Abstract
The relationship between depression and severe non-alcoholic fatty liver disease (NAFLD) has not been clearly defined. We conducted a longitudinal cohort study and a two-sample Mendelian randomization (MR) analysis to assess the association of depression with severe NAFLD risk. We used individual data from the UK Biobank study with 481,181 participants, and summary data from published genome-wide association studies. The association between depression and severe NAFLD was assessed using Cox proportional hazards regression analysis. Two-sample MR for depression with NAFLD was conducted, the principal analysis employed the inverse variance weighted (IVW) approach. In the observational study, after a median follow-up of 13.46 years, 4,563 participants had severe NAFLD. In multivariable-adjusted model, participants with depression had an increased risk of severe NAFLD (hazards ratio:1.21, 95% confidence interval (CI):1.09–1.34), as compared to those without depression. In subgroup analyses, the association between depression and severe NAFLD risk was generally observed across different subgroups. For the MR, result also showed that genetically predicted depression was causally associated with a higher risk of NAFLD (odds ratio:1.55, 95%CI:1.10–2.19) in IVW. Our study revealed a prospective association of depression with severe NAFLD, thus potentially necessitating clinical monitoring of individuals with depression for severe NAFLD.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide with a global prevalence of 25% and the leading cause of cirrhosis and hepatocellular carcinoma, including steatosis with or without mild inflammation (non-alcoholic fatty liver, NAFL) to non-alcoholic steatohepatitis (NASH)1,2. Depression is one of the leading mental illnesses, with an estimated 5% of adults globally suffering from it3.
There are growing epidemiological evidences that depression is associated with various diseases including diabetes4, hypertension5, cardiovascular disease6, which are also commonly accompanied by NAFLD. However, previous observational studies on the association between depression and NAFLD have mostly been cross-sectional studies with inconsistent results7,8,9,10,11,12,13,14. There is only one relevant longitudinal study on this topic, which based on 142,005 participants in median follow-up of 4.0 years, reported that depression was associated with an increased risk of hepatic steatosis (hazards ratio (HR):1.06, 95% confidence interval (CI):1.01–1.10)8. Conventional observational studies remain prone to residual unmeasured confounding and reverse causality biases, which can be reduced by Mendelian randomization (MR) design15. MR explores the effect of exposures on outcomes by using genetic variants as instrumental variables (IVs), the alleles of this exposure-associated genetic variant are randomly allocated and not subject to reverse causation16, and provides new insights into the potential causal association. However, two previous MR studies on this topic have also showed conflict results17,18. Notably, a meta-analysis showed that 18.21% of patients with NAFLD had depression, and in more severe stages of the disease, such as NASH, depression reached 40.68%19. However, research on the relationship between depression and severe NAFLD is limited.
Therefore, we used data from a large prospective cohort, UK Biobank individual-level dataset, to assess whether the onset of depression was associated with severe NAFLD. Furthermore, we conducted a two-sample MR study to explore whether there is potential causal association between depression and NAFLD risk.
Methods
Study population and design
We first used data from the UK Biobank study (application number 84980) to evaluate the association of depression with severe NAFLD. The details about the UK Biobank study design and data collection have been described elsewhere20. Briefly, it is an ongoing, multi-center prospective cohort study of over half a million participants aged 40–69 years in UK from 2006 to 2010 (https://www.ukbiobank.ac.uk/). At one of 22 assessment centers in England, Scotland, and Wales, participants completed touch-screen and nurse-led questionnaires, underwent physical assessments, and provided biological samples. The UK Biobank study has approval from the North West Multi-centre Research Ethics Committee, all methods were performed in accordance with the relevant guidelines and regulations, and all participants provided written informed consent.
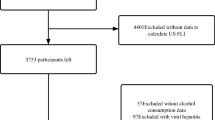
To analyze the association of depression with incident severe NAFLD, we included 481,181 participants after excluding participants with liver disease or alcohol/drug use disorders at baseline, without related exact date for liver disease or alcohol/drug use disorders, and new-onset severe NAFLD within 5 years follow-up (Fig. 1). The baseline liver disease and alcohol/drug use disorders were ascertained through data linkage to primary care and hospital inpatient records. The details of international classification of diseases, 10th revision (ICD-10) codes used to define them are listed in Supplementary Table S1, based on the latest Expert Panel Consensus Statement21. The conversion between the READ codes and ICD-10 codes was done using the UK Biobank’s look-up table.
Study design overview. Abbreviations: NAFLD, non-alcoholic fatty liver disease; SNP, single nucleotide polymorphisms. There are three assumptions of mendelian randomization design. The first assumption is that the genetic variants used as instrumental variables should be robustly associated with the exposure; the second assumption is that the used genetic variants should not be associated with any confounders; and the third assumption is that the selected genetic variants should affect the risk of the outcome merely through the risk factor, not via alternative pathways.
Assessment of depression
The diagnosis of depression at baseline was defined as the ICD-10 codes F32 (depressive episode), F33 (recurrent depressive disorder), F34 (persistent mood [affective] disorders), F38 (other mood [affective] disorders), and F39 (unspecified mood [affective] disorder), as identified from the part of mental and behavioral disorders in the “First occurrences fields” of the UK Biobank study (data category ID: 1712), which included data from primary care, hospital inpatient records, self-reported medical condition, and death registers. Participants with diagnosis date of depression prior to the baseline assessment center attendance date were considered had depression at baseline.
Severe NAFLD ascertainment and follow-up
Severe NAFLD was defined as hospitalization or death due to NAFLD or NASH22, which was ascertained using data linkage to hospital inpatient records (data field ID: 41270) and death registries (data field ID: 40001 and 40002). NAFLD (including NASH) was defined as ICD-10 K76.0 (fatty [change of] liver, not elsewhere classified) and K75.8 (other specified inflammatory liver diseases) in the most recent Expert Panel Consensus Statement21. Specifically, in the UK Biobank study, dates and causes of hospital admissions were obtained from record linkage to the Hospital Episode Statistics for England (England), the Scottish Morbidity Records (Scotland) and the Patient Episode Database for Wales (Wales), and dates and causes of death were obtained from death certificates held by the National Health Service (NHS) Information Centre (England and Wales) and the NHS Central Register Scotland (Scotland). Participants were followed up from the date of baseline assessment for UK Biobank until the date of the first diagnosis of severe NAFLD, loss to follow-up, death, or the end of the follow-up (2022-10-31 for England, 2021-07-31 for Scotland and 2018-02-28 for Wales), whichever came first.
Measurements of covariates
Several covariates were included in our study, and their selection was informed by previous literature and theory7,8,9,10,11,12,13,14,23,24,25,26,27,28,−29. Information on sex (male, female), age at recruitment (years), race (white, non-white), qualification (no qualifications, any other qualification, college or university degree), frequency of alcohol intake (never, special occasions only, one to three times a month, once or twice a week, three or four times a week, daily or almost daily), smoking status (never, former, current), oily fish intake (never, less than once a week, once a week, 2–4 times a week, 5–6 times a week, once or more daily) and processed meat intake (never, less than once a week, once a week, 2–4 times a week, 5–6 times a week, once or more daily), and physical activity level (low, moderate, high, missing) is from the baseline questionnaires. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (< 18.5, 18.5–24.9, 25.0–29.9, ≥ 30.0 kg/m2). Townsend deprivation index was used to indicate socioeconomic status, with a high score indicating a high socioeconomic deprivation30. The information for medication history of antidepressant use was obtained through self-report in verbal interview at baseline, we defined antidepressant use as self-reported use of at least one selective serotonin reuptake inhibitor or other antidepressant medication as previous algorithms31. Hyperglycemia/diabetes was defined as glycated hemoglobin (HbA1c) ≥ 42.0 mmol/mol32 or self-report of a physician diagnosis of diabetes. Elevated blood pressure (BP)/hypertension was defined as a systolic blood pressure ≥ 130 mmHg and/or a diastolic blood pressure ≥ 85 mmHg33 or self-report of a physician diagnosis of hypertension. Two measures of systolic and diastolic BP were obtained, and the average values were calculated. Hypertriglyceridemia was defined as ≥ 1.7 mmol/L and low high-density lipoprotein cholesterol was defined as < 1.3 mmol/L in women and < 1.0 mmol/L in men33.
Mendelian randomization analysis
In addition to the analyses of observational study, we performed a two-sample MR analysis to evaluate the causal effect of depression on the risk of NAFLD. The MR study was based on summary-level data of genome-wide association analyses on depression and NAFLD from published genome-wide association studies (GWAS). We used the hitherto largest published GWAS for depression that was conducted in a population of European-descent, which included 807,553 individuals (246,363 cases and 561,190 controls)34. In total, 67 single nucleotide polymorphisms (SNPs) associated with depression at the genome-wide significance threshold (P < 5 × 10− 8) and without linkage disequilibrium (LD, defined as r2 > 0.001 and clump distance < 10,000 kb) were identified, additionally, the SNPs not founded in the NAFLD GWAS and palindromic SNPs were disregarded (Supplementary Fig. S1). The strength of included SNPs was evaluated by calculating the F-statistic, and F-statistics > 10 were typically used as the cutoff for powerful IVs. Summary-level data for the associations of depression associated SNPs with NAFLD were obtained from the FinnGen consortium (R8 release, https://www.finngen.fi/en) which included 342,499 individuals (1,908 cases and 340,591 controls of European descent). No overlapping participating studies were shared between the depression and NAFLD GWASs. Data sources and instrument IVs are listed in Supplementary Table S2 and Supplementary Table S3. The STROBE-MR checklist is detailed in Supplementary material 1.
Statistical analysis
In the observational study using data from the UK Biobank study, the cumulative incidence of severe NAFLD according to the occurrence of baseline depression was estimated by using R packages “survival” and “survminer”. The log-rank test was used for comparing survival distributions between groups. The proportional hazard assumption was checked using Schoenfeld residuals. Cox proportional hazard regression models were performed to calculate the HR and 95% CI to assess the associations of depression with severe NAFLD risk. In our multivariable model, we adjusted for age at recruitment, sex, BMI, race, Townsend deprivation index, qualification, frequency of alcohol intake, smoking status, oily fish intake, processed meat intake, physical activity level, antidepressant use, hyperglycemia/diabetes, elevated BP /hypertension, hypertriglyceridemia and low high-density lipoprotein cholesterol. P values for interaction were tested by introducing a product term of the two variables examined in the regression models. Subgroup analyses were also conducted across categories of sex (male, female), age at recruitment (< 60, ≥ 60 years), race (non-white, white), smoking status (never, ever), frequency of alcohol intake (non-excessive alcohol intake, excessive alcohol intake), BMI (< 25, ≥ 25 kg/m2), antidepressant use (yes, no), hyperglycemia/diabetes (yes, no) and elevated BP/hypertension (yes, no), respectively.
To confirm the robustness of our results, we performed nine sensitivity analyses: (i) excluding 53 deaths without prior hospitalization; (ii) excluding people who with missing data for covariates; (iii) the analysis based on covariates with missing data imputed by the multiple imputation method with chained equations for 5 imputations; (iv) excluding people who drinking more than 14 units/week or who with missing data about the total average weekly units of alcohol; (v) widening the definition of NAFLD to include a broader definition that includes K76.8 (other specified diseases of the liver), K76.9 (liver disease, unspecified) as well as unspecified liver diseases: K74.0 (hepatic fibrosis), K74.1 (hepatic sclerosis), K74.2 (hepatic fibrosis with hepatic sclerosis), and K74.6 (other and unspecified cirrhosis of the liver); (vi) using primary care records, hospital admissions and death records to ascertain NAFLD; (vii) defining baseline depression using the recommendations of Smith DJ et al.35, which has been used in some papers36,37,38; (viii) using depression as a time-dependent independent variable; (ix) to prevent over-adjustment of potential mediators28,39,40,41,42,43,44,45,46,47,48, only age at recruitment, sex, race, Townsend deprivation index, qualification, oily fish intake and processed meat intake were included as covariates in the model.
Missing rates of covariates ranged from 0.001% for age at recruitment to 19.905% for physical activity level (Supplementary Table S4). Missing values were replaced by the sex-specific median and mode values in the study population for continuous and categorical variables, respectively. Given that the missing rate was high for physical activity level, this covariate was imputed using the missing-indicator method.
For the MR, inverse variance weighted (IVW) was our primary analytical method, assuming that all SNPs are valid IVs49. To examine the consistency of the associations, four sensitivity analyses were conducted, including the weighted median50, maximum likelihood51, MR pleiotropy residual sum and outlier (MR-PRESSO)52 methods as well as leave-one-out analysis. The weighted median method could provide consistent estimates when more than half of the weight in the analysis comes from valid genetic instruments50. The maximum likelihood method may provide more reliable estimates when measurement error occurs in the SNP-exposure effects51. MR-PRESSO employs a global test to detect the presence of horizontal pleiotropy and a distortion test to identify outlier instruments, and remove them before applying IVW52. Leave-one-out analysis where one SNP was removed at a time and IVW was conducted based on the remaining SNPs. Moreover, the Cochran’s Q statistic (IVW) and Rucker’s Q statistic (MR-Egger) were used to detect the heterogeneity of our MR analysis, MR-Egger intercept test and global test of MR-PRESSO were employed to detect for the presence of pleiotropy. An online tool (https://shiny.cnsgenomics.com/mRnd/) was used to assess the statistical power to identify the difference. The statistical power of depression on NAFLD is 90% when the type I error rate is 0.05. All MR analysis was performed by using R packages “TwoSampleMR” (version 0.5.6).
All statistical analyses were performed using R software 4.1.2. A two-sided P < 0.05 was considered statistically for all the tests, if not specified.
Results
Table 1 summarizes baseline characteristics of included participants by baseline depression status. Over a median follow-up of 13.46 years (interquartile range, 12.72–14.10 years), a total of 481,181 participants including 38,206 depression cases were prospectively followed up in the study. Over this period, 4,563 participants (4,510 hospitalizations and 53 deaths without prior hospitalization) were recorded as developing severe NAFLD. Participants who suffered from depression tended to be younger, more likely to be female and smokers, more likely to have a BMI of 30 or greater, had a higher prevalence of antidepressant use, hyperglycemia/diabetes, hypertriglyceridemia and low high-density lipoprotein cholesterol and more likely to be deprived. They also had a lower prevalence of elevated BP/hypertension, a lower physical activity level, were less likely to have processed meat, oily fish and alcohol intake at baseline.
In our multivariable-adjusted model, participants with depression had an increased risk of severe NAFLD (HR:1.21, 95%CI:1.09–1.34), as compared to those without depression (Table 2). The cumulative incidence rate of severe NAFLD during the follow-up was higher in participants with depression compared with those without depression (Fig. 2). In subgroup analyses, we didn’t observe significant interactions across sex, age at recruitment, race, smoking status, frequency of alcohol intake, BMI, antidepressant use, hyperglycemia/diabetes, elevated BP/hypertension and depression (all P for interaction > 0.05), the association between depression and severe NAFLD risk was generally observed across different subgroups (Supplementary Fig. S2). The associations between depression and higher severe NAFLD risk were robust to a range of sensitivity analyses (Supplementary Table S5). The results did not changed too much after excluding those who dead due to severe NAFLD but without prior hospitalization (HR: 1.21, 95% CI: 1.09–1.34), excluding people who drinking more than 14 units/week or who with missing data about the total average weekly units of alcohol (HR: 1.18, 95% CI: 1.03–1.35), widening the definition of NAFLD (HR: 1.11 ,95% CI: 1.02–1.21), using primary care records, hospital admissions and death records to ascertain NAFLD (HR: 1.27, 95% CI: 1.15–1.40), or defining baseline depression using the recommendations of Smith DJ et al. (HR: 1.35, 95% CI: 1.17–1.56), or using depression as a time-dependent independent variable (HR:1.16, 95% CI: 1.06–1.27). The results of other sensitivity analyses were similar.
The MR estimates from different methods of assessing the causal effect of depression on NAFLD were presented in Table 3. Using IVW, genetic liability to depression was significantly associated with an increased risk of NAFLD (odds ratio (OR): 1.55, 95%CI: 1.10–2.19). The estimates remained directionally consistent in the weighted median (OR: 1.85, 95%CI: 1.14–3.00), maximum likelihood (OR: 1.58, 95%CI: 1.12–2.22), MR-PRESSO (OR: 1.55, 95%CI: 1.10–2.19) methods. Heterogeneity tests highlighted the non-existence of heterogeneity (IVW, Q (df) = 69.8 (66), P = 0.352; MR-Egger, Q (df) = 69.2 (65), P = 0.337). No horizontal pleiotropy was detected in the MR-Egger test (MR-Egger intercept = 0.019, P = 0.474) and MR-PRESSO test (global test P = 0.363). No outlier SNPs were detected in MR-PRESSO analysis, suggesting limited evidence of pleiotropic bias. Leave-one-out analysis result which was shown in Supplementary Fig. S3 demonstrated that there was no potentially influential SNP driving the causal link between depression and NAFLD.
Discussion
Using the large UK Biobank dataset and analyzing a sample of more than 480,000, our study suggests an association between depression and higher severe NAFLD risk in people without manifest liver disease, the results of further MR analysis also support this finding, which may help to advance the intervention and treatment of both diseases.
The finding of our study that depression was associated with a higher severe NAFLD risk, which is consistent with several previous studies7,8,9,10. Two cross-sectional studies from the 2016 Korea National Health and Nutrition Examination Survey7, the 2007–2016 National Health and Nutrition Examination Survey (NHANES)9 reported that compared to subjects without depression, those with depression were more likely to have NAFLD. Another cross-sectional study which based on the Ravansar Non-Communicable Disease cohort investigation, reported that the odds of NAFLD in female participants with depression were 71% higher than in non-depressed participants10.The only longitudinal cohort study also found that depression was associated with an increased risk of hepatic steatosis (HR:1.06, 95%CI:1.01–1.10)8. In addition, one cross-sectional study conducted from the 2005–2010 NHANES reported that depression was not independently associated with NAFLD at the population level11, another small sample size study was also failed to detect a positive association between depression and NAFLD14. Our MR analysis using the latest database also supported that depression was associated with increased risk of NAFLD, providing additional evidence to previous inconsistent findings17,18.
There are some biologically plausible mechanisms for the association between depression and NAFLD. Chronic inflammation, obesity, diabetes, the increased cortisol and epinephrine levels, may provide a plausible explanation for the association between depression and NAFLD53. Pathobiological factors associated with depression, such as increased monoamine oxidase-A enzyme activities, may enhance cellular oxidative stress, thereby negatively affecting NAFLD13. What’s more, depression may lead to immune-mediated destruction of pancreatic β-cells54, which can lead to insulin resistance and diabetes mellitus, both of which are risk factors for NAFLD.
The large sample size, in addition to a prospective study design, provides a higher level of evidence than retrospective studies or cross-sectional studies. Moreover, by constructing IVs as measures of exposure, the MR analysis further supports our finding at the genetic level. However, several limitations should be considered. Firstly, in our study, severe NAFLD was identified according to ICD-10 codes in hospitalized medical records and death records, rather than via the gold diagnostic criteria including hepatic image or histology from liver biopsy, besides, there were no biomarkers of liver disease severity such as Fibrosis-4 index for liver fibrosis, NAFLD Fibrosis Score, or aspartate aminotransferase-to-platelet ration index in the UK Biobank study, or information for estimating them was only available at baseline assessment. Therefore, our main analysis included the advanced or severe cases of NAFLD primarily due to this disposal. To address this issue, we carried out a sensitivity analysis by using primary care records, hospital admissions and death records to ascertain NAFLD, more less severe cases can be included, and the pattern of association was similar with our main analysis result. What’s more, some NAFLD cases in the community may not take medical consultation, a proportion of NAFLD cases in this large population-based cohort might remain undiagnosed, leading to an underestimation of the NAFLD incidence rate. Secondly, we acknowledge the new terminology proposal issued to replace NAFLD with metabolic dysfunction-associated fatty liver disease (MASLD)55, however, we decided to use NAFLD rather than MASLD because information on cardiometabolic criteria required for its definition were not available during the follow-up. Given a body of evidence that the prevalence of the two diseases is comparable and almost all patients with NAFLD meet MASLD criteria56,57,58,59,60, it is reasonable to consider that our finding remains valid under the new MASLD definition. Thirdly, although we carefully controlled for many potential confounders, there would still be some potential covariates (unmeasured or unknown), so residual confounders cannot be ruled out, therefore, we used MR analysis for additional causal inference. Fourthly, some of the information in our study was based on self-report, such as frequency of alcohol intake, etc., so recall and misclassification bias may be present. Fifthly, the UK Biobank study participants are not representative of the UK general population owing to evidence of a healthy volunteer selection bias61. Sixthly, a major limitation of MR design is horizontal pleiotropy, which means that the used IVs exert effects on the outcomes not via the exposure but via alternative pathways. In our study, since we observed no indications of horizontal pleiotropy, the bias caused by pleiotropic effects should be minimal. Finally, we limited our MR analysis to individuals of European descent may limit the generalizability of our finding to other populations.
In summary, in this large-scale prospective cohort study of the UK population, depression was associated with an increased risk of severe NAFLD, our further MR analysis also supported this finding. This suggests that depression should be considered more broadly as a target for prevention of severe NAFLD. In addition, clinical monitoring of depressed patients for severe NAFLD may be required.
Data availability
The data that support the finding of this observational study are available from UK Biobank (application number 84980), but there are restrictions on their availability, researchers can apply to use the UK Biobank (https://www.ukbiobank.ac.uk/) resource and access the data used. The data that support the finding of this MR study are available from online: depression (https://www.nature.com/articles/s41593-018-0326-7) and NAFLD (https://www.finngen.fi/en). The GWAS datasets used during the MR study are available from the corresponding author on reasonable request.
References
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6, 578–588. https://doi.org/10.1016/s2468-1253(21)00020-0 (2021).
Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet 397, 2212–2224. https://doi.org/10.1016/s0140-6736(20)32511-3 (2021).
WHO. Depression. https://www.who.int/news-room/fact-sheets/detail/depression (2023).
Tabák, A. G., Akbaraly, T. N., Batty, G. D. & Kivimäki, M. Depression and type 2 diabetes: A causal association? Lancet Diabets Endocrionol. 2, 236–245. https://doi.org/10.1016/s2213-8587(13)70139-6 (2014).
Meng, L., Chen, D., Yang, Y., Zheng, Y. & Hui, R. Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. J. Hypertens. 30, 842–851. https://doi.org/10.1097/HJH.0b013e32835080b7 (2012).
Shao, M. et al. Depression and cardiovascular disease: Shared molecular mechanisms and clinical implications. Psychiatry Res. 285, 112802. https://doi.org/10.1016/j.psychres.2020.112802 (2020).
Lee, J. W. & Park, S. H. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. J. Affect. Disord. 278, 259–263. https://doi.org/10.1016/j.jad.2020.09.073 (2021).
Cho, I. Y. et al. Depression and increased risk of non-alcoholic fatty liver disease in individuals with obesity. Epidemiol. Psychiatric Sci. 30, e23. https://doi.org/10.1017/s204579602000116x (2021).
Kim, D. et al. Depression is associated with non-alcoholic fatty liver disease among adults in the United States. Aliment. Pharmacol. Ther. 50, 590–598. https://doi.org/10.1111/apt.15395 (2019).
Kamari, N. et al. Fatty liver index relationship with biomarkers and lifestyle: Result from RaNCD cohort study. BMC Gastroenterol. 23. https://doi.org/10.1186/s12876-023-02785-5 (2023).
Lee, K., Otgonsuren, M., Younoszai, Z., Mir, H. M. & Younossi, Z. M. Association of chronic liver disease with depression: A population-based study. Psychosomatics 54, 52–59. https://doi.org/10.1016/j.psym.2012.09.005 (2013).
Elwing, J. E., Lustman, P. J., Wang, H. L. & Clouse, R. E. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom. Med. 68, 563–569. https://doi.org/10.1097/01.psy.0000221276.17823.df (2006).
Youssef, N. A. et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 33, 1062–1070. https://doi.org/10.1111/liv.12165 (2013).
Surdea-Blaga, T. & Dumitraşcu, D. L. Depression and anxiety in nonalcoholic steatohepatitis: Is there any association? Romanian J. Intern. Med. = Revue Roumaine de Med. Interne. 49, 273–280 (2011).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ (Clinical Res. ed.) 362, k601. https://doi.org/10.1136/bmj.k601 (2018).
Sekula, P., Del Greco, M. F., Pattaro, C. & Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. JASN 27, 3253–3265. https://doi.org/10.1681/asn.2016010098 (2016).
Zhang, X., Wu, P. & Lin, Y. Genetic correlation, yet no causal association exists between nonalcoholic fatty liver disease and depression. J. Affect. Disord. 316, 243–244. https://doi.org/10.1016/j.jad.2022.08.033 (2022).
Chen, D., Zhang, Y., Huang, T. & Jia, J. Depression and risk of gastrointestinal disorders: A comprehensive two-sample mendelian randomization study of European ancestry. Psychol. Med. 1–13. https://doi.org/10.1017/s0033291723000867 (2023).
Xiao, J. et al. Is fatty liver associated with depression? A Meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front. Med. 8, 691696. https://doi.org/10.3389/fmed.2021.691696 (2021).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Hagström, H. et al. Administrative coding in electronic health care record-based research of NAFLD: An expert panel consensus statement. Hepatol. (Baltimore Md) 74, 474–482. https://doi.org/10.1002/hep.31726 (2021).
Petermann-Rocha, F. et al. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 76, 1021–1029. https://doi.org/10.1016/j.jhep.2022.01.010 (2022).
VanderWeele, T. J. Principles of confounder selection. Eur. J. Epidemiol. 34, 211–219. https://doi.org/10.1007/s10654-019-00494-6 (2019).
Zelber-Sagi, S. et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 68, 1239–1246. https://doi.org/10.1016/j.jhep.2018.01.015 (2018).
Nucci, D., Fatigoni, C., Amerio, A., Odone, A. & Gianfredi, V. Red and processed meat consumption and risk of depression: A systematic review and Meta-analysis. Int. J. Environ. Res. Public Health 17. https://doi.org/10.3390/ijerph17186686 (2020).
Mongan, D. et al. Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: Cross-sectional and longitudinal associations in a general population cohort. Translational Psychiatry. 11, 321. https://doi.org/10.1038/s41398-021-01425-4 (2021).
Tan, L. J. & Shin, S. Effects of oily fish and its fatty acid intake on non-alcoholic fatty liver disease development among South Korean adults. Front. Nutr. 9, 876909. https://doi.org/10.3389/fnut.2022.876909 (2022).
Shaheen, A. A., Kaplan, G. G., Sharkey, K. A., Lethebe, B. C. & Swain, M. G. Impact of major depression and antidepressant use on alcoholic and non-alcoholic fatty liver disease: A population-based study. Liver Int. 41, 2308–2317. https://doi.org/10.1111/liv.14973 (2021).
Pae, C. U. et al. Pharmacological treatment of chronic fatigue syndrome: Focusing on the role of antidepressants. Expert Opin. Pharmacother. 10, 1561–1570. https://doi.org/10.1517/14656560902988510 (2009).
Foster, H. M. E. et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: A prospective analysis of the UK Biobank cohort. Lancet Public. Health 3, e576–e585. https://doi.org/10.1016/s2468-2667(18)30200-7 (2018).
Martin, D. J. et al. Cardiometabolic disease and features of depression and bipolar disorder: Population-based, cross-sectional study. Br. J. Psychiatry J. Mental Sci. 208, 343–351. https://doi.org/10.1192/bjp.bp.114.157784 (2016).
WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation (World Health Organization, 2011).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645. https://doi.org/10.1161/circulationaha.109.192644 (2009).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352. https://doi.org/10.1038/s41593-018-0326-7 (2019).
Smith, D. J. et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: Cross-sectional study of 172,751 participants. PloS One. 8, e75362. https://doi.org/10.1371/journal.pone.0075362 (2013).
Wu, M. et al. Fine particulate matter, vitamin D, physical activity, and major depressive disorder in elderly adults: Results from UK Biobank. J. Affect. Disord. 299, 233–238. https://doi.org/10.1016/j.jad.2021.12.009 (2022).
Graham, N. et al. Impact of major depression on cardiovascular outcomes for individuals with hypertension: Prospective survival analysis in UK Biobank. BMJ Open 9, e024433. https://doi.org/10.1136/bmjopen-2018-024433 (2019).
Sarkar, C., Webster, C. & Gallacher, J. Residential greenness and prevalence of major depressive disorders: A cross-sectional, observational, associational study of 94 879 adult UK Biobank participants. Lancet Planet. Health. 2, e162–e173. https://doi.org/10.1016/s2542-5196(18)30051-2 (2018).
Cebrino, J. & de la Portero, S. Diet quality and sociodemographic, lifestyle, and health-related determinants among people with depression in Spain: New evidence from a cross-sectional population-based study (2011–2017). Nutrients 13 https://doi.org/10.3390/nu13010106 (2020).
Mannan, M., Mamun, A., Doi, S. & Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatry. 21, 51–66. https://doi.org/10.1016/j.ajp.2015.12.008 (2016).
Pan, A. et al. Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes care. 35, 1171–1180. https://doi.org/10.2337/dc11-2055 (2012).
Blomdahl, J., Nasr, P., Ekstedt, M. & Kechagias, S. Moderate alcohol consumption is associated with significant fibrosis progression in NAFLD. Hepatol. Commun. 7, e0003. https://doi.org/10.1097/hc9.0000000000000003 (2023).
Jung, H. S. et al. Smoking and the risk of non-alcoholic fatty liver disease: A cohort study. Am. J. Gastroenterol. 114, 453–463. https://doi.org/10.1038/s41395-018-0283-5 (2019).
Vilar-Gomez, E. et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatol. (Baltimore Md) 75, 1491–1506. https://doi.org/10.1002/hep.32207 (2022).
Li, L. et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Reviews: Official J. Int. Association Study Obes. 17, 510–519. https://doi.org/10.1111/obr.12407 (2016).
Angelico, F. et al. Non-alcoholic fatty liver syndrome: A hepatic consequence of common metabolic diseases. J. Gastroenterol. Hepatol. 18, 588–594. https://doi.org/10.1046/j.1440-1746.2003.02958.x (2003).
Yuan, M. et al. Hypertension and NAFLD risk: Insights from the NHANES 2017–2018 and mendelian randomization analyses. Chin. Med. J. 137, 457–464. https://doi.org/10.1097/cm9.0000000000002753 (2024).
Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabets Endocrionol. 2, 901–910. https://doi.org/10.1016/s2213-8587(14)70032-4 (2014).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. https://doi.org/10.1002/gepi.21758 (2013).
Yavorska, O. O. & Burgess, S. Mendelian Randomization: An R package for performing mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739. https://doi.org/10.1093/ije/dyx034 (2017).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7. https://doi.org/10.7554/eLife.34408 (2018).
Wootton, R. E. et al. Evaluation of the causal effects between subjective wellbeing and cardiometabolic health: Mendelian randomisation study. BMJ (Clinical Res. ed.). 362, k3788. https://doi.org/10.1136/bmj.k3788 (2018).
Huang, X., Liu, X. & Yu, Y. Depression and Chronic Liver diseases: Are there Shared underlying mechanisms? Front. Mol. Neurosci. 10, 134. https://doi.org/10.3389/fnmol.2017.00134 (2017).
Jha, M. K., Qamar, A., Vaduganathan, M., Charney, D. S. & Murrough, J. W. Screening and management of depression in patients with cardiovascular disease: JACC State-of-the-art review. J. Am. Coll. Cardiol. 73, 1827–1845. https://doi.org/10.1016/j.jacc.2019.01.041 (2019).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556. https://doi.org/10.1016/j.jhep.2023.06.003 (2023).
Hagström, H., Vessby, J., Ekstedt, M. & Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 80, e76–e77. https://doi.org/10.1016/j.jhep.2023.08.026 (2024).
Perazzo, H., Pacheco, A. G. & Griep, R. H. Changing from NAFLD through MAFLD to MASLD: Similar prevalence and risk factors in a large Brazilian cohort. J. Hepatol. 80, e72–e74. https://doi.org/10.1016/j.jhep.2023.08.025 (2024).
De, A., Mehta, M. & Duseja, A. Substantial overlap between NAFLD and MASLD with comparable disease severity and non-invasive test performance: An analysis of the Indian Consortium on MASLD (ICOM-D) cohort. J. Hepatol. 81, e162–e164. https://doi.org/10.1016/j.jhep.2024.05.027 (2024).
Yang, A., Zhu, X., Zhang, L. & Ding, Y. Transitioning from NAFLD to MAFLD and MASLD: Consistent prevalence and risk factors in a Chinese cohort. J. Hepatol. 80, e154–e155. https://doi.org/10.1016/j.jhep.2023.09.033 (2024).
Song, S. J., Lai, J. C., Wong, G. L., Wong, V. W. & Yip, T. C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 80, e54–e56. https://doi.org/10.1016/j.jhep.2023.07.021 (2024).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034. https://doi.org/10.1093/aje/kwx246 (2017).
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 84980, and we are grateful to UK Biobank participants. Besides, our MR was based on publicly available GWAS databases, we thank all investigators for sharing these data.
Author information
Authors and Affiliations
Contributions
L.L and X.R.Z had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. L.L conceived and designed the study, and supervised it. X.R.Z conducted statistical analysis and drafted the manuscript. All authors were involved in acquisition, analysis or interpretation the data, and critical revision of the manuscript for important intellectual content. X.H.Z provided administrative, technical, or material support. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The UK Biobank study has approval from the North West Multi-centre Research Ethics Committee, all methods were performed in accordance with the relevant guidelines and regulations, and all participants provided written informed consent. As our MR study was based on publicly available data, no additional ethical approval or consent was required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, X., Liao, J., Liu, L. et al. Association of depression with severe non-alcoholic fatty liver disease: evidence from the UK Biobank study and Mendelian randomization analysis. Sci Rep 14, 28561 (2024). https://doi.org/10.1038/s41598-024-79100-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79100-z