Abstract
DNA methylation is known to be involved in tumor progression. This is the first study to perform an extensive methylation analysis of plasma circulating tumor DNA (ctDNA) using targeted bisulfite sequencing in gastric cancer (GC) patients to evaluate the usefulness of ctDNA methylation as a new biomarker. Sixteen patients who received chemotherapy for recurrent GC were included. After confirmation of the methylation status of 63 genes using the Cancer Genome Atlas (TCGA) dataset, the methylation status in paired tumor and non-tumor tissues and plasma were investigated using targeted bisulfite sequencing in these genes. Forty-four of the 63 genes were significantly hypermethylated in GC patients in the TCGA cohort. Of these 44 genes, hierarchical clustering showed that five (SPG20, FBN1, SDC2, TFPI2, SEPT9) were particularly hypermethylated in tumor compared to non-tumor tissues in our GC cohort. In plasma methylation analysis, patients with high methylation of these genes had significantly worse overall survival than those with low methylation (log-rank P = 0.009). In a patient who underwent blood sampling at multiple points, the methylation levels of these five genes varied closely with clinical tumor status. The plasma ctDNA methylation levels of these five genes could be useful as a noninvasive prognostic biomarker for GC.
Similar content being viewed by others
Introduction
Globally, surgery has been the standard curative treatment for gastric cancer (GC), but many patients experience postoperative recurrence, and the prognosis is still poor despite various chemotherapies have been developed1,2,3,4,5,6. Several approaches thus far have been used to evaluate GC progression, including imaging examinations and the measurement of serum tumor markers7. However, the sensitivity of tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) is only about 40–50%, even in stage IV patients8,9. In addition, frequent use of computed tomography is not suitable for tumor status evaluation because of its low sensitivity for the detection of peritoneal metastases and the risk of radiation exposure10,11. Therefore, there is an urgent need to develop minimally invasive, accurate biomarkers for GC that can be used in real time.
Liquid biopsy is broadly defined as the analysis of tumor material that has been obtained in a minimally invasive manner through the sampling of blood or other bodily fluids12,13. Circulating tumor DNA (ctDNA), which is tumor-derived DNA that has been released into the blood through tumor cell necrosis or apoptosis has been well studied. The amount of ctDNA in the blood has been reported to correlate with disease progression status in various cancer types14,15,16,17. In a previous study, we performed targeted deep sequencing of plasma cell–free DNA in GC patients and demonstrated a correlation between tumor status and mutations of TP53-ctDNA18. However, TP53 mutations are found in only 56% of GC patients19; therefore, ctDNA analysis targeting specific gene mutations may be impractical for clinical use.
To overcome the limitations of ctDNA analysis in terms of identifying specific gene mutations, we focused on the detection of DNA methylation as a regulatory mechanism for epigenetic gene modification in oncogenesis20. Although several studies have explored the potential use of DNA methylation as a biomarker for prognostic purposes, the tissue methylation levels in these studies were mainly determined using invasive surgical methods21,22. Other studies analyzed ctDNA methylation in plasma from GC patients using methylation-specific PCR (MSP) or digital PCR, but they only evaluated a limited range of CpG sites in a small number of genes23,24,25. Here, with the aim of identifying new biomarkers for monitoring tumor status and predicting prognosis, we investigated the methylation status in paired tumor and non-tumor (T/N) tissues and plasma of patients with recurrent GC using target bisulfite sequencing of a broad range of CpG sites in 63 genes.
Results
Methylation status of the 63 genes in the TCGA cohort
The methylation status of the 63 genes in GC patients was analyzed using the TCGA-STAD dataset. After downloading methylation β-value data of the 63 genes from the Illumina Human Methylation 27 and/or 450 platforms, we confirmed that 44 of these genes were significantly hypermethylated in tumor tissues compared with non-tumor tissues (Supplementary table S1), and we analyzed these 44 genes in this study.
Methylation status of the 44 TCGA-derived genes in our cohort
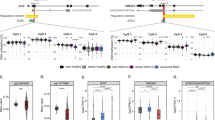
We conducted methylation status profiling of the 44 genes selected from the TCGA cohort using targeted bisulfite sequencing of DNA samples from 10 paired T/N tissues in our cohort. Figure 1 shows a heatmap of the ratio of the methylation β-value in tumor tissues to that in non-tumor tissues (T/N ratio) for the 44 genes in our cohort. Hierarchical clustering analysis of all 44 genes revealed four major clusters (A, B, C, and D). The cluster-A genes (SPG20, FBN1, SDC2, TFPI2, SEPT9) showed the highest mean T/N ratio (cluster-A, 3.424; cluster-B, 1.693; cluster-C, 1.827; cluster-D, 1.120).
Heatmap of the methylation β-value ratio between tumor and non-tumor tissues for the 44 genes in our cohort. Heatmap of the methylation β-value ratio between tumor and non-tumor tissues (T/N ratio) for 44 genes that were determined to be specifically hypermethylated in GC tumors in the TCGA cohort. The T/N ratio is expressed as − 1.2 to 1.2 by ordinary logarithmic conversion.
Methylation status analysis in plasma
We next performed methylation analysis of the 44 genes using targeted bisulfite sequencing of DNA samples from the plasma of 16 patients with postoperative recurrent GC. Figure 2 shows a heatmap of the methylation β-values of these 44 genes. In hierarchical clustering analysis of these genes, the 16 patients were divided into highly and poorly methylated groups, in which the mean β-values of the 44 genes were 0.1599 and 0.0710, respectively. Hierarchical clustering analysis of the five genes in cluster-A (SPG20, FBN1, SDC2, TFPI2, SEPT9) resulted in categorization of the patients into the same two groups, with mean β-values of the five genes of 0.2159 and 0.0418, respectively (Fig. 3). Even when the 16 patients were divided into two groups using the overall mean of 0.0854 as the cutoff, the same patients were included in the highly methylated group (Supplementary Table S2).
Heatmap of cluster-A gene (SPG20, FBN1, SDC2, TFPI2, and SEPT9) methylation levels in the plasma of 16 GC cases at the time of postoperative recurrence. Mean methylation levels of the five cluster-A genes (SPG20, FBN1, SDC2, TFPI2, and SEPT9) are shown as a heatmap. Methylation levels are expressed as β-values, representing continuous measurements from 0 (completely unmethylated, shown in green) to 1 (completely methylated, shown in red).
Prediction of OS by plasma methylation status
We used the Kaplan–Meier method to assess the groups obtained in the hierarchical clustering analysis of the five cluster-A genes. The highly methylated group showed significantly worse OS from the date of blood sampling than the poorly methylated group (log-rank P = 0.009) (Fig. 4a). On the other hand, when the cases were divided into two groups by the serum CEA or CA19-9 level measured at the same time as ctDNA blood sampling, with a cutoff of twice the upper normal limit, the high CEA or CA19-9 group and the normal CEA or CA19-9 group showed no statistical difference in OS (log-rank P = 0.208) (Fig. 4b).
Relationship between methylation status and clinical tumor status
The relationships between the methylation levels of the five genes and various points in the clinical course in Case No. 3 are presented in Fig. 5. This patient had clinical stage III GC with lymph node metastasis and was treated with neoadjuvant chemotherapy followed by total gastrectomy with R0 resection. Adjuvant chemotherapy was administered, but a CT scan performed 1 year after surgery revealed peritoneal and bilateral ovarian recurrences. In this patient, blood sampling was performed at five time points, as described in the 'Patients and samples’ subsection of the Methods section. Plasma before preoperative chemotherapy had the highest mean β-value of the five significant genes, at 0.054, but this value decreased to 0.026 after preoperative chemotherapy, and remained stable during postoperative chemotherapy. However, at the time of recurrence, the methylation level again increased to 0.039. In this patient, neither CEA nor CA19-9 showed elevation above the upper normal limit at any time point.
Discussion
This study demonstrated that five hypermethylated genes (SPG20, FBN1, SDC2, TFPI2, SEPT9) were particularly hypermethylated in advanced GC tissues compared with non-tumor tissues in both the TCGA dataset and our cohort. Additionally, the patients with higher ctDNA methylation levels of these five genes had a significantly worse prognosis. Although the number of cases studied was small, the results suggest that ctDNA methylation levels of these five genes in plasma closely correlate with clinical tumor status. Considering that two currently available tumor markers, CEA and CA19-9, did not clearly indicate prognosis, the plasma ctDNA methylation status of these five genes could be a more sensitive biomarker for monitoring tumor status.
Since the 2010s, we have conducted liquid biopsy research on gastrointestinal malignancies using ctDNA obtained from peripheral blood. We initially analyzed TP53-ctDNA using targeted deep sequencing of plasma cell–free DNA in GC patients, and found that the TP53-ctDNA mutant fraction correlated well with tumor disease status18. In a second study, we investigated secondary C-KIT mutations in the plasma of patients with imatinib-resistant gastrointestinal stromal tumors, and the ctDNA fraction changed with tumor status26. However, the major problem with the methods used in these two studies was that low concentrations of ctDNA could not be detected. To achieve more sensitive and versatile ctDNA detection, our next study employed NGS with molecular barcodes (MB)27 and targeted TP53-ctDNA of esophageal squamous cell carcinoma. The maximum frequency of background errors decreased from 3.22% without MB to 0.08% with MB, indicating marked improvement in the sensitivity of ctDNA detection. TP53 mutations are found in 90% of patients with esophageal squamous cell carcinoma, but in only 24% of patients with GC18; this suggests that the detection of ctDNA mutations may be impractical for malignancies like GC in which gene mutations are not very specific. Therefore, in the current study we focused on ctDNA methylation status as a regulatory mechanism for epigenetic gene modification in oncogenesis.
Studies of liquid biopsy and ctDNA methylation have recently been gaining attention in several cancers, but thus far not in GC28,29,30. Yan et al. used digital PCR technologies to examine plasma from 148 GC patients who underwent chemotherapy and found that hypermethylation of SFRP2 was associated with worse survival23. Hu et al. reported that the methylation status of THBS1 detected with quantitative MSP of preoperative peripheral blood was significantly associated with adverse prognosis24. Although the digital PCR and MSP techniques used in these previous studies were simple and inexpensive, they allow for a very limited number of genes and CpG sites to be examined. Therefore, we utilized the QIAseq Targeted DNA panels as the method for a larger number of CpG sites and cancer-specific genes related to DNA methylation. Currently, sequencers using bisulfite conversion and microarrays are available for the detection of DNA methylation status in the human genome on a single-nucleotide basis. While microarrays are inexpensive and can analyze a large number of samples, only certain methylated CpG sites can be measured31. On the other hand, targeted bisulfite sequencing has the advantage of evaluating a wider range of methylated CpG sites and are reported no difference in the performance compared to microarrays32. Although in this study we analyzed 63 genes that were included in the QIAseq Targeted DNA panels, many of these genes were also found to be associated with GC, and in most of them the hypermethylated status was confirmed in the TCGA cohort20,21.
In this study, we highlighted five particularly hypermethylated genes. SPG20 encodes a multifunctional protein that is involved in intracellular epidermal growth factor receptor trafficking33. Furthermore, downregulation of SPG20 may cause carcinogenesis34, and methylation-mediated gene silencing of SPG20 has been demonstrated previously in GC33,35. FBN1 is involved in the production of the extracellular matrix component fibrillin-1, and its blood and stool methylation status has been reported as a potential biomarker in colorectal cancer (CRC)36,37. SDC2 is a transmembrane protein involved in cell proliferation, cell migration and cell–matrix interactions38. In GC cells, SDC2 plays a regulatory role in migration and invasion39. Two studies reported that plasma and stool tests of SDC2 methylation were useful in screening for GC and CRC40,41. Regarding TFPI2, some reports suggested that hypermethylation of its promoter CpG island was more prevalent in primary GC tissues than normal tissues42,43. In addition, tissue and plasma TFPI2 methylation was reported to be a prognostic marker in GC patients44,45. TFPI2 is also one of the top-performing biomarkers in GC methylation studies with a large number of cases46. SEPT9 is an important component of the cytoskeleton and influences cell polarization, intracellular material transport, cell cycle regulation, and cell apoptosis47,48. Many biomarker studies using SEPT9 methylation have been reported previously in various tumor types49,50,51. In GC, Lee et al. showed that patients with plasma SEPT9 methylation before treatment were more likely to develop distant metastasis and to have worse disease-free survival than those without such methylation52. In addition, plasma SEPT9 methylation was shown to be a useful diagnostic biomarker even in early-stage GC53. The literature reports on the five aforementioned genes strongly support the findings in this study indicating that the blood methylation levels of these genes could become important biomarkers for GC. Their relevance to the mechanism of tumor progression remains largely unexplored and requires further study.
This study also analyzed ctDNA methylation in a series of GC patients before and after preoperative chemotherapy, before and after surgery, and at postoperative recurrence, and showed that the methylation levels of these five genes were closely correlated with disease status. In various tumors, quantifying ctDNA through the methylation analysis of some genes correlates with total tumor burden and can therefore be used for the prognosis and disease monitoring biomarker30. Some studies have investigated the relationship between ctDNA methylation and clinical disease course in CRC30,54,55,56, but few have examined this issue in GC patients. Regarding the efficacy of chemotherapy in metastatic CRC patients, a prospective study evaluated NPY methylation demonstrated that the rate of change in ctDNA methylation before and after chemotherapy was associated with the early prediction of treatment benefit30,54. In a study evaluating changes in ctDNA methylation of GRI4A before and after curative resection in CRC patients, the methylation rate decreased by 4.5 fold in metastatic CRC patients55. Our present report suggests that ctDNA methylation may reflect tumor disease status in GC patients as well as in those with CRC. Furthermore, several studies reported that ctDNA methylation biomarkers are more effective for predicting recurrence than existing CEA or imaging tests in CRC patients56. In our case No. 3, the ctDNA methylation of the five genes showed a correlation with tumor disease progression although CEA and CA19-9 did not change. We consider that our research suggests the possibility of ctDNA methylation as a marker for predicting recurrence in GC patients, but further research would be needed to compare to existing modalities.
There are several limitations to this study. First, the number of examined cases was small. However, there are still few methylation-related biomarker studies targeting a broad range of CpG sites specifically in patients with recurrent GC, and this study provides new insights despite the limited sample size. Second, we analyzed the methylation status of only 63 genes, and other genes may be suitable as biomarkers. Further studies on DNA methylation in GC are needed to understand the roles of other genes and their mechanisms in cancer progression. Third, the patient population is very heterogeneous, with different treatment regimens as well as varying metastasis. However, we believe that ctDNA methylation is not influenced by the patient’s metastatic sites or treatment regimens. Indeed, previous methylation-related biomarker research has also included patients across various stages and metastatic levels56,57,58. In addition, patients with GC present with different recurrence patterns, and chemotherapy regimens are diverse as well. For these reasons, we comprehensively included these patients and demonstrated that those with higher ctDNA methylation levels of these five genes had a significantly worse prognosis, regardless of the metastatic regions or treatment regimens. Finally, targeted bisulfite sequencing is very costly and time consuming, making it difficult to implement in clinical practice. As a solution to these problems, we plan to use digital PCR in a larger number of cases to study the methylation status of the five genes selected in this study and to demonstrate their usefulness as biomarkers.
In conclusion, we demonstrated that the methylation status of five particularly hypermethylated genes (SPG20, FBN1, SDC2, TFPI2, SEPT9) in plasma reflected tumor status and prognosis in recurrent GC. This is the first study to perform an extensive methylation analysis of plasma ctDNA in patients with GC using targeted bisulfite sequencing to identify potential biomarkers.
Methods
Patients and samples
This study included 16 patients who received chemotherapy for recurrent GC at Osaka University Hospital between July 2019 and August 2021 (Table 1). Plasma samples were collected from all 16 patients (Cases No. 1 – No. 16). In Case No. 3, blood samples were obtained and stored at five time points, as follows: before neoadjuvant chemotherapy, before and after surgery, during adjuvant chemotherapy, and at the time of recurrence. Clinical staging was based on the 15th edition of the Japanese Classification of Gastric Carcinoma. Written informed consent was obtained from all patients before sampling. This study was performed in accordance with the Declaration of Helsinki and approved by the Osaka University Research Ethics Committee (No. 894).
DNA preparation
Paired T/N tissues were obtained from formalin-fixed, paraffin-embedded (FFPE) slides of stomach specimens resected during the primary surgery from 10 patients (Cases No. 1 – No. 10), in which a sufficient amount of DNA for analysis could be isolated. DNA from tissue samples was isolated using the GeneRead DNA FFPE Kit (QIAGEN, Hilden, Germany). Blood samples were collected in 10-mL ethylenediaminetetraacetic acid disodium salt, 2-hydrate (EDTA-2Na) vacutainer tubes. Plasma was separated from blood cells via centrifugation at 1600 g for 10 min at 4 °C, followed by re-centrifugation at 16,000 g for 10 min at 4 °C. The centrifugation was conducted within 2 h and the plasma samples were stored at − 80 °C until DNA extraction. DNA from plasma samples was isolated using the Apostle MiniMax™ High Efficiency cfDNA Isolation Kit (Beckman Coulter, CA, USA).
Methylation panel and the Cancer Genome Atlas (TCGA) cohort
The QIAseq Targeted DNA panels (DHS-002Z, QIAGEN, Hilden, Germany) targets thousands of CpG sites in cancer-specific 63 genes (Supplementary table S3). To confirm the methylation status of these 63 genes in GC, we used TCGA-Stomach Adenocarcinoma (STAD) dataset and the UCSC Xena browser (https://xena.ucsc.edu). Gene methylation levels were scored using a β-value between 0 (completely unmethylated) and 1 (completely methylated). The 63 genes were analyzed by downloading the methylation β-value data of each case from the Illumina Human Methylation 27 and 450 platforms (https://xenabrowser.net). The mean methylation β-value was calculated for each of the 63 genes. Since no specific selection criteria exist for identifying genes with higher methylation in tumor tissues compared to non-tumor tissues using the β-value, we used the T/N ratio to analyze the differences. To broadly narrow down the list of higher methylated genes in GC, genes in the TCGA cohort were selected on the basis of the following: (i) the ratio of the β-value in tumor tissues to that in non-tumor tissues (β-value T/N ratio) > 1, and (ii) a P value < 0.05 as determined by the Wilcoxon rank sum test comparing tumor and non-tumor tissues.
Bisulfite conversion and next-generation sequencing (NGS)
Briefly, 5 ng plasma DNA and 200 ng FFPE DNA were bisulfite-converted with the EPITECT Fast Bisulfite Sequencing conversion kit (QIAGEN). The bisulfite-converted DNA was used as the input template to create targeted libraries as per the QIAseq Targeted Methyl Panel protocol. Sequencing was then performed on an Illumina Miseq 2 × 150 bp with V2 chemistry (Illumina, CA, USA). Paired-end reads were mapped to the human genome reference (hg19). Data were analyzed by the GeneGlobe Data Analysis Center.
Methylation β-value
For each CpG site, the DNA methylation level, defined as a β-value, was calculated as the methylated coverage (the number of reads with evidence of methylation at that position) divided by the context coverage (the number of reads conforming to the selected methylation context)32. The region involved in protein coding was selected as the gene type. The analysis area was ± 1,500 base pairs upstream and downstream from the transcription start site (TSS). Methylation values were defined as missing when the context coverage was less than 10X. Finally, the β-value of each gene was calculated as the mean β-value of the included CpG sites. Hypermethylated genes in GC were assessed by comparing the β-values in tumor and non-tumor tissues. Selection of particularly hypermethylated genes was based on hierarchical clustering in the heatmap of the methylation β-value T/N ratio.
Statistics
Hierarchical clustering was performed using Ward’s minimum variance method. Overall survival (OS) was defined as the interval from the date of blood sampling to the date of death from any cause. Survival rates were estimated using the Kaplan–Meier method and compared using the log-rank test. All statistical analyses were performed using JMP PRO software (JMP version 17.0.0; SAS Institute, Cary, NC).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hashimoto, T., Kurokawa, Y., Mori, M. & Doki, Y. Update on the treatment of gastric cancer. JMA J 1, 40–49 (2018).
Kurokawa, Y. et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): A phase 3, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 3, 460–468 (2018).
Kurokawa, Y. et al. Five-year follow-up of a randomized clinical trial comparing bursectomy and omentectomy alone for resectable gastric cancer (JCOG1001). Br. J. Surg. 110, 50–56 (2022).
Yanagimoto, Y., Kurokawa, Y. & Doki, Y. Essential updates 2021/2022: Perioperative and surgical treatments for gastric and esophagogastric junction cancer. Ann. Gastroenterol. Surg. 7, 698–708 (2023).
Kurokawa, Y. et al. Phase 2 trial of neoadjuvant docetaxel, oxaliplatin, and S-1 for clinical stage III gastric or esophagogastric junction adenocarcinoma. Ann. Gastroenterol. Surg. 7, 247–254 (2023).
Kurokawa, Y. et al. Docetaxel plus S-1 versus cisplatin plus S-1 in unresectable gastric cancer without measurable lesions: a randomized phase II trial (HERBIS-3). Gastric Cancer 24, 428–434 (2021).
Smyth, E. C. et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v38–v49 (2016).
Shimada, H., Noie, T., Ohashi, M., Oba, K. & Takahashi, Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 17, 26–33 (2014).
Wada, N. et al. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19–9 levels in gastric cancer cases. Surg. Today 47, 227–232 (2017).
Zhao, L., Li, M., Zhang, S. & Liu, Y. Plasma-methylated SEPT9 for the noninvasive diagnosis of gastric cancer. J. Clin. Med. Res. 11 (2022).
Dong, D. et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 30, 431–438 (2019).
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 36, 1631–1641 (2018).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Hou, J., Li, X. & Xie, K.-P. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol. Cancer 20, 34 (2021).
Nagasaka, M. et al. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol. Cancer 20, 82 (2021).
Lim, S. Y., Lee, J. H., Diefenbach, R. J., Kefford, R. F. & Rizos, H. Liquid biomarkers in melanoma: detection and discovery. Mol. Cancer 17, 8 (2018).
Dawson, S.-J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Hamakawa, T. et al. Monitoring gastric cancer progression with circulating tumour DNA. Br. J. Cancer 112, 352–356 (2015).
van Beek, E. J. A. H. et al. Rates of TP53 mutation are significantly elevated in African American patients with gastric cancer. Ann. Surg. Oncol. 25, 2027–2033 (2018).
Zeng, Y. et al. DNA methylation: An important biomarker and therapeutic target for gastric cancer. Front. Genet. 13, 823905 (2022).
Matsusaka, K. et al. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 71, 7187–7197 (2011).
Anderson, B. W. et al. Detection of gastric cancer with novel methylated DNA markers: Discovery, tissue validation, and pilot testing in plasma. Clin. Cancer Res. 24, 5724–5734 (2018).
Yan, H. et al. Value of plasma methylated SFRP2 in prognosis of gastric cancer. Dig. Dis. Sci. 66, 3854–3861 (2021).
Hu, X.-Y. et al. Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer. J. Clin. Lab. Anal. 35, e23936 (2021).
Karamitrousis, E. I. et al. Prognostic role of RASSF1A, SOX17 and Wif-1 promoter methylation status in cell-free DNA of advanced gastric cancer patients. Technol. Cancer Res. Treat. 20, 1533033820973279 (2021).
Wada, N. et al. Detecting secondary C-KIT mutations in the peripheral blood of patients with imatinib-resistant gastrointestinal stromal tumor. Oncology 90, 112–117 (2016).
Hagi, T. et al. Molecular barcode sequencing for highly sensitive detection of circulating tumor DNA in patients with esophageal squamous cell carcinoma. Oncology 98, 222–229 (2020).
Jensen, L. H. et al. NPY gene methylation as a universal, longitudinal plasma marker for evaluating the clinical benefit from last-line treatment with regorafenib in metastatic colorectal cancer. Cancers 11 (2019).
Boeckx, N. et al. Mutation and methylation analysis of circulating tumor DNA can be used for follow-up of metastatic colorectal cancer patients. Clin. Colorectal Cancer 17, e369–e379 (2018).
Janssens, K. et al. NPY methylated ctDNA is a promising biomarker for treatment response monitoring in metastatic colorectal cancer. Clin. Cancer Res. 29, 1741–1750 (2023).
Zhu, Z. et al. MethMarkerDB: A comprehensive cancer DNA methylation biomarker database. Nucleic Acids Res. https://doi.org/10.1093/nar/gkad923 (2023).
Yu, C. et al. Repeatability of methylation measures using a QIAseq targeted methyl panel and comparison with the Illumina HumanMethylation450 assay. BMC Res. Notes 14, 394 (2021).
Zhang, H. et al. Detection of aberrant hypermethylated spastic paraplegia-20 as a potential biomarker and prognostic factor in gastric cancer. Med. Oncol. 31, 830 (2014).
Lind, G. E. et al. SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene 30, 3967–3978 (2011).
Wei, K.-L. et al. Methylomics analysis identifies a putative STAT3 target, SPG20, as a noninvasive epigenetic biomarker for early detection of gastric cancer. PLoS ONE 14, e0218338 (2019).
Kerslake, R. et al. A pancancer overview of FBN1, asprosin and its cognate receptor OR4M1 with detailed expression profiling in ovarian cancer. Oncol. Lett. 22, 650 (2021).
Li, W.-H. et al. Detection of SNCA and FBN1 methylation in the stool as a biomarker for colorectal cancer. Dis. Markers 2015, 657570 (2015).
Oh, T. et al. Genome-wide identification and validation of a novel methylation biomarker, SDC2, for blood-based detection of colorectal cancer. J. Mol. Diagn. 15, 498–507 (2013).
Wang, J. et al. Expression of Syndecan-2 in gastric adenocarcinoma and its effect on tumorigenesis in vitro. Transl. Cancer Res. 6, 658–666 (2017).
Ma, L. et al. A novel stool methylation test for the non-invasive screening of gastric and colorectal cancer. Front. Oncol. 12, 860701 (2022).
Guo, J., Li, J., Chang, J., Wang, L. & Xi, Y. Value of methylation status of RPRM, SDC2, and TCF4 genes in plasma for gastric adenocarcinoma screening. Int. J. Gen. Med. 16, 673–681 (2023).
Takada, H. et al. Tissue factor pathway inhibitor 2 (TFPI2) is frequently silenced by aberrant promoter hypermethylation in gastric cancer. Cancer Genet. Cytogenet. 197, 16–24 (2010).
Hu, H. et al. The role of TFPI2 hypermethylation in the detection of gastric and colorectal cancer. Oncotarget 8, 84054–84065 (2017).
Jee, C. D., Kim, M. A., Jung, E. J., Kim, J. & Kim, W. H. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur. J. Cancer 45, 1282–1293 (2009).
Hibi, K. et al. Detection of TFPI2 methylation in the serum of gastric cancer patients. Anticancer Res. 31, 3835–3838 (2011).
Ren, J. et al. Genome-scale methylation analysis of circulating cell-free DNA in gastric cancer patients. Clin. Chem. 68, 354–364 (2022).
Nakos, K., Rosenberg, M. & Spiliotis, E. T. Regulation of microtubule plus end dynamics by septin 9. Cytoskeleton 76, 83–91 (2019).
Li, W. et al. SNHG3 affects gastric cancer development by regulating SEPT9 methylation. J. Oncol. 2022, 3433406 (2022).
Li, B. et al. SEPT9 gene methylation as a noninvasive marker for hepatocellular carcinoma. Dis. Markers 2020, 6289063 (2020).
Dietrich, D. et al. Circulating cell-free SEPT9 DNA methylation in blood is a biomarker for minimal residual disease detection in head and neck squamous cell carcinoma patients. Clin. Chem. 69, 1050–1061 (2023).
Jin, S. et al. Efficient detection and post-surgical monitoring of colon cancer with a multi-marker DNA methylation liquid biopsy. Proc. Natl. Acad. Sci. USA 118 (2021).
Lee, H. S. et al. Circulating methylated septin 9 nucleic Acid in the plasma of patients with gastrointestinal cancer in the stomach and colon. Transl. Oncol. 6, 290–296 (2013).
Cao, C.-Q., Chang, L. & Wu, Q. Circulating methylated Septin 9 and ring finger protein 180 for noninvasive diagnosis of early gastric cancer. Transl. Cancer Res. 9, 7012–7021 (2020).
Thomsen, C. B. et al. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 12, 1758835920918472 (2020).
Lukacova, E. et al. Hypermethylated GRIA4, a potential biomarker for an early non-invasive detection of metastasis of clinically known colorectal cancer. Front. Oncol. 13, 1205791 (2023).
Wang, W. et al. Recurrence risk assessment for stage III colorectal cancer based on five methylation biomarkers in plasma cell-free DNA. J. Pathol. 259, 376–387 (2023).
Liu, Y. et al. Methylation of serum SST gene is an independent prognostic marker in colorectal cancer. Am. J. Cancer Res. 6, 2098–2108 (2016).
Pedersen, S. K. et al. Detection of methylated BCAT1 and IKZF1 after curative-intent treatment as a prognostic indicator for colorectal cancer recurrence. Cancer Med. 12, 1319–1329 (2023).
Acknowledgements
We received funding from MEXT KAKENHI Grant Number JP23K08169. We would like to thank Dr. Kyoko Masumi-Koizumi for her excellent assistance, and all patients, without whom this study would not have been possible.
Author information
Authors and Affiliations
Contributions
S.N., Y.K., T.H., and R.Y. designed the overall experiments and had unrestricted access to all data. S.N. and Y.K. performed statistical analyses and drafted the manuscript. S.N., Y.K., T.T., T.S., K.Y., K.M., K.Y., K.T., T.M., and K.N. collected patient samples. H.E. and Y.D. provided critical reading of the manuscript and insightful comments. All authors read and approved the final draft of the manuscript and take full responsibility for its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nagano, S., Kurokawa, Y., Hagi, T. et al. Extensive methylation analysis of circulating tumor DNA in plasma of patients with gastric cancer. Sci Rep 14, 30739 (2024). https://doi.org/10.1038/s41598-024-79252-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79252-y