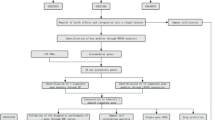
Abstract
Allergic rhinitis (AR) resulted in impairing human health and quality of life seriously. There is currently no definitive remedy for AR. Recent studies have shown that autophagy may regulate airway inflammation. Our comprehension of autophagy and its molecular mechanism in the field of AR condition remains incomplete. Our research endeavors to bridge this knowledge deficit by investigating the correlation between AR and autophagy. The AR-related gene expression profile GSE50223 was screened and downloaded. The “limma” package of R software was utilized to identify differentially expressed genes associated with autophagy. GO, KEGG, and Gene set enrichment analyses were conducted. A PPI network of differentially expressed autophagy-related genes were established and further identified through the CytoHubba algorithm. A receiver operating characteristic curve analysis was employed to evaluate the diagnostic effectiveness of the hub genes and to examine the relationship between autophagy-related genes and AR. Finally, qRT-PCR was carried out to confirm the chosen autophagy-related genes using clinical samples. 21 autophagy-related genes in allergic rhinitis were identified. BECN1, PIK3C3, GABARAPL2, ULK2, and UVRAG were considered as significant differentially expressed autophagy-related genes. However, additional molecular biological experiments will be necessary to elucidate the underlying mechanism connecting autophagy and AR.
Similar content being viewed by others
Introduction
Allergic rhinitis is a common allergic condition that impacts the nasal passages. AR is a medical condition in which immune responses are triggered by environmental stimuli such as fungal spores, tree pollen animal dander, or house dust mites1. Mediators and cytokines released by these immune cells stimulate eosinophil adhesion, enhance mucus production, lead to fibrosis, induce goblet cell hyperplasia, promote vasodilation, increase airway hyperresponsiveness, and cause tissue damage2,3,4. Symptoms of AR encompass rhinorrhea, sneezing, and pruritus, along with lower respiratory tract infections, nasal congestion and sinusitis5. This condition imposes a substantial health burden on individuals as it adversely impacts their quality of life. Estimates of its prevalence exhibit a broad range, but reliable epidemiological studies indicate that approximately 20 to 30% of adults and potentially up to 40% of children experience this condition6. However, the etiology and pathogenesis of this disease remains unclear. Current therapeutic approaches primarily focus on symptom management in the case of AR, with no definitive cure available7.
Recent studies have indicated that autophagy may regulate airway inflammatory responses through its modulation of systems, genetic polymorphisms, involvement in immune responses, and airway remodeling8. Autophagy is a cellular death programming distinct from apoptosis9. Autophagy is a fundamental physiological process which maintains cellular balance and supports the survival of normal, functioning cells. In typical physiological circumstances, cells uphold a relatively modest baseline level of autophagy. However, under stress conditions such as infection, oxidative stress, or starvation, cellular autophagy levels increase rapidly10. Extensive research has demonstrated a close relationship between cellular autophagy, the immune system, and inflammatory responses11,12,13. Previous studies suggested that autophagy primarily affects immune-mediated diseases through endogenous antigen presentation. More recent research has revealed that autophagy also exerts a regulatory influence on the differentiation, maturation, and functions of immune cells11.
The pathogenesis of AR is mainly attributed to imbalances in immune cells14. Autophagy has been linked to a multitude of biological processes, encompassing immunity, aging, differentiation, and development. Moreover, it has been shown to have an impact on the development of allergic diseases and the remodeling of airways. Despite significant advancements in the field of AR, the involvement of autophagy and its underlying molecular mechanisms in AR remain elusive. Consequently, we are conducting research to unravel the connection between AR and autophagy, with the goal of shedding light on the underlying mechanisms and identifying potential targeted treatments.
Materials and methods
Patient dataset
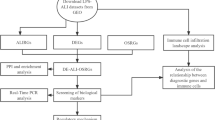
The AR-related dataset GSE5022315 was obtained from the GEO (Gene Expression Omnibus) database (https://www.ncbi.nlm.nih.gov/geo/) using the “GEO query” package16, containing 21 allergic rhinitis samples and 21 control samples, derived from Homo sapiens with data platform of GPL6884-11607. PBMCs from 21 patients (P) and 21 healthy controls (H) were challenged with grass pollen for 7 days. Diluent challenged control samples were obtained from all subjects.
A set of 232 genes linked to cellular autophagy were obtained from HADb (Human Autophagy Database) database (http://www.autophagy.lu/), a comprehensive repository of autophagy-related information. This database contains curated data on autophagy-related genes, proteins, pathways, and associated diseases in humans. The information within the HADb database is available for free download and use.
Data processing and identification of differentially expressed autophagy-related genes (DEAGs)
Utilizing R software (version 4.05, https://www.r-project.org/), we applied the “limma” package to perform data correction and normalization on the GSE50223 dataset. The “EdgeR” package was used to identify distinctions between specimens from AR and control groups, focusing on significantly DEAGs. The selection criteria were: p < 0.05 and |fold change (FC)| > 0. Within this group, genes exhibiting a logFC > 0 and p-value < 0.05 are categorized as upregulated differentially expressed genes (DEGs), whereas those with logFC < 0 and p-value < 0.05 are classified as downregulated DEGs. Using “pheatmap” and “ggplot2” packages of R software, a volcano plot was created to visualize the DEAGs, and a heatmap was used to display the top 50 up-regulated and the top 50 down-regulated DEAGs.
Functional enrichment (GO) and pathway enrichment (KEGG) analysis
Gene Ontology (GO)17 functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG)18 pathway analysis were performed to investigate the major biological characteristics with the R package (http://bioconductor.org/)19 “Cluster Profiler”. GO analyses were carried out to explore the underlying biological processes and KEGG analysis was employed to identify potential signaling pathways associated with these DEAGs. A statistical significance threshold of p < 0.05 was applied for the analyses.
Gene set enrichment analysis (GSEA)
Gene set enrichment analysis (GSEA) was carried out to identify the significant enrichment signaling pathways associated with the most significant autophagy-related genes in AR. Enrichment analyses were carried out to ascertain if a set of predefined biological processes exhibited enrichment, and the enrichment score was utilized to evaluate the statistical significance of the findings. The top five terms from the KEGG analyses were presented, showcasing KEGG pathways with notable enrichment outcomes, considering an adjusted p-value threshold of < 0.05.
Protein–protein interaction (PPI) network of autophagy-related genes and hub genes
The STRING database is an online search tool employed for analyzing known proteins and predicting protein-protein interaction (PPI) networks, including both direct and indirect protein interactions, along with functional associations20 (https://string-db.org/). Protein nodes that exhibited no interactions with others were omitted. The PPI network model was visualized using Cytoscape21 (version 3.9.1).
Hub genes hold a significant role in various biological processes. Using the PPI network, hub genes were selected based on their network. Cytoscape software (version 3.9.1), along with the cytoHubba (version: 0.1) and MCODE (version: 2.0.0) plug-ins, were applied to identify crucial targets or subnetworks within the complex networks.
Immune cell infiltration
The Cell-type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) is an analytical method to estimate the cellular composition of intricate tissues in 22 subsets of immune cells. The LM22 gene file of CIBERSORT was employed to define 22 immune cell subsets and the gene expression profiles of the GSE50223 dataset were uploaded to CIBERSORT to evaluate immune cell infiltration of the treat and control groups. The CIBERSORT results were visually represented using the “ggplot2” package within the R software.
ROC
ROC curve analysis was carried out to evaluate the prognostic significance of hub genes in AR. The analysis employed 1-specificity (true positive rate, TPR) on the x-axis and sensitivity (false positive rate, FPR) on the y-axis. Subsequently, the area under the curve (AUC) values and their corresponding 95% confidence intervals (CI) were calculated for the genes. Genes with AUC values exceeding 0.7 were considered to possess stronger predictive capabilities for AR prognosis.
Real-time fluorescence quantitative polymerase chain reaction (qRT‐PCR)
As reported in authoritative journal articles, the inferior turbinate was frequently used as the control reference in previous studies20,21. In this study, a total of 8 tissues from allergic rhinitis (AR) patients and 8 normal mucosal tissues were acquired from Wuhan Union Hospital for subsequent qRT-PCR analysis. All patients had signed informed consent forms prior to surgery. The samples were extracted and promptly frozen in liquid nitrogen, then stored in a -80 °C low-temperature refrigerator for future investigations. Total RNA was extracted from samples with TRIzol™ Reagent (Invitrogen). RNA was reverse-transcribed using PrimeScript™ RT Master Mix (Takara). Real-time PCR was performed with TB Green™ Premix EX Taq™ II (Takara). The expression levels of mRNAs were normalized by GAPDH. Relative expression levels were determined using the comparative Ct method (2 − ΔΔCt), and the significance of mRNA expression differences was assessed with a two-tailed Student’s t-test in GraphPad Prism (version 8.0). The primer sequences are provided below:
-
BECN1
-
Forward: 5′-AAACCAGATGCGTTATGCCC-3′.
-
Reverse: 5′-CCCAGCCTGAAGTTATTGATTGTG-3′.
-
GABARAPL2
-
Forward: 5′-CCACTCGCTGGAACACAGAT-3′.
-
Reverse: 5′-CATAGTTAGGCTGGACTGTGGG-3′.
-
UVRAG
-
Forward: 5′-TGGAAGAGACAGCCACTTACG-3′.
-
Reverse: 5′-CTCATTCTCAGAGCTGGCCC-3′.
-
PIK3C3
-
Forward: 5′-GGAACAACGGTTTCGCTCTTT-3′.
-
Reverse: 5′-TGTCAATCTATCCAGCCAATCTACT-3′.
-
ULK2
-
Forward: 5′-TCAAGCATCTTCCAACCTGTTAG-3′.
-
Reverse: 5′-TAAACTGTCTGTGCTGCCCTGAT-3′.
Statistical analysis
All analyses were performed using R version 4.0.5, and a P-value < 0.05 was considered statistically significant.
Results
Identification of autophagy-related genes in allergic rhinitis
Within dataset GSE50223, a total of 1769 genes (|logFC| > 0, p-value < 0.05) exhibiting differential expression was identified, consisting of 918 upregulated genes (logFC > 0, p-value < 0.05) and 851 downregulated genes (logFC < 0, p-value < 0.05). Additionally, a comprehensive list of 232 genes linked to cellular autophagy was extracted from HADb. Next, an intersection analysis between the DEGs in dataset GSE50223 and autophagy-related genes was conducted, resulting in 21 significantly differentially expressed autophagy-related genes that are intricately linked to cellular autophagy of AR, including HGS, PIK3C3, BECN1, EDEM1, TMEM49, VEGFA, SIRT2, UVRAG, CALCOCO2, BNIP3, ULK2, GABARAPL2, DLC1, PEX14, CDKN1B, EIF2AK3, ZFYVE1, TM9SF1, CASP4, NPC1, PELP1, including 11 up-regulated genes and 10 down-regulated genes. Up-regulated genes were PIK3C3, BECN1, EDEM1, SIRT2, UVRAG, ULK2, DLC1, EIF2AK3, ZFYVE1, CASP4, PELP. Down-regulated genes were PEX14, CALCOCO2, CDKN1B, TMEM49, TM9SF1, NPC1, VEGFA, BNIP3, HGS, GABARAPL2 (Fig. 1).
Functional enrichment analysis of autophagy-related genes in allergic rhinitis
GO annotation and KEGG signaling pathway enrichment analyses were conducted to analyze the relationships between the 21 autophagy-related genes in AR and their involvement in biological processes (BP), cellular components (CC) and molecular functions (MF), cellular components (CC). The outcomes of the GO enrichment analysis (p < 0.05) indicated significant enrichment of autophagy-related genes in AR within various biological processes such as macroautophagy, regulation of autophagy, response to nutrient levels and cellular component disassembly. Additionally, these genes were enriched in cellular components such as autophagosome and vacuolar membrane. Furthermore, they were associated with molecular functions such as ubiquitin—like protein (Fig. 2A,B). In the subsequent KEGG pathway analysis, it was observed that the autophagy-related genes exhibited significant enrichment in several pathways, including Autophagy— animal, Mitophagy—animal, Shigellosis, and Pathways of neurodegeneration—multiple diseases (Fig. 2C-D).
GSEA enrichment analysis
The GSEA enrichment results indicated that genes in treat group were predominantly enriched in DNA Replication, proteasome, spliceosome, terpenoid backbone biosynthesis, T-cell receptor signaling pathway, etc. (Fig. 3A) The genes in control group were primarily enriched in complement and coagulation cascades, glycolysis gluconeogenesis, lysosome, toll like receptor signaling pathway, nod like receptor signaling pathway, etc. (Fig. 3B).
Protein–protein interaction (PPI) network of allergic rhinitis autophagy-related genes and hub genes
By utilizing the STRING database, the interactions among AR and autophagy-related genes were analyzed to explore the PPI network of these genes. Afterward, the interacting proteins was imported into Cytoscape software to create a visual representation (Fig. 3C). Genes that did not have any interactions were removed, resulting in a PPI network consisting of a total of 18 genes. The interaction between target proteins and these proteins was illustrated using nodes and edges, with the intensity of color and the size of the node reflecting higher Degree values, indicating increased connectivity among nodes.
Using Cytohubba plug-in in Cytoscape software, top 10 hub genes were identified by their degree of connectivity including PIK3C3, BECN1, VEGFA, UVRAG, CALCOCO2, BNIP3, ULK2, GABARAPL2, ZFYVE1, VMP1.
Immune infiltration analysis
Immune infiltration analysis was conducted to identify the potential involvement of specific immune cell types in the development of AR. As depicted in Fig. 4A, in comparison to control group, the score for NK cells resting was notably lower (p < 0.05), whereas the score for T cells follicular helper was significantly higher (p < 0.05) in the treat group. No significant differences were observed in the proportions of other immune cell types between the treat and control groups. Figure 4B provides a visualization of the proportions of the 22 different types of immune cells.
ROC
In the results of ROC curve analysis, based on the AUC value, it was observed that BECN1 (AUC = 0.780) exhibited the highest prognostic capability among AR patients, followed by PIK3C3 (AUC = 0.768), GABARAPL2 (AUC = 0.709), ULK2 (AUC = 0.717), UVRAG (AUC = 0.713) (Fig. 4C). The AUC values for the remaining five genes were all less than 0.7, signifying a limited accuracy in predicting the prognosis of patients with AR. Taking into account these data as well as previous research, BECN1, PIK3C3, GABARAPL2, ULK2, and UVRAG were identified as the key genes for subsequent analysis.
PCR
Finally, we validated the expression of BECN1, PIK3C3, GABARAPL2, ULK2, and UVRAG in clinical specimens by qRT-PCR. Consistent with our previous bioinformatics analysis, significant differences in relative expression levels between normal nasal mucosa samples and AR samples. The expression of BECN1 (p < 0.05), PIK3C3 (p < 0.05), ULK2 (p < 0.05), and UVRAG (p < 0.05) were observed increased in AR specimens (Fig. 5A–D), and the level of GABARAPL2(p < 0.05) was significantly decreased in AR specimens (Fig. 5E).
Discussion
AR is a highly prevalent condition that is not life-threatening, but imposes a considerable socioeconomic burden on affected individuals and significantly reduces their overall quality of life21. Current pharmacological treatments, allergen immunotherapy, and surgical interventions are commonly utilized to manage AR symptoms, but the treatment outcomes are not satisfactory.
According to the 2023 international consensus statement on AR, oral H1 antihistamines, intranasal corticosteroid sprays, combination intranasal corticosteroid and intranasal antihistamine treatments, and intranasal saline are strongly recommended for AR management. Nevertheless, it is essential to acknowledge that these treatments may be associated with certain adverse effects, including central nervous system effects, anticholinergic side effects, nasal irritation, cough, ear fullness, taste disturbances, and undesirable local effects like increased frequency of epistaxis, among others22. Biologic therapies like Omalizumab treatment have shown symptom improvement, but US FDA did not approve the indication due to the local injection site reactions and the potential risk of anaphylaxis. Allergen Immunotherapy offers the advantage of initiating and maintaining immunological changes. Both sublingual immunotherapy and subcutaneous immunotherapy can lead to common local reactions and, in rare instances, systemic reactions. These systemic reactions, if not managed correctly, have the potential to be severe and even fatal.
For allergic rhinitis (AR) patients who have not experienced sufficient relief with medical treatment, particularly in cases of persistent rhinorrhea, alternative options like cryoablation and radiofrequency ablation of the posterior nasal nerve, as well as procedures like Vidian neurectomy or posterior nasal neurectomy, may be considered. Vidian neurectomy and posterior nasal neurectomy have shown effectiveness in relieving rhinorrhea symptoms. However, it’s essential to consider potential complications, such as epistaxis, temporary facial discomfort and swelling, headaches, dry eye with reduced tear production, numbness in the lip and palate, nasal dryness, and the risk of damage to other nerves. Additionally, the long-term results of these procedures may be limited22.
Autophagy plays a fundamental role in intracellular degradation, targeting a wide range of microorganisms (including bacteria, fungi, viruses, and protozoa/protists), as well as proteins and damaged organelles that the proteasome cannot effectively dismantle. It constitutes a cellular process dedicated to recycling and breaking down damaged or superfluous cellular components. It stands as a vital mechanism for upholding cellular homeostasis and promoting cell survival. Autophagy has been associated with multiple aspects of allergic diseases, including allergic rhinitis. Several autophagy-related genes have been identified to contribute to the pathogenesis and regulation of allergic rhinitis. Therefore, investigating the connection between autophagy and allergic rhinitis holds great promise in elucidating the underlying mechanisms and discovering precise targeted treatments.
Our study involved an extensive bioinformatics analysis to provide a comprehensive understanding. First, 1769 significant DEAGs were identified from datasets GSE50223. Second, HGS, PIK3C3, BECN1, EDEM1, TMEM49, VEGFA, SIRT2, UVRAG, CALCOCO2, BNIP3, ULK2, GABARAPL2, DLC1, PEX14, CDKN1B, EIF2AK3, ZFYVE1, TM9SF1, CASP4, NPC1, PELP1 were identified as 21 autophagy-related genes in allergic rhinitis between from both datasets and the HADb database. Third, functional enrichment analyses and GSEA enrichment analysis emphasized that the significantly DEAGs could potentially be associated with various related KEGG pathways. Fourth, BECN1, PIK3C3, GABARAPL2, ULK2, and UVRAG were considered as significant differentially expressed autophagy-related genes. Fifth, the experimental verification indicated that BECN1, PIK3C3, GABARAPL2, ULK2, and UVRAG genes were the important DEAGs of AR.
The genes responsible for regulating autophagy, known as autophagy-related (Atg) genes, predominantly group into one of five ‘core machinery’ components that operate in mostly sequential stages during the formation of autophagosomes. These components include the BECN1/beclin1-PIK3C3/Vps34 lipid kinase complex, the Atg9 trafficking system, the Atg12–Atg5 and Atg8–PE ubiquitin-like conjugation systems, and the ULK protein kinase complex23.
BECN1, alternatively referred to as beclin1, serves as a crucial protein involved in autophagy processes24, it is present in numerous human and murine tissues and is predominantly found within cytoplasmic structures, including the mitochondria, the perinuclear membrane, and the endoplasmic reticulum. The Beclin-1/class III PI3K complex has a crucial function in initiating the nucleation of autophagosomal membranes25. The expression levels of Beclin-1 mRNA and protein are significantly increased in the upper airways of individuals with AR, and this upregulation is closely linked to changes in markers of airway remodeling. Additionally, robust expression of Beclin-1 in cilia lining large airway epithelial cells is predominantly observed in patients with AR, where signs of airway remodeling are apparent. These findings suggest that autophagy is implicated in AR by promoting airway remodeling processes26,27.
The Vps34p-Vps30p complex, in conjunction with its mammalian counterpart PIK3C3-BECN1 (Beclin), governs the production of phosphoinositide signals that promote the inception of the primary autophagosome28. Among the paramount kinase groups overseeing autophagy, phosphatidylinositol 3-kinases (PtdIns3Ks) and phosphoinositide 3-kinases (PI3Ks) are of utmost significance. The catalytic subunit PIK3C3/Vps34, belonging to class III PtdIns3K, associates with BECN1 and PIK3R4 to trigger the generation of phosphatidylinositol 3-phosphate (PtdIns3P). This molecule plays a crucial role in initiating and driving the autophagy process forward29. PI3-Kinase governs the degranulation of eosinophils and neutrophils in individuals afflicted with AR and allergic asthma30. Effective inhibition of the PI3K/AKT signaling pathway led to a notable reduction in allergic responses in individuals with AR31.
Our study successfully identified and confirmed autophagy-related genes associated with allergic rhinitis, shedding light on the connection between autophagy and this condition. BECN1, PIK3C3, GABARAPL2, ULK2 and UVRAG were recognized as significant autophagy-related genes of allergic rhinitis. A review of scientific literature via the PubMed and Web of Science databases revealed a notable scarcity of articles pertaining to GABARAPL2, ULK2 and UVRAG with AR. There’s no report regarding GABARAPL2, ULK2 and UVRAG in AR patients were identified.
Conclusion
We are the pioneering researchers to investigate autophagy and its underlying molecular mechanism in allergic rhinitis, employing an extensive bioinformatics analysis. Nevertheless, it’s essential to acknowledge the presence of certain limitations in this study. To gain a more profound understanding, further molecular biological experiments are indispensable in elucidating the underlying mechanisms.
Data availability
The datasets presented in this study can be found in online repositories. The original contributions presented in the study are included in this article. Further inquiries can be directed to the corresponding authors.
Change history
30 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-88036-x
Abbreviations
- AR:
-
Allergic rhinitis
- DEGs:
-
differentially expressed genes
- DEAG:
-
differentially expressed autophagy-related gene
- GEO:
-
Gene expression omnibus
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- GSEA:
-
Gene set enrichment analysis
- PPI:
-
Protein–protein interaction
- HADb:
-
Human autophagy database
- ATG:
-
Autophagy-related genes
References
Hammad, H. & Lambrecht, B. N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8 (3), 193–204 (2008).
Holgate, S. T. Pathogenesis of asthma. Clin. Exp. Allergy . 38 (6), 872–897 (2008).
Lambrecht, B. N. & Hammad, H. The immunology of asthma. Nat. Immunol.. 16 (1), 45–56 (2015).
Bradding, P., Walls, A. F. & Holgate, S. T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol.. 117 (6), 1277–1284 (2006).
Brozek, J. L. et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 140 (4), 950–958 (2017).
Meltzer, E. O. Allergic rhinitis: Burden of illness, quality of life, comorbidities, and control. Immunol. Allergy Clin. N. Am. 36 (2), 235–248 (2016).
Reitsma, S., Subramaniam, S., Fokkens, W. W. J. & Wang, Y. Recent developments and highlights in rhinitis and allergen immunotherapy. Allergy 73 (12), 2306–2313 (2018).
Jyothula, S. S. & Eissa, N. T. Autophagy and role in asthma. Curr. Opin. Pulm Med. 19 (1), 30–35 (2013).
Moretti, L., Yang, E. S., Kim, K. W. & Lu, B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist. Updat. 10 (4–5), 135–143 (2007).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell. 11 (4), 728–741 (2011).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature. 20 (7330), 323–335 (2011).
Riva, N. et al. A comparative study using thrombin generation and three different INR methods in patients on vitamin K antagonist treatment. Int. J. Lab. Hematol. 39 (5), 482–488 (2017).
Wang, F., Li, B., Schall, N., Wilhelm, M. & Muller, S. Assessing autophagy in mouse models and patients with systemic Autoimmune diseases. Cells 28(3), 6 (2017).
Bousquet, J. et al. Allergic Rhinitis and its impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy . 63 (Suppl 86), 8–160 (2008).
Nestor, C. E. et al. DNA methylation changes separate allergic patients from healthy controls and may reflect altered CD4 + T-cell population structure. PLoS Genet.. 10 (1), e1004059 (2014).
Davis, S. & Meltzer, P. S. GEOquery: a bridge between the Gene expression Omnibus (GEO) and BioConductor. Bioinf.. 15 (14), 1846–1847 (2007).
Gene Ontology, C. Gene Ontology Consortium: going forward. Nucleic Acids Res.. 43 (Database issue), D1049–1056 (2015).
Ogata, H. et al. KEGG: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res.. 1 (1), 29–34 (1999).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16 (5), 284–287 (2012).
Kim, H. J., Kim, J. H., Han, S. A. & Kim, W. Compositional alterations of the nasal microbiome and Staphylococcus aureus-characterized dysbiosis in the nasal mucosa of patients with allergic rhinitis. Clin. Exp. Otorhinolaryngol. 15 (4), 335–345 (2022).
Wu, A. C., Dahlin, A. & Wang, A. L. The role of environmental risk factors on the development of Childhood Allergic Rhinitis. Child. (Basel) 17(8), 8 (2021).
Wise, S. K. et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023. Int. Forum Allergy Rhinol. 13 (4), 293–859 (2023).
Agrotis, A., Pengo, N., Burden, J. J. & Ketteler, R. Redundancy of human ATG4 protease isoforms in autophagy and LC3/GABARAP processing revealed in cells. Autophagy. 15 (6), 976–997 (2019).
Feng, W. et al. Dissection of autophagy in human platelets. Autophagy. 10 (4), 642–651 (2014).
Kihara, A., Kabeya, Y., Ohsumi, Y. & Yoshimori, T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-golgi network. EMBO Rep. 2 (4), 330–335 (2001).
Li, J. & Li, Y. Autophagy is involved in allergic rhinitis by inducing airway remodeling. Int. Forum Allergy Rhinol. 9 (11), 1346–1351 (2019).
McAlinden, K. D. et al. Autophagy activation in asthma airways remodeling. Am. J. Respir. Cell. Mol. Biol. 60 (5), 541–553 (2019).
Simonsen, A. & Tooze, S. A. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J. Cell. Biol. 21 (6), 773–782 (2009).
Yu, X., Long, Y. C. & Shen, H. M. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy. 11 (10), 1711–1728 (2015).
Kampe, M. et al. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflamm.. 35 (1), 230–239 (2012).
Lin, H., Zheng, C., Li, J., Yang, C. & Hu, L. Lentiviral shRNA against KCa3.1 inhibits allergic response in allergic rhinitis and suppresses mast cell activity via PI3K/AKT signaling pathway. Sci. Rep.. 14, 5:13127 (2015).
Acknowledgements
Thanks to all the authors for their contributions to this study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Natural Science Foundation of Hubei Province (2024AFB615, ZLQ), Natural Science Foundation of Hubei Province (2022CFB087, ZT), National Natural Science Foundation of China (82201301, CH, 81771005, ZT), the Research Grant of Union Hospital, Tongji Medical College, HUST (F016.02004.21003.126, ZT), and Open Project of Key Laboratory of Molecular Imaging (2022fzyx015, TZ).
Author information
Authors and Affiliations
Contributions
Among the authors in the list, TZ and LZ conceived and performed bioinformatics analysis. TZ, HC, and LW co-wrote the manuscript. LZ, TZ, and JL undertook a manuscript review. JC have contributed to the revision and refinement of the article. All authors contributed to and revised the final manuscript.
Corresponding authors
Ethics declarations
Statement
All experimental protocols were approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (UHCT-IEC-SOP-016-03-01). All methods were carried out in accordance with relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article Tao Zhou, Hua Cai and Lisha Wu were omitted as equally contributing authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, T., Cai, H., Wu, L. et al. Bioinformatics analysis and identification of underlying biomarkers potentially linking allergic rhinitis and autophagy. Sci Rep 14, 27624 (2024). https://doi.org/10.1038/s41598-024-78375-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78375-6