving functional health literacy and investigating how enhanced health literacy can boost screening compliance and promote healthier lifestyles. However, as mentioned in the introduction, positive screens may represent a teachable moment for promoting healthy lifestyle changes21,22,23,24,25. This lifestyle change is particularly notable in lung cancer screening using low-dose computed tomography (LDCT). Individuals with abnormal LDCT findings indicating potential lung cancer concerns tend to have higher cessation rates compared to those without such findings23. This lifestyle change is likely to exhibit a dose-response relationship in terms of the number and severity of abnormal findings23. Additionally, a similar dose-response relationship for lifestyle changes is observed in individuals with abnormal endoscopic screening results22.
In addition to the advantages of a large sample size and the “all-versus-none” intervention design, along with the significant findings highlighted above, this study has several limitations that warrant attention. First, as an exploratory analysis based on an intervention study rather than an independent RCT, it does not directly evaluate whether occasional positive screening reduces the risk of CVD mortality. Consequently, some degree of missing information or misclassification of CVD mortality is inevitable. Second, data on previous CVD were derived from self-reported information by participants, which may lead to potential inaccuracies in classification. Third, the participants in the PLCO trial were aged 55 to 74 years, limiting the ability to assess the association between positive screenings and CVD mortality risk in younger populations. Fourth, due to the complexity of non-compliance scenarios in the PLCO screening trial, we did not analyze the impact of non-compliance with different screening methods on the results of this study. Future research with more sophisticated design are needed to further explore the role of non-compliance in the association between positive screening results and CVD mortality. Finally, due to the lack of information on changes in lifestyle factors other than smoking and BMI, we are unable to determine whether positive screening affects other aspects of healthy lifestyles. Furthermore, due to the lack of data on short-term changes (e.g., < 3 years) in smoking and BMI, we are also unable to assess the short-term impact of positive screening on these two factors.
In conclusion, positive screenings, akin to a cancer diagnosis, can elevate the risk of CVD mortality due to increased psychological stress. However, occasional positive screenings following multiple tests are associated with a reduced risk of CVD mortality, regardless of prior CVD, cancer diagnosis, or other competing risks. Given the shared risk factors and common mechanisms underlying the multimorbidity of cancer and CVD, comprehensive health management that integrates lifestyle interventions is essential to mitigate the combined burden of these diseases. Further research with more refined study designs is necessary to validate these findings.
Data availability
The study taken from publicly available data [PLCO trial database, https://cdas.cancer.gov/plco/].
Abbreviations
- CVD:
-
Cardiovascular disease
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- PLCO:
-
Prostate, Lung, Colorectal and Ovarian Cancer
- XRY:
-
X-ray
- FSG:
-
Flexible sigmoidoscopy
- PSA:
-
Prostate-specific antigen
- DRE:
-
Digital rectal examinations
- CA-125:
-
Cancer antigen 125
- TVU:
-
Transvaginal ultrasound
- ASU:
-
Annual study update
- NDI:
-
The National Death Index
- ICD-9:
-
The 9th edition of International Classification of Diseases
- DRP:
-
Death Review Process
- K-M:
-
Kaplan-Meier
References
Zhao, D., Liu, J., Wang, M., Zhang, X. & Zhou, M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 16, 203–212 (2019).
Liu, S. et al. Burden of Cardiovascular diseases in China, 1990–2016: findings from the 2016 global burden of Disease Study. JAMA Cardiol. 4, 342–352 (2019).
Xu, C. et al. Increased serum levels of aldehydes are associated with cardiovascular disease and cardiovascular risk factors in adults. J. Hazard. Mater. 400, 123134 (2020).
Lugun, J., Ghosh, D., Anand, A., Chakraborty, B. & Ghosh, S. Prevalence of CVD risk factors among some tribal and nontribal populations of Jharkhand - A comparative survey. Spat. Spatiotemporal Epidemiol. 37, 100419 (2021).
Pilleron, S. et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int. J. Cancer. 144, 49–58 (2019).
Karlstaedt, A., Moslehi, J. & de Boer, R. A. Cardio-onco-metabolism: metabolic remodelling in cardiovascular disease and cancer. Nat. Rev. Cardiol. 19, 414–425 (2022).
Narayan, V., Thompson, E. W., Demissei, B., Ho, J. E. & Januzzi, J. L. Jr. Ky. Mechanistic biomarkers informative of both Cancer and Cardiovascular Disease: JACC State-of-the-art review. J. Am. Coll. Cardiol. 75, 2726–2737 (2020).
Koene, R. J., Prizment, A. E. & Blaes, A. Konety. Shared Risk factors in Cardiovascular Disease and Cancer. Circulation. 133, 1104–1114 (2016).
Fang, F. et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl. J. Med. 366, 1310–1318 (2012).
Fang, F. et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J. Natl. Cancer Inst. 102, 307–314 (2010).
Fall, K. et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: prospective cohort study. PLoS Med. 6, e1000197 (2009).
Force, U. S. P. S. T. et al. Screening for prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 319, 1901–1913 (2018).
Henderson, J. T. & Webber, E. M. Sawaya. Screening for ovarian Cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 319, 595–606 (2018).
Brownlee, S. et al. Evidence for overuse of medical services around the world. Lancet. 390, 156–168 (2017).
Nelson, H. D. et al. Harms of breast Cancer screening: systematic review to Update the 2009 U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 164, 256–267 (2016).
Kivimaki, M. & Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018).
Osborne, M. T., Shin, L. M., Mehta, N. N. & Pitman, R. K. Fayad and A. Tawakol. Disentangling the links between psychosocial stress and Cardiovascular Disease. Circ. Cardiovasc. Imaging. 13, e010931 (2020).
Nudy, M., Asmaro, R., Jiang, X. & Schnatz, P. F. The association between incidentally found breast arterial calcification on routine screening mammography and the development of coronary artery disease and stroke: results of a 10-year prospective study. Menopause. 29, 1375–1380 (2022).
Tailor, T. D. et al. Cardiovascular Risk in the Lung Cancer Screening Population: a Multicenter Study evaluating the Association between Coronary Artery Calcification and preventive statin prescription. J. Am. Coll. Radiol. 18, 1258–1266 (2021).
Ruparel, M. et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 74, 1140–1146 (2019).
Tammemagi, M. C., Berg, C. D., Riley, T. L., Cunningham, C. R. & Taylor, K. L. Impact of lung cancer screening results on smoking cessation. J. Natl. Cancer Inst. 106, dju084 (2014).
Knudsen, M. D. et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin. Gastroenterol. Hepatol. 20, e1240–e1249 (2022).
Slatore, C. G., Baumann, C., Pappas, M. & Humphrey, L. L. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann. Am. Thorac. Soc. 11, 619–627 (2014).
Pineiro, B., Simmons, V. N., Palmer, A. M. & Correa, J. B. Brandon. Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review. Lung Cancer. 98, 91–98 (2016).
Townsend, C. O. et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 103, 2154–2162 (2005).
Prorok, P. C. et al. Design of the prostate, lung, colorectal and ovarian (PLCO) Cancer Screening Trial. Control Clin. Trials. 21, 273S–309S (2000).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S. & Ntzani, E. E. Ioannidis. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 350, g7607 (2015).
Schoen, R. E. et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl. J. Med. 366, 2345–2357 (2012).
Kachuri, L. et al. Pan-cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat. Commun. 11, 6084 (2020).
Hackshaw, A., Cohen, S. S., Reichert, H., Kansal, A. R. & Chung, K. C. Ofman. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br. J. Cancer. 125, 1432–1442 (2021).
Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759 (2020).
Renzi, C. et al. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat. Rev. Clin. Oncol. 16, 746–761 (2019).
Kuan, V. et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit. Health. 5, e16–e27 (2023).
Xu, X., Mishra, G. D., Dobson, A. J. & Jones, M. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med. 15, e1002516 (2018).
Boudoulas, K. D., Triposkiadis, F., Gumina, R., Addison, D. & Iliescu, C. Boudoulas. Cardiovascular Disease, Cancer, and Multimorbidity interactions: clinical implications. Cardiology. 147, 196–206 (2022).
Barnes, P. J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir J. 45, 790–806 (2015).
Han, Y. et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J. 42, 3374–3384 (2021).
Chen, Y. et al. Association of Cardiovascular Risk Assessment with early colorectal neoplasia detection in Asymptomatic Population: a systematic review and Meta-analysis. Clin. Epidemiol. 12, 865–873 (2020).
Niederseer, D. et al. Cardiovascular risk and known coronary artery Disease are Associated with Colorectal Adenoma and Advanced Neoplasia. J. Am. Coll. Cardiol. 69, 2348–2350 (2017).
Osibogun, O., Ogunmoroti, O. & Michos, E. D. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 30, 399–404 (2020).
Poeta do Couto, C., Policiano, C., Pinto, F. J., Brito, D. & Caldeira, D. Endometriosis and cardiovascular disease: a systematic review and meta-analysis. Maturitas. 171, 45–52 (2023).
Hsiang, C. W. et al. Detection of left ventricular systolic dysfunction using an Artificial Intelligence-enabled chest X-Ray. Can. J. Cardiol. 38, 763–773 (2022).
Patane, S. & Marte, F. Prostate-specific antigen kallikrein: from prostate cancer to cardiovascular system. Eur. Heart J. 30, 1169–1170 (2009).
Patane, S. & Marte, F. Prostate-specific antigen kallikrein and acute myocardial infarction: where we are. Where are we going? Int. J. Cardiol. 146, e20–22 (2011).
Patane, S., Marte, F., Sturiale, M., Grassi, R. & Patane, F. Significant coronary artery disease associated with coronary artery aneurysm and elevation of prostate-specific antigen during acute myocardial infarction. Int. J. Cardiol. 141, e39–42 (2010).
Chima, C. C., Abdelaziz, A. & Asuzu, C. Beech. Impact of health literacy on Medication Engagement among adults with diabetes in the United States: a systematic review. Diabetes Educ. 46, 335–349 (2020).
Zhang, N. J., Terry, A. & McHorney, C. A. Impact of health literacy on medication adherence: a systematic review and meta-analysis. Ann. Pharmacother. 48, 741–751 (2014).
Bostock, S. & Steptoe, A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 344, e1602 (2012).
Kobayashi, L. C., Wardle, J. & von Wagner, C. Limited health literacy is a barrier to colorectal cancer screening in England: evidence from the English Longitudinal Study of Ageing. Prev. Med. 61, 100–105 (2014).
Acknowledgements
This research was conducted using the PLCO resource under application number PLCO-1125. The authors thank the National Cancer Institute for access to NCI’s data collected by the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
Role of the funder/sponsor
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
This work was supported by the Chinese National Key Research and Development Project [grant number 2021YFC2500400]; Tianjin Health Committee Foundation [grant number TJWJ2021MS008].
Author information
Authors and Affiliations
Contributions
Dr Huang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.Concept and design: Huang, Yang.Acquisition, analysis, or interpretation of data: Ji, Zhang, Duan, Liu, Zhang, Feng, Li, Fan, Liu, Zhang, Yang, Lyu, Song, Song, Li, Huang.Drafting of the manuscript: Ji, Zhang, Huang.Critical revision of the manuscript for important intellectual content: Yang, Lyu, Song, Song, Li, Huang.Statistical analysis: Ji, Zhang.Obtained funding: Song, Huang.Administrative, technical, or material support: Huang.Supervision: Song, Song.The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ji, Y., Zhang, Y., Duan, H. et al. Decreased risk of cardiovascular disease mortality associated with occasional positive screens following cancer screenings. Sci Rep 15, 1927 (2025). https://doi.org/10.1038/s41598-024-78252-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78252-2