Abstract
Humidity influences the life table parameters and foraging behaviours of various terrestrial arthropods. The soil mite, Blattisocius mali Oudemans is a potential biological control agent of some acarid mites, moths, and nematodes. In the current study, we investigated the functional response of B. mali preying on the eggs of the mould mite Tyrophagus putrescentiae Schrank (Acari: Acaridae) at different humidity levels between 33% and 92%. To determine the type of functional response, we used logistic regression and a generalized functional response equation suggested by Real. The functional response parameters were estimated using models proposed by Hassell and Cabello et al. Blattisocius mali exhibited Type II functional response at 33% and Type III at other tested humidities (52%, 72%, 82%, and 92%). The potential for prey mortality (α) was the highest, i.e., 0.05923, and the handling time was the shortest, i.e., 0.00463 day, at 92% humidity, indicating the highest efficiency of B. mali at this humidity. Our findings revealed that B. mali was more efficient at higher humidity levels as compared to lower humidity levels. Humidity affected the predation rate and might have played an important role in stabilizing the predator–prey system by shifting the functional response with humidity.
Similar content being viewed by others
Introduction
The concentration of carbon dioxide in the atmosphere is steadily increasing, leading to a rise in global atmospheric temperature by 2.0–4.5 °C by the year 2100. This rise in temperature results in the modification of precipitation patterns1. The warming and alterations in precipitation can directly impact soil temperature, moisture2,3, and humidity4. Humidity in turn can constrain the growth and population dynamics of various microarthropods at optimal temperatures, especially if the animal expends energy to balance hydration at the expense of reproduction5. Humidity plays a significant role in determining the activity, distribution patterns, and species richness of terrestrial arthropods6. Terrestrial arthropods include mites are particularly susceptible to water loss due to their high surface-to-volume ratio7. Fluctuations in humidity can cause biochemical, physiological, and behavioural changes in arthropods6,8 including their functional responses.
The functional response is an important aspect of predator-prey or parasitoid-host interaction and is crucial for the dynamics of populations and communities of animals9,10,11,12,13. It represents the relationship between the number of prey or hosts successfully attacked by a predator or parasitoid and the density of prey in the environment. There are four types of functional responses: Type I, where the consumption rate increases linearly until reaching a constant plateau; Type II, where the consumption rate approaches the asymptote in a hyperbolic manner as the prey density increases; Type III, where the consumption rate initially increases until reaching the inflection point of a sigmoid curve, followed by a decrease in the proportion of prey killed; and less commonly observed, Type IV, known as the domed type, where the efficiency of predation decreases instead of increasing at certain prey densities10,12,14,15. Among insect and mite predators, all types of functional responses have been documented16,17,18,19,20,21,22. However, as Hassell23 pointed out, the occurrence of Type III may be underestimated under laboratory conditions, and it may be a more common response type than previously believed.
The effectiveness of a predator can be assessed by examining the parameters of functional response, which include the predator’s instantaneous attack rate, the handling time12, and the predator’s potential for prey mortality24. Natural enemies that have high attack rates and short handling times are considered to be the most successful agents for biological control17,25. On the other hand, if a predator is highly efficient and causes significant mortality while reducing prey survival, the potential for causing mortality will be high, and vice-versa24. The type and parameters of functional response in invertebrate predators can be affected by various factors, such as temperature, prey, and predator types, predator age, voracity, hunger level, etc26,27,28.
Studies have indicated that rising temperatures have the potential to alter the functional response pattern from Type II to Type III20,21,22 and from Type III to Type II29,30,31. However, there are instances where the functional response type remained unchanged under temperature variations32,33,34,35. Furthermore, shifts from Type III to Type II, induced by warming, may result in the destabilization of predator-prey interactions and the potential extinction of prey species under highly unfavourable conditions32. All these diverse findings suggest that temperature may affect different predator-prey systems differently, depending on their sensitivity to varying temperature levels. Likewise, humidity may affect predator-prey interactions differently.
The mould mite Tyrophagus putrescentiae Schrank (Acari: Acaridae) is an omnivorous acarid mite, common in-house dust, soil with decomposed plant material, and vertebrate nests. It is also a pest of various stored food products and crop plants such as cucumber, gerbera, or bulbs of many ornamental plants36,37 and the heavy reliance on chemical pesticides for its control raises environmental and health concerns38. To combat these problems, low-risk methods, such as biological control have been considered as a possible alternative to chemical pesticides. Production of natural enemies and their utilization in pest control is one of the most important strategies in Integrated Pest Management programmes39.
This study aimed to examine the impact of five levels of relative humidity on the functional response of the predatory mite Blattisocius mali Oudemans towards its prey T. putrescentiae. Blattisocius mali, a type of soil mite, has garnered attention recently due to its potential to control acarid mites, nematodes, and moth pests. It belongs to the family Blattisociidae, in which several species i.e. B. dentriticus Berlese, B. tarsalis Berlese, and B. keegani Fox, have been reported to be the potential biocontrol agents of stored food pests including the mold mite, T. putrescentiae40. By feeding on a mixture of mould mite eggs and larvae, the life table parameters of B. mali were much higher than those of B. dentriticus, also examined in this respect41. Apart from acarid mites, B. mali has been reported to be a potentially effective predator of nematodes42, the eggs of moths43,44 as well as phytophagous insects such as thrips and mites such as spider mites45. Moreover, recent findings revealed that B. mali not only could disperse using drosophilids but also feed on them during transportation and after disembarkment, also prey upon their eggs and larvae45,46,47.
To date, there is no report on the influence of humidity on the predation of soil mites towards different prey densities. Among various climate factors, moisture seems to have the most significant impact on soil organisms, particularly those occupying higher trophic levels such as predatory mites or omnivorous nematodes. These organisms have been observed to significantly increase in abundance in response to available soil moisture48. As humidity has been shown to significantly affect the survival and speed of development of other Blattisocius species, i.e., B. dendriticus and B. keegani49,50, we expected that humidity might be a key factor influencing the dynamics of B. mali and T. putrescentiae interactions and stability of that system. In this paper, we pose a question on to what extent the possible deleterious effect of extreme values of humidity may affect the behaviour of a predatory mite and this way also the functioning of the whole predator-prey system. To determine the changes in the functional response of B. mali solely with humidity, we selected the immobile egg stage as a prey, since, the mobile stages, i.e., larva, protonymph, deutonymph, and adult, of the T. putrescentiae might change the behaviour with changing moisture, and additionally affect predator’s response. Apart from classical parameters of functional responses, instantaneous attack rate, and handling time12, we also compared another parameter, the potential of mortality (α) proposed by Cabello et al.24 to provide a more comprehensive biological interpretation of predator’s behaviour exhibiting Type III functional response.
Results
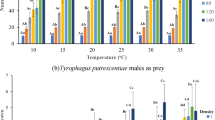
The statistical analysis revealed a significant effect of both humidity (χ2 = 1627.23; df = 4; P < 0.001) and the density of T. putrescentiae eggs offered (χ2 = 829.38; df = 6; P < 0.001) on the mean number of eggs eaten by B. mali. Additionally, the interaction between humidity and density was found to be significant (χ2 = 829.38; df = 6; P < 0.001), implying that the mean number of eggs eaten by the predator depended not only on the specific level of humidity but also on the density of the prey. At low prey densities of 10 and 20 eggs, the mean numbers of eggs eaten by B. mali females were similar at tested humidity levels (Fig. 1). However, in other prey densities, the predators ate significantly fewer eggs at the lowest humidity of 33% compared to higher humidity levels. While there was no significant difference in the number of prey consumed within the humidity range of 52–92% for densities of 40 and 160 eggs, there was a noticeable trend of increasing consumption rate with higher humidity levels for other prey densities.
Logistic regression between the initial egg densities offered and the proportion of eggs eaten revealed that the linear coefficients were positive and the quadratic coefficients were negative at the levels of humidity, 52%, 72%, 82%, and 92%; indicating a Type III functional response. In contrast, both the linear and quadratic coefficients were positive at a humidity level of 33%, making it difficult to determine the exact type of functional response at this level (Table 1). Therefore, the additional method proposed by Real51 was also used to clarify the type of functional response which showed that the value of ‘q’ was not significantly different from zero and Th was greater than zero, at a humidity of 33%; indicating a Type II response. However, at humidity levels of 52%, 72%, 82%, and 92%, both q and Th were found to be greater than zero, indicating a Type III functional response (Table 2).
The shape of the functional response curve exhibited a hyperbolic pattern at a humidity level of 33%, indicating a Type II functional response. However, at higher humidity levels, the curve displayed a nearly sigmoid shape, indicating a Type III functional response (Fig. 2). The functional response curves were drawn based on the models proposed by Hassell12 at 33% humidity, and both Hassell12 and Cabello et al.24 at higher humidities. The curve drawn based on Hassell’s12 model showed that the number of eggs eaten increased with increasing egg densities hyperbolically at 33% humidity. Whereas, the curves drawn based on models suggested by Hassell12 and Cabello et al.24 showed similar results at other humidity levels, i.e., the number of eggs eaten increased with increasing egg densities following the sigmoid shape (Fig. 3).
Estimates of functional response parameters, determined from the model proposed by Hassell12, revealed that B. mali exhibited longer handling times at lower humidities as compared to higher humidities. The handling time was significantly longer at 33% and 52% humidities but decreased with an increase in humidity up to 92% humidity (Table 3). The instantaneous attack rate (a) for Type II was 1.51 day− 1 at 33% humidity and the Functional Response Ratio [FRR52) was 196.5396 day− 2. The predation rate of B. mali was significantly affected by humidity levels (χ2 = 38.18; df = 4; P < 0.01) and increased with increasing humidity levels (Table 3). The parameters estimated from the model suggested by Cabello et al.24 showed that B. mali exhibited a higher potential for prey mortality and shorter handling time at higher humidities as compared to lower humidity levels. The potential for prey mortality was the highest at 92%, followed by 82%, 72%, and 52% humidity levels (Table 4). Conversely, the handling time was the lowest at 92% humidity and increased with the declining humidity up to 52%. Humidity significantly affected both the FRRs52 (χ2 = 35.231; df = 3; P < 0.01) and the predation rate (χ2 = 42.045; df = 3; P < 0.001), which increased clearly with increasing the humidity levels (Table 4).
The effect of five humidity levels and seven densities of Tyrophagus putrescentiae eggs on the mean number (± 95% CI) of the T. putrescentiae eggs eaten by Blattisocius mali. Different lowercase and uppercase letters indicate significant differences between means (P < 0.05) for various prey densities within each humidity level or among different humidity levels, respectively.
Discussion
The results of this study showed that humidity had a significant impact on the functional response of B. mali when preying on T. putrescentiae eggs. When tested across a range of humidity levels from 33% to 92% RH, the predatory females consistently displayed a Type III response, except at the lowest humidity level of 33% where they exhibited a Type II response. At this low humidity level, the predation rate of the mite was the lowest for most prey densities. Additionally, the handling time was significantly longer at 33% and 52% humidity levels compared to higher levels of humidity. Conversely, the potential for prey mortality was much lower at 52% humidity compared to higher humidities. As the humidity increased further, the handling time decreased, reaching its shortest value at 92%. Furthermore, at this particular humidity level, the potential for mortality of T. putrescentiae eggs was the highest for this predatory mite.
Edaphic predatory mites have been observed to exhibit different responses to varying humidity levels to ensure their survival and growth49,50. Although humidity has a direct effect on the survival of soil predatory mites, there is no report on the effect of humidity on the foraging behaviour including the functional response of soil mites. To the best of our knowledge, this paper represents the first report on the effect of humidity on the predation of the soil mite.
According to previous studies, predatory mites predominantly have displayed either Type II or Type III functional responses19. However, there has been limited investigation into the functional responses of predatory soil mites, unlike the well-studied plant-dwelling phytoseiids. The soil mites, B. tarsalis and Macrocheles robustulus Berlese (Mesostigmata: Macrochelidae) exhibited Type II response when exposed to eggs of the potato tuber moth (PTM), Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae)43. Similarly, B. tarsalis showed Type II response to eggs of the Guatemalan potato tuber moth Tecia solanivora Povolny (Lepidoptera: Gelechiidae)53; Stratiolaelaps scimitus Womersley (Mesostigmata: Laelapidae) exhibited Type II to pupae of the western flower thrips Frankliniella occidentalis Pergande (Thysanoptera: Thripidae)54 while S. scimitus and Macrocheles mammifer Berlese (Acari: Macrochelidae) to pupae of the Asian bean thrips Megalurothrips usitatus Bagnall (Thysanoptera: Thripidae)55. The macrochelid mite Macrocheles muscaedomesticae Scopoli (Acari: Macrochelidae) exhibited Type III functional response at 27 °C and Type II at 33 °C when exposed to varying densities of eggs of the house flies Musca domestica L. (Diptera: Muscidae)31.
Type II and Type III functional responses differ significantly about the stability of the predator–prey system. In the case of Type II, prey density is reduced in the following generations and finally becomes extinct due to negatively density-dependent predation. By contrast, in the sigmoid response, predation is initially positively density-dependent up to some threshold which may contribute to the stability of predator-prey system23,56. Invertebrate predators could exhibit different types of functional responses based on various factors, including temperature, prey and predator types, predator age, voracity, hunger level, etc26,27,28. As shown by the study of Daugaard et al.26 on climate warming effects in ciliates, an increase in temperature could lead to the shift from Type III to Type II functional response, resulting in destabilization of the predator-prey system. Contrarily, the results of our research suggested that low humidity levels could potentially destabilize the interaction between B. mali and T. putrescentiae. Several reports indicated that B. mali might inhabit environments with relatively high humidity. For example, in Finland, this predator was carried on the body of several species of fruit flies of the genus Drosophila, which inhabited moist riverside habitats57. Moreover, in grain or dried fruit storage rooms, B. mali often co-occurred with acarid mites, such as T. putrescentiae, Acarus siro Latreille, Carpoglyphus lactis Linnaeus, and Glycyphagus destructor Schrank58,59,60,61. Acarid mites are generally hygrophilous and susceptible to low relative humidity due to their weakly sclerotized cuticles that favour water loss through the body surface. The optimal humidity for the survival of T. putrescentiae lies between 80% and 90% RH at 25 ºC62, which also coincides with the highest probability for prey mortality and the shortest handling time of the T. putrescentiae eggs by B. mali, which was observed in this study. In contrast, the lowest relative humidity at which T. putrescentiae development was possible was found to be 65% at temperatures ranging from 15 ºC to 25 ºC63. Also in B. mali, the functional response parameters gradually deteriorated as the humidity decreased until reaching 52%. However, only at 33% relative humidity, there was a significant decrease in the number of eggs eaten by a predator for most prey densities, which might be related to a significant reduction of its activity to conserve energy and reduce water deficit.
Arthropods can cope with the risk of dehydration in different ways. The adaptations can be morphological (body size, integument composition), physiological (e.g. development time, respiration rate), or behavioural (e.g. aggregating, quiescence, free water uptake; increased prey consumption, transfer to sites with lower saturation deficit)7,8. A decrease in the activity of B. mali females and their foraging in low humidity could have a significant adaptive value due to the limited availability of their prey under such conditions. However, studies have shown that both male and female mould mites can survive short drops in relative humidity to 15% for 24–48 h (such as during migration). If they are unable to escape or find moisture for longer periods, both the mites and their eggs will become desiccated, as demonstrated by Eaton and Kells63.
As the previous studies showed, phytoseiids may behave completely differently from B. mali. Mori and Chant16 studied the functional response of Phytoseiulus persimilis Athias-Henriot (Mesostigmata: Phytoseiidae) to mixed stages of the two-spotted spider mite, Tetranychus urticae Koch (Trombidiformes: Tetranychidae), at 50%, 70%, and 100% RH while Döker et al.64 examined Neoseiulus californicus (McGregor) (Mesostigmata: Phytoseiidae) about the eggs of T. urticae, in a humidity range of 30–90%RH similar to that in our study. Both phytoseiid species are associated with Tetranychus species. Phytoseiulus persimilis is a specialized predator of Tetranychus species while N. californicus is a selective predator of tetranychid mites and well adapted to heavy webbing found within their colonies65. Phytoseiulus persimilis showed generally a less common ‘domed’ functional response16 while N. californicus displayed a Type II response64. Interestingly, in both species, a decrease in humidity led to an increase in prey consumption. In P. persimilis, the highest prey consumption was at 30% RH, while in N. californicus the attack rate was the highest at 50% RH, which has been explained by the authors of both studies as adaptation aimed at preventing water loss. Conversely, at very high humidity levels, the activity of phytoseiids decreased, leading to reduced prey consumption. As in the case of B. mali, the predation rate depended on the potential availability of prey. Contrary to mold mites, high humidity inhibited the development of spider mite populations, while low humidity favoured their increased growth rate65.
Predators exhibiting Type III response are especially promising biological control agents due to their ability to consume prey in a density-dependent manner, which may stabilize the prey-predator interaction, and regulate the population of prey species67,68. Similar hopes were raised by B. mali, which in this study, exhibited Type III functional response to increasing densities of T. putrescentiae eggs within relatively a broad range of humidity favouring the development of the prey. The estimates of parameters of functional response by both Hassell12 and Cabello et al.24 models showed similar trends across different levels of humidity. It confirmed the usefulness of the model by Cabello et al.24 and the parameter α, i.e. potential of mortality, in further research on the behaviour of predators exhibiting Type III functional response. The instantaneous attack rate, a, is a parameter characterizing Type I, and together with handling time, it also describes Type II functional response12. However, in the sigmoid response, apart from handling time there are also two model parameters, b, and c, that are constants and have no biological meaning. Instead, the potential of mortality, proposed by Cabello et al.24 for Type III, is the affinity of a predator at which a predator causes the mortality of a prey. While a high attack rate means that the predator is adept at quickly removing prey from the areas where it is foraging, a high potential of mortality means that the predator has a very high degree of efficacy, causing high mortality of prey24. In our findings, the shortest handling time and the highest potential of mortality were observed at 92% humidity which was not only optimal humidity for mould mites but also seemed to be optimal for B. mali foraging. However, when the two parameters were amalgamated in the novel FRR (i.e. a/ Th or α/Th), which considers the joint effects of the attack rate or potential of mortality and handling time parameters52, we found a clear and significant increase in FRR over increasing humidity levels. It is more important that under suitable conditions of food, temperature, and humidity, eggs make up nearly 50% of the population of T. putrescentiae69.
Although our study suggests a high potential of the predator to reduce the population of T. putrescentiae at higher humidities, research is necessary on other prey stages, which behaviour, e.g., emission of alarm pheromones, clumping to reduce water loss, or burrowing in food to limit respiration62,63,70, may substantially hinder predation and also influence both functional response and its parameters. While the findings provide valuable insights into the potential efficacy of B. mali against T. putrescentiae, the scope of the study may limit its ability to fully assess its effectiveness under Real-world conditions. Future studies are needed to determine the numerical response, interference, and efficiency of converting ingested food into egg biomass (ECI) to gain a more thorough understanding of its effectiveness as a biocontrol agent against mould mites. Moreover, further tests should be performed to confirm that the shift from Type III to Type II functional response at low humidity may destabilize the system between B. mali and T. putrescentiae or other prey types.
Methods
Mites
The initial culture of T. putrescentiae reared on instant dry bakers’ yeast and wheat bran (50/50% by weight), was taken from the mass rearing of the Department of Plant Protection, Warsaw University of Life Sciences, Warsaw, Poland. Tyrophagus putrescentiae adults were chosen and reared using the same quantity of yeast and wheat bran (50/50% by weight) in glass Petri dishes (90 mm in diameter) for obtaining 24-h eggs following the method developed by Pirayeshfar et al.71. Petri dishes were placed on top of water-saturated foam located inside larger plastic containers (120 mm diameter, 200 mm high), which were half-filled with water and covered with a lid containing small pores for ventilation. The foam was covered with wet tissue paper to prevent the mites from escaping35. The cultures were maintained in a Sanyo Environmental Test Chamber (Panasonic MLR-350) in darkness, at 26 °C and 95 ± 5% RH.
The culture of B. mali was maintained on various stages of T. putrescentiae in wheat bran in the laboratory of the Department of Plant Protection at Warsaw University of Life Sciences, Warsaw, Poland47,62,63. The species of predatory mite was previously identified morphologically and confirmed molecularly by DNA barcoding45,72. The rearing unit consisted of soaked foam platforms (220 mm × 150 mm × 25 mm), which were covered with foil and placed within broader vessels filled up with water. The cultures of B. mali were maintained in a Sanyo Environmental Test Chamber (Panasonic MLR-352-PE), at 23 °C, with a photoperiod of 16/8 h (L/D) and a humidity of 85 ± 5%.
Experimental set-up
The experimental unit consisted of a Plexi-glass cage (38 mm × 30 mm × 4 mm), in which a round-shaped hole (8 mm) was drilled. A piece of white filter paper was attached to the lower surface of the cell and a suitable glass coverslip (18 mm × 18 mm) was placed on its upper surface using paraffin wax to prevent the escape of predatory mites. Before 24 h of choosing the female predator, the colony was always fed with sufficient amount of the mixed life stages of T. putrescentiae reared on yeast. The well-fed female predator was randomly chosen from the colony and exposed to seven densities, 10, 20, 40, 60, 80, 120, or 160 of 24-h T. putrescentiae eggs at five different levels of humidity, 33%, 52%, 72%, 82%, and 92%, and at a constant temperature of 25 °C and photoperiod of 16 L:8D h in a cooled incubator (MIR-154-PE) for 24 h. The humidity levels of 33%, 52%, 72%, 82%, and 92% were maintained by the solutions of MgCl2, Mg (NO3)2, NaCl, KCl, and KNO3, respectively in a desiccator73. The eggs of T. putrescentiae were separated from other stages by sieving the rearing colonies through a 100 μm mesh screen74. The eggs were transferred to the chamber/cell of the cage using a fine paintbrush and were scattered evenly on the base of the chamber at all densities. After 24 h, the predators were removed and the number of eggs eaten was counted by excluding the remained eggs. Cages from which a live B. mali was not recovered, because of loss or death, were not included in the analysis. Each egg density was replicated twenty times at each humidity level.
Functional response
To analyze the effect of humidity and density of prey on the consumption of the eggs of T. putrescentiae by B. mali, Generalized Linear Models (GLM) with Poisson probability distribution were applied. As a post hoc test, we used Tukey’s linear contrast.
The functional response data were analyzed in two stages. First, we determined the type of functional response and then estimated the parameters of the functional response. The type of the functional response was determined by, logistic regression of the proportion of prey killed as a function of initial density72 and the generalized functional response equation of Real41. The polynomial logistic regression equation with binomial distribution (Eq. 1) to determine the type of functional response was fitted as under:
where \(\:\frac{{N}_{a}}{{N}_{0}}\) is the proportion of prey eaten, Na is the number of prey eaten, N0 is the initial number of prey density offered, \(\:{P}_{0}\) is the intercept, \(\:{P}_{1}\), \(\:{P}_{2}\), and \(\:{P}_{3}\) are the linear, quadratic, and cubic coefficients, respectively. The coefficients were estimated using the maximum likelihood method. The values of linear and quadratic coefficients indicate the type of functional response. Type I responses are described by an intercept or constant positive slope (\(\:{P}_{0}\)). In the Type II responses, the linear coefficient (P1) \(\:{P}_{0}\) is negative and the proportion of prey eaten declines monotonically with the initial number of prey offered. Whereas, the linear coefficient (P1) \(\:{P}_{0}\) is positive and the quadratic coefficient (\(\:{P}_{2})\) is negative in Type III responses, which are characterized by an increase in the proportion of prey eaten with the prey density offered up to an inflection point and then decreases74.
The modified Holling disc equation proposed by Real51 (Eq. 2) was as under75:
where Na is the number of prey eaten, N0 is the initial number of prey densities offered, a is the predator’s instantaneous attack rate or searching efficiency (The rate of successful search), Th is the handling time (Time spent by the predator in subduing, pursuing, eating, and digesting the prey), T is the time length of the assay, and q is the scaling component that determines the shape of the curve. The functional response can be a Type I (linear, q = 0 and Th = 0), Type II (hyperbolic curve, q = 0, Th > 0), or a Type III (sigmoid curve, q > 0, Th > 0).
After determining the correct shape of the functional response, the functional response parameters, i.e., instantaneous attack rate (a), handling time (Th), and the potential for prey mortality (α), were estimated after fitting to proper models. Data were fitted to the equations proposed by Hassell12 (Eqs. 3 and 4) and Cabello et al.24 (Eq. 5), using non-linear least square regression, as the depleted preys were not replaced throughout the experiment:
where Na is the number of prey eaten, N0 is the initial number of prey density offered, a is the predator’s instantaneous attack rate, Th is the handling time, P is the number of predators used, T is the time length of the assay, α is the potential of mortality of the predator, and b and c are the constants that relate a and N0 in Type III functional response as a= \(\:\frac{b{N}_{0}}{1\:+c{N}_{0}}\). In our experiment, P = 1 and T= 1 day. The parameters, Th and α among different humidities, were obtained using a non-linear least square regression procedure and were compared based on their confidence intervals (± 95% CI) obtained, i.e., if the CI does not overlap, the difference between the means is significant (P < 0.05)76. To calculate Confidence Intervals (CI), we used the permutation test described by Ernst77. We obtained two different kinds of functional responses at different levels of humidity. Therefore, the differences in attack rate among different humidities were not estimated, as the instantaneous attack rate was a constant in Type II functional response but it was a variable that varied with prey densities in Type III functional response10. To amalgamate and further compare the functional response parameters a, α, and Th among humidity levels, the functional response ratio (FRR)52 was estimated using either the attack rate (a) or potential of prey mortality (α) divided by the handling time (Th). The FRR has advantages due to combining either a or α with Th, as high values for a or α and low values for Th would result in high predatory impacts on the ecosystem. Moreover, the predation rate of the predator was estimated by dividing the duration of assay (T) by the handling time (Th). One-way Kruskal–Wallis’s rank sum tests were used to test whether FRRs and the predation rate differed across humidity levels. We used Dunn test as post hoc test with Bonferroni corrections for comparison. In addition, the proportion of prey eaten by the predator at different densities was analyzed using Generalized Linear Models (GLM) with gamma probability distribution. All statistical analyses were performed using R version 4.3.0 (The R Foundation for Statistical Computing, Vienna, Austria)78.
Data availability
The data used in this study are available by email request to the corresponding author (email: [email protected]).
References
IPCC [Intergovernmental Panel on Climate Change]. Climate Change 2007: The Physical Science Basis (Cambridge University Press, 2007).
van Straalen, N. M. Adaptive significance of temperature responses in Collembola. Acta Zool. Fennica. 195, 135–142 (1994).
Uvarov, A. V. Effects of diurnal temperature fluctuations on population responses of forest floor mites. Pedobiologia. 47, 331–339 (2003).
Thompson, J. P., Rostad, H. E., Macdonald, B. J. & Whish, J. P. M. elevated temperature reduces survival of peak populations of root-lesion nematodes (Pratylenchus thornei) after wheat growth in a vertisol. Biol. Fertil. Soils. 54, 243–257 (2018).
Colloff, M. J. Dust mites. Exp. Appl. Acarol. 52, 449–450 (2010).
Chown, S. L., Sørensen, J. G. & Terblanche, J. S. Water loss in insects: an environmental change perspective. J. Insect Physiol. 57, 1070–1084 (2011).
Walzer, A. et al. Intraspecific variation in humidity susceptibility of the predatory mite neoseiulus californicus: survival, development and reproduction. Biol. Control. 41, 42–52 (2007).
Benoit, J. B., McCluney, K. E., DeGennaro, M. J. & Dow, J. A. T. Dehydration dynamics in Terrestrial arthropods: from Water sensing to Trophic interactions. Annu. Rev. Entomol. 68, 129–149 (2023).
Solomon, M. E. The natural control of animal populations. J. Anim. Ecol. 18, 1 (1949).
Holling, C. S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 91, 293–320 (1959).
Holling, C. S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 (1959).
Hassell, M. P. Functional responses. In The Dynamics of Arthropod Predator-Prey Systems 13, 28–49 (Princeton University Press, 1978).
Krebs, C. J. Some historical thoughts on the functional responses of predators to prey density. Front. Ecol. Evol. 10, 1052289 (2022).
Holling, C. S. Principles of insect predation. Ann. Rev. Entomol. 6, 163–182 (1961).
Köhnke, M. C., Siekmann, I., Seno, H. & Malchow, H. A type IV functional response with different shapes in a predator–prey model. J. Theor. Biol. 505, 110419 (2020).
Mori, H. & Chant, D. A. The influence of prey density, relative humidity, and starvation on the predacious behaviour of phytoseiulus persimilis Athias-Henriot (acarina: phytoseiidae). Can. J. Zool. 44, 483–491 (1966).
Fathipour, Y. & Maleknia, B. Mite Predators. In Ecofriendly Pest Management for Food Security (ed. Omkar) 329–366 (Elsevier, 2016).
Fathipour, Y., Karimi, M., Farazmand, A. & Talebi, A. A. Age-specific functional response and predation rate of Amblyseius Swirskii (Phytoseiidae) on two-spotted spider mite. Syst. Appl. Acarol. 22 (2), 159–169 (2017).
Fathipour, Y., Karimi, M., Farazmand, A. & Talebi, A. A. Age-specific functional response and predation capacity of Phytoseiulus Persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia. 58 (1), 31–40 (2018).
Mohaghegh, D. C. Functional response of the predators Podisus maculiventris (say) and Podisus Nigrispinus (Dallas) (Heteroptera, Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera, Noctuidae): effect of temperature. J. Appl. Entomol. 125, 131–134 (2001).
Kharboutli, M. S. & Mack, T. P. Effect of temperature, humidity, and Prey Density on feeding rate of the Striped Earwig (Dermaptera: Labiduridae). Environ. Entomol. 22, 1134–1139 (1993).
Wang, B. & Ferro, D. N. Functional responses of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) to Ostrinia nubilalis (Lepidoptera: Pyralidae) under Laboratory and Field conditions. Environ. Entomol. 27, 752–758 (1998).
Hassell, M. P., Lawton, J. H. & Beddington, J. R. Sigmoid functional responses by invertebrate predators and parasitoids. J. Anim. Ecol. 46, 249 (1977).
Cabello, T., Gámez, M. & Varga, Z. An improvement of the Holling type III functional response in entomophagous species model. J. Biol. Syst. 15, 515–524 (2007).
Pervez, A. & Omkar Functional responses of coccinellid predators: an illustration of a logistic approach. J. Insect Sci. 5 (2005).
Daugaard, U., Petchey, O. & Pennekamp, F. Warming can destabilise predator-prey interactions by shifting the functional response from type III to type II. J. Anim. Behav. 88, 1575–1586 (2019).
Hassanpour, M., Mohaghegh, J., Iranipour, S., Nouri-Ganbalani, G. & Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 18, 217–224 (2011).
Nunes, G. S. et al. Temperature-dependent functional response of Euborellia annulipes (Dermaptera: Anisolabididae) preying on Plutella xylostella (Lepidoptera: Plutellidae) larvae. J. Therm. Biol. 93, 102686 (2020).
Taylor, D. & Collie, J. Effect of temperature on the functional response and foraging behaviour of the sand shrimp Crangon septemspinosa preying on juvenile winter flounder Pseudopleuronectes americanus. Mar. Ecol. Prog. Ser. 263, 217–234 (2003).
Madbouni, M. A., Samih, M. A., Namvar, P. & Biondi, A. Temperature-dependent functional response of Nesidiocoris tenuis (Hemiptera: Miridae) to different densities of pupae of cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 114, 325–331 (2017).
Shiralizadeh, R., Esfandiari, M., Shishehbor, P. & Farahi, S. Effect of temperature on the functional response of the predatory mite Macrocheles muscaedomesticae (Acari: Macrochelidae) by feeding on eggs of the house fly, Musca domestica (Diptera: Muscidae). Plant. Prot. (Sci. J. Agric.). 44 (2), 19–31 (2021).
Ahn, J. J., Kim, K. W. & Lee, J. H. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol. 134, 98–104 (2010).
Jalali, M. A., Tirry, L. & De Clercq, P. Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 55, 261–269 (2010).
Dong, H., Liu, Q., Xie, L., Cong, B. & Wang, H. Functional response of Wolbachia -infected and uninfected Trichogramma Dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) to Asian corn borer, Ostrinia Furnacalis Guenée (Lepidoptera: Pyralidae) eggs. J. Asia Pac. Entomol. 20, 787–793 (2017).
Islam, Y. et al. Functional response of Harmonia axyridis preying on Acyrthosiphon pisum nymphs: the effect of temperature. Sci. Rep. 11, 13565 (2021).
Zhang, Z. Q. Acarid mites. Part II pest mites. In Mites of Greenhouses-identification, Biology and Control, vol. 8, 141–158 (CABI, 2003).
Hubert, J. et al. Mites as selective fungal carriers in stored grain habitats. Exp. Appl. Acarol. 29, 69–87 (2003).
Platts-Mills, T. A., Vaughan, J. W., Carter, M. C. & Woodfolk, J. A. The role of intervention in established allergy: avoidance of indoor allergens in the treatment of chronic allergic disease. J. Allergy Clin. Immunol. 106 (5), 787–804 (2000).
Sorenson, J. G., Addison, M. F. & Terblanche, J. S. Mass rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Prot. 38, 87–94 (2012).
de Moraes, G. J., Venancio, R., dos Santos, V. L. V. & Paschoal, A. D. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as biological control agents of pest organisms. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms 33–75 (Springer International Publishing, 2015).
Pirayeshfar, F., Safavi, S. A., Moayeri, H. R. S. & Messelink, G. J. Provision of astigmatid mites as supplementary food increases the density of the predatory mite Amblyseius Swirskii in greenhouse crops, but does not support the omnivorous pest, western flower thrips. BioControl. 66, 511–522 (2021).
Abbas, A. A., Yassin, E. M. A., El-Bahrawy, A. F., El-Sharabasy, H. M. & Kamel, M. S. Biology of Blattisocius Mali (Oudemans) (Acari: Gamasida: Ascidae) feeding on different diets under laboratory conditions. EVMSPJ. 16, 92–101 (2020).
Gallego, J. R., Caicedo, O., Gamez, M., Hernandez, J. & Cabello, T. Selection of predatory mites for the biological control of potato tuber moth in stored potatoes. Insects. 11, 196 (2020).
Solano-Rojas, Y. et al. Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius Mali (Mesostigmata: Blattisociidae) in the Post-harvest Biological Control of the Potato Tuber moth (Lepidoptera: Gelechiidae): Use of Sigmoid functions. Agriculture. 12, 519 (2022).
Michalska, K. et al. Preliminary studies on the Predation of the Mite Blattisocius Mali (Acari: Blattisociidae) on various life stages of Spider Mite, Thrips and Fruit fly. Insects. 14, 747 (2023).
Michalska, K., Mrowi´nska, A., Studnicki, M. & Jena, M. K. Feeding behaviour of the mite Blattisocius Mali on eggs of the fruit flies Drosophila melanogaster and D. Hydei. Diversity. 15, 652 (2023).
Michalska, K., Mrowińska, A. & Studnicki, M. Ectoparasitism of the flightless Drosophila melanogaster and D. Hydei by the mite Blattisocius Mali (Acari: Blattisociidae). Insects. 14, 146 (2023).
Sylvain, Z. A. et al. Soil animal responses to moisture availability are largely scale, not ecosystem dependent: insight from a cross-site study. Glob. Chang. Biol. 20 (8), 2631–2643 (2014).
Rivard, I. Influence of humidity on the predaceous mite melichares dentriticus (Berlese) (Acarina: Aceosejidae). Can. J. Zool. 40, 761–766 (1962).
Barker, P. S. Bionomics of Blattisocius keegani (Fox) (Acarina, Ascidae) a predator on eggs of pests of stored grains. Can. J. Zool. 45, 1093–1099 (1967).
Real, L. A. The kinetics of functional response. Am. Nat. 111, 289–300 (1977).
Cuthbert, R. N., Dickey, J. W., Coughlan, N. E., Joyce, P. W. & Dick, J. T. The Functional Response Ratio (FRR): advancing comparative metrics for predicting the ecological impacts of invasive alien species. Biol. Invasions 21, 2543-2547 (2019).
Gavara, J., Cabello, T., Gallego, J. R. & Hernández-Suarez, E., Piedra-Buena Díaz, A. Evaluation of the egg predator Blattisocius tarsalis (Mesostigmata: Blattisociidae) for the biological control of the potato tuber moth Tecia solanivora under storage conditions. Agriculture. 12, 920 (2022).
Wu, S. et al. Evaluation of Stratiolaelaos Scimitus and Neoseiulus barkeri for biological control of thrips on greenhouse cucumbers. Biocontrol Sci. Technol. 24 (10), 1110–1121 (2014).
Zhu, J. et al. Functional response of two soil-dwelling predatory mites, Macrocheles mammifer (Berlese) (Acari: Macrochelidae), and Stratiolaelaps Scimitus (Womersley) (Acari: Laelapidae), on thrips Megalurothrips Usitatus (Bagnall) (Thysanoptera: Thripidae). Acarologia. 63 (4), 1039–1047 (2023).
Murdoch, W. W. & Oaten, A. Predation and population stability. Adv. Ecol. Res. 9, 1–125 (1975).
Lehtinen, P. T., Aspi, J. A. & Phytoseiid mite Paragarmania mali, associated with drosophilid flies. In The Acari Physiological and Ecological Aspects of Acari-Host Relationships 537–544 (European Association of Acarologists: Krynica, 1992).
Hughes, A. The mites of stored food and houses. Tech. Bull. Min. Agric. Fish. Lond. 73, 145 (1976).
Turk, F. A. & Turk, S. M. LV.—Studies of Acari. —7th series: records and descriptions of mites new to the British fauna, together with short notes on the biology of sundry species. Ann. Mag. Nat. Hist. 5 (53), s484 (1952).
Palyvos, N. E., Emmanouel, N. G. & Saitanis, C. J. Mites associated with stored products in Greece. Exp. Appl. Acarol. 44(3), 213–221 (2008).
Dizlek, H., Karagoz, M., Faraji, F. & Cakmak, I. Mites in dried figs of Turkey; diversity, species composition and density. Syst. Appl. Acarol. 24 (6), 992–997 (2019).
Sánchez-Ramos, I., Álvarez-Alfageme, F. & Castañera, P. Effects of relative humidity on development, fecundity and survival of three storage mites. Exp. Appl. Acarol. 41, 87–100 (2007).
Eaton, M. & Kells, S. A. Use of vapor pressure deficit to predict humidity and temperature effects on the mortality of mold mites, Tyrophagus putrescentiae. Exp. Appl. Acarol. 47, 201–213 (2009).
Doker, I., Kazak, C. & Karut, K. Functional response and fecundity of a native Neoseiulus californicus population to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at extreme humidity conditions. Syst. Appl. Acarol. 21, 1463 (2016).
McMutry, J. A. & de Moraes, G. J. Famah Sourassou, N. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18, 297–320 (2013).
Messelink, G. & Leman, A. Integrated control of plant-feeding mites. IOBC-WPRS Bull. 149, 101–102 (2020).
Fernández-Arhex, V. & Corley, J. C. The functional response of parasitoids and its implications for biological control. Biocontrol Sci. Technol. 13, 403–413 (2003).
Xiao, Y. & Fadamiro, H. Y. Functional responses and prey-stage preferences of three species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus Citri (Acari: Tetranychidae). Biol. Control. 53, 345–335 (2010).
Boczek, J. Mite pests in stored food. Ecol. Manag. Food Ind. Pests. Arlingt. FDA Tech. Bull. 4, 57–79 (1991).
Kuwahara, Y., Ishii, S. & Fukami, H. Neryl formate: alarm pheromone of the cheese mite, Tyrophagus putrescentiae (Schrank) (Acarina, Acaridae). Experientia. 31, 1115–1116 (1975).
Pirayeshfar, F., Safavi, S. A., Moayeri, S., Messelink, G. J. & H. R. & The potential of highly nutritious frozen stages of Tyrophagus putrescentiae as a supplemental food source for the predatory mite Amblyseius Swirskii. Biocontrol Sci. Technol. 30, 403–417 (2020).
Dabert, M., Bigoś, A. & Witaliński, W. DNA barcoding reveals andropolymorphism in Aclerogamasus species (Acari: Parasitidae). Zootaxa. 3015, 13 (2011).
The Engineering Tool Box. Saturated Salt Solutions—Controlling Air Humidity. https://www.engineeringtoolbox.com/salt-humidity-d_1887.html) (Accessed 2014).
Juliano, S. A. Non-linear curve fitting: predation and functional response curves. In Design and Analysis of Ecological Experiments (eds Scheiner, S. M. & Gurevitch, J.) 178–196 (Oxford University Press, 2001).
Pritchard, D. W., Paterson, R. A. & Bovy, H. C. Barrios-O’Neill, D. frair: an R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 8, 1528–1534 (2017).
Mahdian, K., Tirry, L. & De Clercq, P. Functional response of Picromerus bidens: effects of host plant. J. Appl. Entomol. 131, 160–164 (2007).
Ernst, M. D. Permutation methods: a basis for exact inference. Statist Sci. 19(4), 676–685 (2004).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Acknowledgements
The authors are highly grateful to Puchalska E., Lewandowski M., Wakuliński W., Witaliński W., and Wit M. for the inspiring discussion about the effect of humidity on growth and survival of soil and plant-dwelling predatory mites. The publication was financed by the Science Development Fund of the Warsaw University of Life Sciences – SGGW.
Funding
The research did not receive any external grants.
Author information
Authors and Affiliations
Contributions
This research was conducted in collaboration among all authors. The authors’ names are given in alphabetical order.Conceptualization, K.M., M.S., and M.K.J.; Designing the methodology, M.K.J., K.M., M.S.; investigation, M.K.J., K.M. and M.S.; software, M.S.; validation, formal analysis, resources, data curation, writing—original draft preparation, writing— review and editing, visualization, funding acquisition, M.K.J., K.M., and M.S.; supervision, project administration, K.M. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jena, M.K., Michalska, K. & Studnicki, M. The impact of humidity on the functional response of Blattisocius Mali (Acari: Blattisociidae) preying on the acarid mite Tyrophagus putrescentiae. Sci Rep 14, 28051 (2024). https://doi.org/10.1038/s41598-024-78997-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78997-w