Abstract
The gut microbiome primarily generates short-chain fatty acids (SCFAs) by fermenting dietary fibers. Though previous studies have linked SCFAs to blood pressure, there remains a lack of research on the relationship between SCFAs levels in the serum of elderly individuals and blood pressure. Based on this, we investigated the associations of serum SCFAs with blood pressure in Chinese older adults in a cross-sectional study. In this report, we recruited 1013 older adults over 60 years of age from June to September 2016 in Lu 'an City, China. Using Ultra High Performance Liquid Chromatography-Quadrupole-Exactive-Orbitrap-Mass Spectrometry (UHPLC-QE-Orbitrap MS), we measured the level of various SCFAs, including acetic acid (AA), propanoic acid (PA), butyric acid (BA), isobutyric acid (iso-BA), valeric acid (VA), isovaleric acid (iso-VA), and caproic acid (CA), in serum samples collected from Chinese elderly adults. The study recruited 1013 older adults in total. Multiple logistic regression analysis shows that AA (OR = 0.696, 95%CI: 0.501–0.966) and VA (OR = 0.713, 95%CI: 0.516–0.985) are negatively associated with hypertension. Linear regression analysis shows a negative correlation between AA (β = -3.89, 95% CI: -7.12 – -0.66) and the systolic blood pressure (SBP) levels, and a significant negative association between iso-VA (β = -2.11, 95% CI: -3.94 – -0.29) and diastolic blood pressure (DBP) levels. Whether in unadjusted or adjusted linear regression models, we all observe significant positive associations between CA and blood pressure levels. In the Bayesian kernel-machine regression (BKMR) models, the trends between the mixture of SCFAs and hypertension, SBP are inverse, but not significant; we also observe a significant negative correlation between AA and SBP, and a significant negative association between iso-VA and DBP levels, while CA is significantly positively correlated with SBP and DBP. Collectively, our results advocate for considering SCFA as a potential intervention to lower blood pressure, and especially AA may be a possible target for research. This may provide new perspectives for understanding the role of SCFAs in hypertension.
Similar content being viewed by others
Introduction
Hypertension is typically associated with diabetes, obesity, and metabolic syndrome, serving as a primary risk factor for cardiovascular diseases1. It is estimated that there are approximately 1.39 billion adults globally are afflicted with hypertension, with three-quarters residing in developing nations and one-quarter in developed countries2. The global burden of hypertension is increasing and it is estimated that by 2025, one-third of the global population will be affected due to accelerated population aging and increasing rates of obesity3. The prevalence of hypertension among Chinese populations was 25.2% in 2012 and increased to 27.5% in 2018. Overall prevalence of hypertension among Chinese populations is on the rise5and this trend will continue for next decades4. In contrast to the high prevalence rate, the hypertension control rate for individuals aged 60 and above in China was low, e.g., 16.1% in 2012 and 18.2% in 2015, indicating that there is still a significant gap between the control rate of hypertension in Chinese elderly people and the requirement for healthy aging6.
The prevalence of hypertension among senior Chinese (aged 60 and above) is 59.2%, and 56.8% of whom have been discharged from hospitals for hypertension5, indicating that the elderly population is more severely affected. In 2017, high systolic blood pressure (SBP) led to the deaths of 2.54 million people in China, with 95.7% of them dying from cardiovascular diseases, highlighting the substantial burden posed by hypertension among the Chinese population7. Treating hypertensive patients can reduce 803,000 cardiovascular events and gain 1.2 million quality-adjusted life years (QALY) annually compared to maintaining the current situation7. Therefore, hypertension is a crucial public health issue currently faced in China.
Short-chain fatty acids (SCFAs) are known to activate various signaling pathways within the host, primarily produced by the metabolism of gut microbiota, with a small amount coming from dietary fats and liver metabolism, directly or indirectly contributing to human health8. Growing experimental evidence from rodents indicates that the gut microbiota is associated with blood pressure and SCFAs can regulate blood pressure9,10,11,12. There are some population studies showing that the level of SCFAs in the feces of hypertensive patients is higher than those of normotensive subjects13,14,15,16, and that the level of SCFAs in the serum of patients with hypertension is lower than healthy individuals13. Besides, increasing the level of SCFAs has the effect of lowering blood pressure17,18. These studies mainly focus on the relationship between SCFAs and blood pressure in the elderly. However, as far as we know, only one study from the Guangxi region has been reported in China. Due to the vast area of China, in order to eliminate the influence brought by geographical differences, we therefore conducted the study again in the Lu’an region in order to obtain comprehensive results and reveal the potential association between SCFAs and blood pressure. Though substantial amounts of studies have been conducted, our comprehension of the significance of SCFAs in human health remains restricted. This report is a cross-sectional study, we used a sample of Chinese older adults to investigate the potential association between serum SCFAs and blood pressure, separately and as the mixture. Our study is not conclusive but provides some new perspectives to understanding the role of SCFAs in human health which may lead to new means for the prevention and treatment of hypertension.
Materials and methods
Study population
We conducted a baseline study from June to September 2016 in Lu’an, China, in a cohort program called Health and Environmentally Controllable Factors in Older Adults. As the largest city in Anhui Province, Lu’an has an area of 15,451 square kilometers, with 5.8361 million people. Elderly individuals in the group were recruited by the local Center for Disease Control and Prevention together with us. Our studies have identified several risk factors related to the health of elderly people, such as hypertension drugs19, antibiotics20,21, and phthalates22.
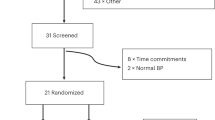
Our previous article describes the details of the study design in detail20. Briefly, we first randomly selected two counties from Lu’an and then randomly selected one community in each county, all participants’ age ≥ 60 years, resided in the local area for at least six months, provided an informed consent, while those with mental illness or limited mobility were excluded. We made phone appointments with 1,217 older adults, 1,080 of whom completed face-to-face interviews. Participants without demographic information (N = 67) were excluded. Finally, 1,013 individuals were included in our study (Fig. 1). The process of conducting the questionnaire survey was thoroughly explained in our previous article23. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Anhui Medical University (No. 20170284).
Blood sample collection and physical measurement
Before collecting blood, all included participants were asked to provide their blood samples in the morning after at least an 8-h overnight fast. Upon arriving at the community health service center in the morning, a 10 ml venous blood sample is placed by a medical professional into serum separation gel tube, and gently mixed by inverting 5–6 times, allowed to stand for 30 min. The collection samples were centrifuged at 4 °C and 1000 g for 5 min. Afterwards, the samples were transported in dry ice to the local Center for Disease Control and Prevention for temporary storage at -20 °C. After all the samples were collected, they were transported to the Experimental Centre of Anhui Medical University by cold-chain truck and stored in a refrigerator at -80 °C until further analysis. When measuring height and weight, participants were required to remove their shoes, hats, and outerwear, wear lightweight clothing, had both heels together, and used a mechanical stadiometer for measuring the height of each participant. Furthermore, a lever scale was utilized to measure the weight of each participant.
A tabletop mercury column sphygmomanometer was used to measure morning blood pressure (BP). Participants were instructed to maintain in a resting condition for more than 5 min before BP was measured. We measured BP in the right arm, and BP values (mmHg) were taken as the average of three times. The following conditions are considered as hypertension: (1) either systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg; (2) a self-reported history of hypertension; or (3) currently taking antihypertensive medications24.
Detection of short-chain fatty acids
There is still lack of a precise method available for detecting serum SCFAs in human , we developed an Ultra High Performance Liquid Chromatography-Quadrupole-Exactive-Orbitrap-Mass Spectrometry (UHPLC-QE-Orbitrap MS) method based on 3-nitrophenylhydrazine derivatization in negative electrospray ionization through parallel reaction monitoring mode for the simultaneous detection of SCFAs in the serum, and the analysis was performed on an Agilent Infinity Lab Proshell 120 EC-C18 column (2.1 mm × 100 mm, 2.7 μm, Agilent, Santa Clara, CA,USA). It demonstrated stability, sensitivity, and accuracy. The specific operation and instruments required for this method to measure SCFAs had been described in detail in our previous study25.
Statistical analysis
When conducting statistical analysis, values below the limit of detection (LOD) of SCFAs are treated as half of the LOD26. We used chi-square tests and t-tests to analyze the differences between the normotensive and hypertensive populations in terms of demographic variables such as gender and age, and analysis of the association between seven SCFAs and hypertension in the elderly using binary logistic regression models. When linear regression models were used to assess the association between SCFAs and blood pressure values, we excluded 278 older adults taking antihypertensive medication because it affects blood pressure values, which may introduce confounding variables that obscure the association between SCFAs and blood pressure values. In addition, these drugs were diverse and might have different effects. We calculated the Spearman correlation coefficients among SCFAs to assess potential confounding factors or collinearity in the regression analysis. To determine whether there is a non-linear and non-additive association between SCFAs and blood pressure, we used a Bayesian kernel machine regression (BKMR) approach, which estimates the multivariate exposure–response function utilizing a kernel machine regression model, allowing specific exposures to be set at particular quantile values and examining the association between residual exposure and results, it also allows a visual representation of the estimated exposure–response function27. We used 100 00 iterations to fit the BKMR models and presented the exposure response function in 4 different visualizations by the BKMR approach: (1) overall effect of SCFAs was obtained through comparing specific percentiles (25th, 50th and 75th percentile) of all the SCFAs with their 50th percentile; (2) univariate exposure–response curves showed the association of one SCFA with the result when all other SCFAs were fixed at their medians of exposure levels; (3) single-exposure effects (95% CI), defined as the change in the odds of hypertension associated with single SCFA as concentrations of other SCFAs set at particular percentiles (i.e., 25th, 50th, and 75th); and (4) bivariate exposure response functions were used to indicate potential interaction effects in SCFAs mixture. In the BKMR models, posterior inclusion probabilities (PIPs) represented the weight of each SCFAs in the health effect.
In addition, we used restricted cubic splines (RCS) to further investigate the potential non-linear relationship between SCFAs and blood pressure. Based on the concentration distribution of SCFAs, we applied 3 knots at specific locations.
In the sensitivity analysis, we included older adults taking antihypertensive drugs to verify the robustness of our main analysis.
To reduce the interference of confounding factors, we included variables such as age (60 ≤ age < 70 years old; age ≥ 70 years old), gender(male; female), education(illiteracy; primary school; middle school; high school and above), household income (< 30,000yuan/year; ≥ 30,000yuan/year; decline to answer), body mass index (BMI = weight (kg)/height^ 2 (m)), smoking status(no and yes), alcohol drinking status(no and yes), diabetes(no and yes) and taking antihypertensive medication(no and yes) as covariates in the models for the analysis. Of these covariates, BMI was divided into four groups (BMI < 18.5 kg/m2;18.5 ≤ BMI < 24 kg/m2; 24 ≤ BMI < 28 kg/m2, BMI ≥ 28 kg/m2). Smoking was defined as “yes” if the participant had smoked more than one cigarette per day and for above six months. Drinking was described as “yes” if the participant had drunk once or more per week and for over six months. Diabetes was determined as a history of self-declared diabetes, insulin or glucose-lowering drug use, or fasting glucose ≥ 7.0 mmol/L. Taking antihypertensive medication was defined as “yes” if the participant took antihypertensive medication at least once a day in last month.
We used the bkmr package and rms package in R software (version 4.3.0) to build the BKMR models and RCS models. We performed all remaining analyses using Statis-tical Package for the Social Sciences (SPSS, version 27.0), and statistical significance was defined as having a p-value less than 0.05.
Results
Characteristics of the study population
The demographic profile of the study participants was presented in Table 1. The study subjects comprised 1,013 elderly individuals with an average age of 71.31 years. The mean SBP was 139.53 ± 20.83 mm Hg and the mean DBP was 80.50 ± 10.70 mm Hg. Among all participants, 55.68% were female, 46.10% were illiterate, 59.52% household income lower than RMB 30,000, and 43.63% BMI in the normal range of values. Most of those subjects were also non-smokers or non-drinkers. Besides, 30.70% had no hypertension and 69.30% had hypertension. Among the hypertensive population, 39.60% were taking antihypertensive medication. Table 1 also showed a comparison of the levels of 7 SCFAs between the normal blood pressure population and the one with hypertension. There were no significant differences in age, gender, education, annual household income, smoking status, drinking status and level of SCFAs. The detection probability (DP) and LODs of the 7 SCFAs were shown in Table 2. The LODs for acetic acid (AA), propanoic acid (PA), butyric acid (BA), isobutyric acid(iso-BA), valeric acid.
(VA), isovaleric acid (iso-VA), and caproic acid (CA) were 2,000 pg/ml, 20 pg/ml, 0.5 pg/ml, 0.2 pg/ml, 10 pg/ml, 1 pg/ml, and 50 pg/ml, respectively, and except the DP of VA was 99.61%, the remaining SCFAs were 100%. The Spearman’s rank correlation coefficients between SCFAs were displayed in Figure S1, and SCFAs were significantly correlated with each other (all P-values were less than 0.05), with Spearman’s rank correlation (rs) values ranging from 0.12 to 0.67.
Associations between single SCFA and blood pressure
The outcome of between single SCFA and hypertension analyses were shown in Table 3. Significant correlations are not found in the unadjusted model nor in the adjusted single-SCFA model.
Following adjustment for covariates and remaining SCFAs, the significant negative association of AA (OR = 0.696, 95%CI: 0.501–0.966) and VA (OR = 0.713, 95%CI: 0.516–0.985) with hypertension are observed (Table 3).
The association between SCFAs and blood pressure were shown in Table 4. After adjusting for age, gender, education, household income, BMI, smoking status, alcohol drinking status, diabetes and other SCFAs, we observe a significant negative association of AA (β = -3.89, 95% CI: -7.12 – -0.66) with the SBP levels, and a significant negative association between iso-VA (β = -2.11, 95% CI: -3.94 – -0.29) and DBP levels. Whether in unadjusted or adjusted linear regression models, we all observe significant positive associations between CA and BP levels.
Associations between the SCFAs mixture and blood pressure
Figure 2A demonstrated the joint effect of the mixture of SCFAs on hypertension, BKMR plots reveal an inverse trend between the mixture of SCFAs and hypertension, even if not statistically significant. The relationships between single SCFA and hypertension were illustrated in Fig. 2B, with the remaining SCFAs set at their median levels, in line with findings from logistic regression models, AA, VA, and iso-VA exhibit an inverse trend with the hypertension. Figure 2C exhibited the change in the odds of hypertension associated with single SCFA as concentrations of other SCFAs set at particular percentiles (i.e., 25th, 50th, and 75th). Eventually, bivariate exposure response functions were used to indicate potential interaction effects in SCFAs mixture (Fig. 2D). The bivariate interaction plots visualized by BKMR do not show a strong interaction between SCFAs and hypertension (Fig. 2C and 2D). The PIPs for conditional inclusion into the BKMR model between SCFAs and hypertension were summarized in Table S1, and CA has the highest PIP value (PIP = 0.275).
Figure S2A showed the analysis of the joint effect of mixture of SCFAs on SBP, with BKMR plots. The result shows that there is an inverse trend between mixture of SCFAs and SBP levels, although not significant. The relationships between single SCFA and SBP were illustrated in Figure S2B, with the remaining SCFAs set at their median levels, which exhibited the single dose–response association and the 95% CI. Similar to the results of the linear regression models, we observe a significant negative correlation between AA and SBP, while CA is significantly positively correlated with SBP. Figure S2C indicated the change in the effect of single SCFA on SBP levels when the remaining SCFAs were kept at specific percentiles (i.e., 25th, 50th, and 75th). Finally, bivariate exposure–response curves for SCFAs were evaluated (Figure S2D). The bivariate interaction plots visualized by BKMR do not show a strong interaction between SCFAs and SBP (Figure S2C and S2D). Table S2 summarized the PIP values between SCFAs and SBP in BKMR model in which AA has the highest PIP value (PIP = 0.156). The joint effect analysis of SCFAs mixture on the DBP was displayed in Figure S3A. The BKMR plots indicate that there is no association between mixture of SCFAs and DBP levels. Figure S3B showed the exposure–response association and 95% CI for single SCFA. Similar to the results of the linear regression models, we observe a significant negative association between iso-VA and DBP levels, and CA is significantly positively correlated with DBP. Figure S3C indicated the variation in the effect of single SCFAs on DBP levels when the remaining SCFAs were kept at specific percentiles (i.e., 25th, 50th, and 75th). Finally, bivariate exposure–response curves for SCFAs were evaluated (Figure S3D). The bivariate interaction plots visualized by BKMR also do not show a strong interaction of SCFAs on diastolic blood pressure (Figure S3C and S3D). The PIPs for conditional inclusion into the BKMR model between SCFAs and DBP were summarized in Table S3, and PA has the largest PIP value (PIP = 0.399).
The results of the RCS were shown in the supplemental material Figure S4-6. SCFAs are not nonlinearly associated with the prevalence of hypertension, SBP and DBP levels (all P for nonlinear > 0.05).
Sensitivity analyses
After excluding 278 older adults who were taking antihypertensive medication, we further analyzed logistic regression and BKMR models with the presence of hypertension as the dependent variable. Similar results to the primary outcome were observed (Table S4 and Figure S7), but the joint effect of SCFAs (Figure S7A) no longer shows an inverse trend with hypertension, which might be due to the insufficient number of cases, making it difficult for us to detect potential association. Whereas, in the linear regression and BKMR models analyses with systolic and diastolic blood pressure as dependent variables, we included older adults who were taking antihypertensive medication, and the results shows that our findings are robust (Table S5 and Figures S8-9).
Associations between 7 SCFAs and the hypertension among older adults were estimated using Bayesian kernel machine regression (BKMR). Adjustment variables for BKMR are consistent with logistic and linear regression models, including age, sex, education, household income, BMI, smoking status, alcohol drinking status and diabetes. (A) overall effect of SCFAs was obtained through comparing specific percentiles (25th, 50th and 75th percentile) of all the SCFAs with their 50th percentile; (B) univariate exposure–response curves showed the association of one SCFA with the hypertension when all other SCFAs were fixed at their medians of exposure levels; (C) single-exposure effects (95% CI), defined as the change in the odds of hypertension associated with single SCFA as concentrations of other SCFAs set at particular percentiles (i.e., 25th, 50th, and 75th); and (D) bivariate exposure response functions were used to indicate potential interaction effects in SCFAs mixture. Abbreviation: AA acetic acid; PA propanoic acid; BA butyric acid; iso-BA isobutyric acid; VA valeric acid; iso-VA isovaleric acid; CA caproic acid.
Discussion
In our study, we used logistic regression and BKMR approaches for comprehensively evaluating the single and joint associations between serum SCFAs and hypertension, and comprehensively assessed single and joint associations between SCFAs and blood pressure values using linear regression and BKMR models. In our study, individual associations of AA and VA with the odds of hypertension in logistic regression models are significantly negative, while their independent associations in BKMR are also negative but not significant. Overall, we can find that SCFAs mixture are negatively linked with the odds of hypertension, although no significance is observed, this may be because single SCFA analyses underestimate the protective effect of SCFAs on blood pressure levels in the elderly and mixture analyses are warranted.
In the linear regression and BKMR models, we observe a significant negative association of AA with the SBP levels, a significant negative association between iso-VA and DBP levels, and CA is significantly positively correlated with SBP and DBP. In addition, the BKMR plots show that there is no association of SCFAs mixture with SBP and DBP levels, but we can observe that the SCFAs mixture has an opposite trend to the SBP (albeit not statistically significant). To our knowledge, this study represents the initial investigation into the correlation between serum SCFAs and BP among elderly Chinese individuals.
Analyses of single SCFA
In our study, we observe that there is a negative correlation between AA, VA and hypertension. Specifically, we observe a significant negative correlation between AA and SBP, and a significant negative association between iso-VA and DBP levels, while CA is significantly positively correlated with SBP and DBP. These results are biologically reasonable. In line with our data, previous studies have shown that supplementation with oral acetate28,29,30and valerate31reduces blood pressure in animal experiments. SCFAs can regulate blood pressure through the G protein-coupled receptors (GPCRs) which play a significant role in the development of hypertension32,33,34. SCFAs were found to interact with at least four GPCRs to affect host blood pressure35, namely GPCR41, 43, 109A, and olfactory G protein-coupled receptor 78 (olfr78). Acetate is one of the most potent agonists of GPCR41 and GPR4336, and AA promotes the release of intracellular calcium ions through the activation of GPCR41 and GPCR43, thereby lowering blood pressure37. The PA can partially regulate the renin levels through olfr78, thus increasing blood pressure and antagonizing the hypotensive effect mediated by GPCR4138. CA were shown to enhance differentiation and proliferation of T helper 1 (Th1) and Th17 cells and to suppress that of Treg lymphocytes, thus supporting inflammation39,40, this could explain why we observe a significant positive correlation between CA and SBP, DBP. In addition, animal studies have shown that SCFAs promote the level of interleukin-10 (IL-10)41, a GPCR43-mediated anti-inflammatory factor, and attenuates the inflammatory response mediated by helper T cells42and regulatory T cells42,43. Furthermore, hyperactivation of the sympathetic nervous system is one of the key hallmarks of hypertension44. It has been shown that SCFAs can directly modulate the sympathetic nervous system via GPCR4145. Therefore, we speculate that AA and VA may negatively regulate blood pressure through above mentioned pathways. In supporting this speculation, a study of long-lived people in Guangxi, China, found that AA counteracts the damage caused by hypertension to maintain a healthy body balance46. A cohort study including participants from six different ethnic groups showed that participants with lower SBP tended to have lower levels of AA in their feces, which may be due to the fact that more SCFAs are absorbed into the body and thus less being excreted out of the body47.
Animal studies have shown that PA and BA both lower blood pressure in mice30,48,49,50. Cross-sectional human studies have also shown that there is a negative correlation between serum BA and hypertension13,51. It has also been shown that butyrate levels are negatively correlated with portal hypertension52and can be potentially used for blood pressure-lowering interventions53, e.g., a randomized, double-blind, placebo-controlled trial has shown that butyrate can reduce blood pressure in patients with type 2 diabetes mellitus54. In line with these reports, our study also show that there is a negative correlation between butyric acid and hypertension in Chinese elderly population, although not statistically significant.
From the current studies conducted in young and older adults, the findings are nearly identical, i.e., AA may be a potential treatment for hypertension; our cohort study was specifically conducted in older adults and called Health and Environmentally Controllable Factors in Older Adults, and therefore there are no data from the younger populations in the same geographic area, and future studies need to focus on this issue. Most notably, our findings may only apply to older adults in Lu 'an City and cannot be generalized to the entire population.
Analyses of SCFAs mixture
Epidemiological studies on the association between SCFAs mixture in serum and blood pressure are limited. As far as we are concerned, previous studies have not addressed this issue. In this study, we applied the BKMR models to explore the correlation between the SCFAs mixture in serum and blood pressure. We observe that the mixture of SCFAs has an inverse trend with hypertension and SBP, but not significant. Yet, the relationship between SCFAs mixture in serum and hypertension, blood pressure still warrants further investigation.
Strengths and limitations
This study has the following advantages: (1) This is the first study to explore the correlation between serum SCFAs and hypertension use elderly residents in China and our results provide initial indications for further studying the influence of serum SCFAs mixture on hypertension; (2) In this report, BKMR model was used to investigate potential non-linear, holistic and interactive relationships between mixture of SCFAs and hypertension, blood pressure; and (3) The chemical properties of SCFAs are unique, and currently there is still lack of a precise method available for detecting serum SCFAs in human, we developed an UHPLC-QE-Orbitrap MS method that demonstrated stability, sensitivity, and accuracy.
Nevertheless, several limitations should be noted. Firstly, owing to the constraints of the cross-sectional design, we were unable to establish a causal relationship between serum SCFAs mixture and hypertension or blood pressure. Secondly, only one-time measurement of serum SCFAs was conducted for elder individuals, which may not adequately illustrate the long-term SCFAs levels in older adults and could introduce measurement bias. Thirdly, we can’t rule out the effects of all confounding factors, such as antibiotics, as most SCFAs in vivo are produced by the fermentation of dietary fibers by the gut microbiome, which may affect the abundance and community of the gut microbiome. Fourthly, we limited our analyses to 7 SCFAs that were most abundant in our samples, although other SCFAs may also be involved in the regulation of blood pressure. Fifthly, the collection or storage of biological samples may affect the stability of SCFAs, which may introduce bias into the measurement of SCFAs concentrations. Sixthly, several lifestyle components were self-reported and thus, recall bias is inevitable. For example, the missing use of medications such as anti-inflammatory drugs and antibiotics is a limitation of our study. Seventhly, in addition to diabetes, some individuals may also suffer from other comorbidities of hypertension, which may prompt study subjects to undergo drug treatment or lifestyle changes. This could lead to changes in their SCFAs concentrations, thereby forming a reverse causal relationship. Finally, our findings may only apply to older adults in Lu 'an City and cannot be generalized to the entire population.
Conclusions
In conclusion, while the precise mechanism underlying SCFAs’ impact on blood pressure remains elusive, our results advocate for considering SCFA as a potential intervention to lower blood pressure, and especially AA may be a possible target for research. Further investigation is warranted to confirm these preliminary findings and to gain deeper insights into the mechanisms through which SCFAs contribute to blood pressure regulation.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to the privacy of our research group, but are available from the corresponding author on reasonable request.
References
Virani, S. S. et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee (2021). Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation, 143(8), e254–e743. https://doi.org/10.1161/CIR.0000000000000950 (2021).
Mills, K. T. et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134(6), 441–450. https://doi.org/10.1161/CIRCULATIONAHA.115.018912 (2016).
Oliveros, E. et al. Hypertension in older adults: assessment, management, and challenges. Clinical cardiology 43(2), 99–107. https://doi.org/10.1002/clc.23303 (2020).
Fang, L. et al. Prevalence and characteristics of hypertension in mainland Chinese adults over decades: a systematic review. Journal of human hypertension 28(11), 649–656.https://doi.org/10.1038/jhh.2014.5 (2014).
National Health Commission of the People’s Republic of China. 2022 China health statistics yearbook. Peking Un-ion Medical College Press, (in Chinese) Available online: http://www.nhc.gov.cn/mohwsbwstjxxzx/tjtjnj/202305/6ef68aac6bd14c1eb9375e01a0faa1fb.shtml (accessed on 15 No-vember 2023) (2022).
Li Suning, Chen Jo, Wang Zengwu, et al. Analysis of the current situation of hypertension among the elderly in China. Chinese Journal of Hypertension, 27(02): 140–148. (in Chinese) https://doi.org/10.16439/j.cnki.1673-7245.2019.02.002 (2019).
Dietary guidelines for adults with hypertension (2023 Edition). Clinical and education in family medicine, 21(06): 484–485. (in Chinese) https://doi.org/10.13558/j.cnki.issn1672-3686.2023.006.002 (2023).
Xu, J., Moore, B. N. & Pluznick, J. L. Short-chain fatty acid receptors and blood pressure regulation: council on hypertension mid-career award for research excellence 2021. Hypertension 79 (10), 2127–2137. https://doi.org/10.1161/HYPERTENSIONAHA.122.18558 (2022).
Mell, B. et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiological genomics 47(6), 187–197. https://doi.org/10.1152/physiolgenomics.00136.2014 (2015).
Hsu, C. N. et al. Targeting on gut microbial metabolite trimethylamine-N-oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Molecular nutrition & food research 63(18). https://doi.org/10.1002/mnfr.201900073 (2019).
Hsu, C. N. et al. Perinatal resveratrol therapy prevents hypertension programmed by maternal chronic kidney disease in adult male offspring: implications of the gut microbiome and their metabolites. Biomedicines 8(12), 567. https://doi.org/10.3390/biomedicines8120567 (2020).
Hsu, C. N. et al. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. The Journal of nutritional biochemistry 108, https://doi.org/10.1016/j.jnutbio.2022.109090 (2022).
Calderón-Pérez, L. et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Scientific reports 10(1), 6436. https://doi.org/10.1038/s41598-020-63475-w (2020).
Huart, J. et al. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension 74(4), 1005–1013. https://doi.org/10.1161/HYPERTENSIONAHA.118.12588 (2019).
de la Cuesta-Zuluaga, J. et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 11(1), 51. https://doi.org/10.3390/nu11010051 (2018).
Huart, J. et al. Human stool metabolome differs upon 24 h blood pressure levels and blood pressure dipping status: a prospective longitudinal study. Metabolites 11(5), 282. https://doi.org/10.3390/metabo11050282 (2021).
De Filippis, F. et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65(11), 1812–1821.https://doi.org/10.1136/gutjnl-2015-309957 (2016).
Chen, L. et al. Modest sodium reduction increases circulating short-chain fatty acids in untreated hypertensives: a randomized, double-blind. Placebo-Controlled Trial. Hypertension 76(1), 73–79. https://doi.org/10.1161/HYPERTENSIONAHA.120.14800 (2020).
Jia-Ning Z, Lin-Sheng Y, Dong-Mei Z, et al. The prevalence and associated factors of antihypertensive medication use in urban and rural community-dwelling elderly with hypertension in Lu’an City. Chinese Journal of Disease Control & Prevention, 21(10): 979–982. (in Chinese)https://doi.org/10.16462/j.cnki.zhjbkz.2017.10.003 (2017).
Li, Z. et al. Antibiotics in elderly Chinese population and their relations with hypertension and pulse pressure. Environmental science and pollution research international 29(44), 67026–67045. https://doi.org/10.1007/s11356-022-20613-3 (2022).
Zhu, Y. et al. Antibiotic body burden of elderly Chinese population and health risk assessment: A human biomonitoring-based study. Environmental pollution 256, https://doi.org/10.1016/j.envpol.2019.113311 (2020).
Li, Y. L. et al. The levels of phthalate exposure and associations with obesity in an elderly population in China. Ecotoxicology and environmental safety 201, https://doi.org/10.1016/j.ecoenv.2020.110749 (2020).
Li, X. D. et al. Adhering to a vegetarian diet may create a greater risk of depressive symptoms in the elderly male Chinese population. Journal of affective disorders 243 , 182–187. https://doi.org/10.1016/j.jad.2018.09.033 (2019).
Williams, B.et al. ESC Scientific Document Group 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J., 39(33), 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Wang, S. et al. An UHPLC-QE-Orbitrap-MS method for accurate quantification of short-chain fatty acids in serum from an older Chinese population. Chromatographia 87(2), 125–136. https://doi.org/10.1007/s10337-023-04304-w (2024).
Shi, Z. X. et al. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk. Environmental science & technology 43(12), 4314–4319. https://doi.org/10.1021/es8035626 (2009).
Bobb, J. F. et al. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environmental health : a global access science source 17(1), 67. https://doi.org/10.1186/s12940-018-0413-y (2018).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135(10), 964–977. https://doi.org/10.1161/CIRCULATIONAHA.116.024545 (2017).
Ganesh, B. P. et al. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72(5), 1141–1150. https://doi.org/10.1161/HYPERTENSIONAHA.118.11695 (2018).
Robles-Vera, I. et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Molecular nutrition & food research 64(6), https://doi.org/10.1002/mnfr.201900616 (2020).
Onyszkiewicz, M. et al. Valeric acid lowers arterial blood pressure in rats. European journal of pharmacology 877, https://doi.org/10.1016/j.ejphar.2020.173086 (2020).
Brinks, H. L. & Eckhart, A. D. Regulation of GPCR signaling in hypertension. Biochimica et biophysica acta 1802(12), 1268–1275. https://doi.org/10.1016/j.bbadis.2010.01.005 (2010).
Drummond, G. R. et al. Immune mechanisms of hypertension. Nature reviews. Immunology 19(8), 517–532. https://doi.org/10.1038/s41577-019-0160-5 (2019).
Vieira-Rocha, M. S. et al. Insights into sympathetic nervous system and GPCR interplay in fetal programming of hypertension: a bridge for new pharmacological strategies. Drug discovery today 25(4), 739–747. https://doi.org/10.1016/j.drudis.2020.01.019 (2020).
Tan, J. K. et al. Metabolite-sensing g protein-coupled receptors-facilitators of diet-related immune regulation. Annual review of immunology 35, 371–402. https://doi.org/10.1146/annurev-immunol-051116-052235 (2017).
van der Hee, B. & Wells, J. M. Microbial regulation of host physiology by short-chain fatty acids. Trends in microbiology 29(8), 700–712. https://doi.org/10.1016/j.tim.2021.02.001 (2021).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. The Journal of biological chemistry 278(28), 25481–25489. https://doi.org/10.1074/jbc.M301403200 (2003).
Pluznick, J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut microbes 5(2), 202–207. https://doi.org/10.4161/gmic.27492 (2014).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43(4), 817–829. https://doi.org/10.1016/j.immuni.2015.09.007 (2015).
Saresella, M. et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. 11, 1390. https://doi.org/10.3389/fimmu.2020.01390 (2020).
Sun, M. et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nature communications 9(1), 3555. https://doi.org/10.1038/s41467-018-05901-2 (2018).
Liu, Y. J. et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10(12), 5225–5241. https://doi.org/10.7150/thno.43716 (2020).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480), 451–455. https://doi.org/10.1038/nature12726 (2013).
Grassi, G., Mark, A. & Esler, M. The sympathetic nervous system alterations in human hypertension. Circulation research 116(6), 976–990. https://doi.org/10.1161/CIRCRESAHA.116.303604 (2015).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proceedings of the National Academy of Sciences of the United States of America 108(19), 8030–8035. https://doi.org/10.1073/pnas.1016088108 (2011).
Zhang, Q. et al. Protection effect of gut microbiota composition and acetate absorption against hypertension-induced damages on the longevity population in Guangxi. China. Frontiers in nutrition 9, 1070223. https://doi.org/10.3389/fnut.2022.1070223 (2023).
Verhaar, B. J. H. et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. European heart journal 41(44), 4259–4267. https://doi.org/10.1093/eurheartj/ehaa704 (2020).
Pluznick, J. L. et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences of the United States of America 110(11), 4410–4415. https://doi.org/10.1073/pnas.1215927110 (2013).
Bartolomaeus, H. et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139(11), 1407–1421. https://doi.org/10.1161/CIRCULATIONAHA.118.036652 (2019).
Wang, L. et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. Journal of hypertension 35(9), 1899–1908. https://doi.org/10.1097/HJH.0000000000001378 (2017).
Kim, S. et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clinical science 132(6), 701–718. https://doi.org/10.1042/CS20180087 (2018).
Juanola, O. et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33(10), 11595–11605. https://doi.org/10.1096/fj.201901327R (2019).
Tilves, C. et al. Increases in circulating and fecal butyrate are associated with reduced blood pressure and hypertension: results from the SPIRIT trial. Journal of the American Heart Association 11(13), https://doi.org/10.1161/JAHA.121.024763 (2022).
Roshanravan, N. et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: a randomized double-blind. Placebo-Controlled Trial. Hormone and metabolic research 49(11), 886–891. https://doi.org/10.1055/s-0043-119089 (2017).
Acknowledgements
We appreciate the participants in the Lu’an Cohort Study. We acknowledge the cooperation of all the participants, staff, and administrators of this study.
Funding
This work was supported by the Research Fund for Scientific Research Level Improvement Plan of Anhui Medical University (2022xkjT007), Research Funds of Center for Big Data and Population Health of IHM (JKS2023018) and National Natural Science Foundation of China (82073558).
Author information
Authors and Affiliations
Contributions
Conceptualization, Jiamou Zhou and Dongmei Zhang; methodology, Jiamou Zhou; software, Jiamou Zhou and Heqiao Zhang; validation, Jiamou Zhou, Heqiao Zhang, Pengcheng Huo, Huiyan Shen and Qian Huang; formal analysis, Jiamou Zhou; re-sources, Linsheng Yang , Annuo Liu , Guimei Chen, Fangbiao Tao; data curation, Kaiyong Liu and Dongmei Zhang; writing—original draft preparation, Jiamou Zhou; writing—review and editing, Jiamou Zhou and Dongmei Zhang; funding acquisition, Kaiyong Liu and Dongmei Zhang. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in accordance with the Decla-ration of Helsinki, and approved by the Ethics Committee of Anhui Medical University (No. 20170284).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Zhang, H., Huo, P. et al. The association between circulating short-chain fatty acids and blood pressure in Chinese elderly population. Sci Rep 14, 27062 (2024). https://doi.org/10.1038/s41598-024-78463-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78463-7
Keywords
This article is cited by
-
Masked hypertension in irritable bowel syndrome: A cause for concern?
Indian Journal of Gastroenterology (2025)
-
Probiotics and Prebiotics in Post-Myocardial Infarction Rehabilitation: Mechanisms, Benefits, and Future Directions
Current Nutrition Reports (2025)