Abstract
Vascular calcification is the main cause of death in patients with chronic kidney disease (CKD). The study aims to investigate the risk factor affecting coronary artery calcification (CAC), and the relationship between serum hypoxia-inducible factor-1α (HIF-1α) levels and CAC in non-dialysis CKD patients. One hundred and twenty-three patients were selected in the final analysis. Spearman rank correlation analysis assessed the correlation between HIF-1α levels and CAC scores. Logistic regression analysis was utilized to assess potential risk variables associated with CAC. A ROC curve was drawn to analyze the diagnostic value of HIF-1α in non-dialysis CKD patients with CAC. Compared to the non-CAC group, the CAC group was older age, with a higher proportion of males, smokers, hypertension and diabetes, lower eGFR and HDL-C, and higher SBP and HIF-1α levels. As the HIF-1α levels went up, the proportion of patients with VC increased, as well as Agatston scores. Spearman rank correlation analysis showed that HIF-1α levels were positively correlated with VC and Agatston scores. Logistic regression analysis indicated that being male (OR = 0.242, P = 0.036), older age (OR = 1.093, P < 0.001), and increased HIF-1α levels (OR = 1.002, P = 0.025) were risk factors for the occurrence of CAC. Furthermore, ROC curve analysis determined that a HIF-1α cutoff value of 451.177 pg·mL-1 provided the best diagnostic value for non-dialysis CAC, with a sensitivity of 65.8% and a specificity of 80.9%. The prevalence of CAC is high in non-dialysis CKD patients, with old age and male gender being traditional risk factors for CAC occurrence. HIF-1α positively correlate with both CAC and VC, which can provide certain diagnostic values for non-dialysis CAC.
Similar content being viewed by others
Introduction
The prevalence of chronic kidney disease (CKD) is increasing gradually with the prolongation of the human lifespan, the improvement of diagnosis levels, and the rising incidence of hypertension and diabetes1. In CKD patients, cardiovascular events are the most common cause of death. Vascular calcification, especially coronary artery calcification (CAC), is a major risk factor contributing to increased risk of cardiovascular events2. The severity of CAC can predict the incidence of cardiovascular events and the mortality of CKD patients345. Unlike atherosclerosis, which leads to intimal calcification, patients with CKD and diabetes experience mainly medial calcification6. Previously, it was widely believed that vascular calcification was a passive result of hydroxyapatite deposition in the vessel wall. However, studies in recent years have found that vascular calcification in CKD patients is a complex process resulting from multiple factors. In addition to traditional risk factors, the transformation of vascular smooth muscle cells (VSMCs) to an osteogenic phenotype, inflammation, oxidative stress, cellular autophagy, and apoptosis play important roles in the process of vascular calcification789.
Hypoxia-inducible factor (HIF) is an important regulator of erythropoiesis and iron metabolism. In addition, it is associated with glucose metabolism, lipid metabolism, vascular neogenesis, inflammatory and oxidative stress, and is involved in bone formation and repair10. In recent years, several in vitro studies have shown that elevation of HIF-1α level is associated with vascular calcification11,12, and clinical study has also found that serum HIF-1α is higher in patients with type 2 diabetes mellitus in the presence of severe CAC13. However, in the field of CKD, studies on HIF-1α promoting calcification have mostly focused on animal models or in vitro experiments. To determine whether there is an association between HIF-1α levels and CAC in patients with nondialysis CKD, and how HIF-1α levels vary by CKD stage, we conducted this study. Here, we compared the clinical characteristics of participants in the CAC group with those in the non-CAC group and assessed the association of cardiac valve calcification (VC) and CKD stage with HIF-1α.
Methods
Study population
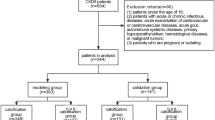
The study retrospectively screened 241 non-dialysis patients with CKD 1 ~ 5 stages admitted at the Department of Nephrology, Hebei General Hospital, from February 1, 2023, to July 31, 2023. According to the exclusion criteria, 118 patients who were not eligible for the study were excluded, resulting in the final analysis enrolling a total of 123 patients (Fig. 1).
Inclusion criteria were as follows: (1) age ≥ 18 years; (2) complete clinical data; (3) met the diagnostic criteria for CKD; (4) had not undergone renal replacement therapy, including hemodialysis, peritoneal dialysis, and renal transplantation. Exclusion criteria were as follows: (1) severe infection within 2 weeks before enrollment; (2) history of blood transfusion within the last 6 months; (3) respiratory failure, pulmonary embolism, or presence of chronic lung disease, including chronic obstructive pulmonary disease and pulmonary hypertension; (4) sleep apnea syndrome; (5) severe arterial stenosis or occlusion; (6) previous coronary stenting; (7) CKD in combination with acute kidney injury (AKI); (8) need for renal replacement therapy within the following 3 months; (9) treatment with erythropoietin or prolyl hydroxylase inhibitors (PHIs) in the 1 month before enrollment; and (10) presence of malignancy or malignant hematologic disease.
The study protocol was approved by the Ethics Committee of Hebei General Hospital (NO.2024131). We confirm that all methods were performed in accordance with the relevant guidelines and regulations. All patients signed an informed consent form.
Data collection
Demographic information was collected from the patient’s electronic medical record system for all eligible participants, including age, gender, height, weight, and history of smoking, drinking, hypertension and diabetes. The patient’s systolic and diastolic blood pressures (SBP and DBP) were measured at admission.
Laboratory data were collected from the patients, including hemoglobin (HGB), white blood cell count (WBC), platelet count (PLT), total protein (TP), serum albumin (ALB), globulin (GLB), alkaline phosphatase (ALP), fasting plasma glucose (FPG), serum phosphorus (P), serum calcium (Ca), serum creatinine (Scr), uric acid (UA), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and lipoprotein(a) (Lp(a)).
Biochemical measurements
The patient’s blood samples were collected after 8 h of water fasting and left at room temperature for 2 h. After separating the red blood cells and serum by centrifuge, the serum samples were divided into 0.5 ml EP tubes and stored at -80 ℃ until use. According to the manufacturer’s instructions, using a human ELISA kit (Elabscience, Wuhan, China) measured HIF-1α levels. Kit detection range: 62.5 ~ 4000 pg/mL. Using a MUTISKAN microplate reader with the wavelength set to 450 nm detected the optical density value (OD) of HIF-1α. Using Origin 9.8 calculated the concentration of HIF-1α.
Measurement of coronary artery calcium score (CACs)
All patients underwent multi-slice spiral computed tomography (MSCT). A clinical imaging physician conducted a blind quantitative analysis of the extent of calcification in CAC patients. The total CAC score was determined based on the Agatston method14. Patients were divided into the coronary artery calcification group (CAC group) and non-coronary artery calcification group (non-CAC group) according to the presence or absence of CAC, and patients with CAC were further classified into mild calcification group (CaCs 1 ~ 100 Hu); moderate calcification group (CaCs 101 ~ 400 Hu), and severe calcification group (CaCs ≥ 400 Hu) according to the calcification score.
Measurement of cardiac valve calcification (CVC)
An experienced sonographer performed echocardiography on the patients using a diagnostic color Doppler ultrasound machine (Philips CX 50 Netherlands) with a probe frequency of 1.0 to 5.0 MHz. Valve calcification (VC) was defined as the presence of one or more bright echoes larger than 1 mm in any valve cusps or mitral annulus. According to the presence or absence of valvular calcification, patients were divided into valvular calcification group (VC group) and non-valvular calcification group (non-VC group).
Calculation formula
(1) BMI = weight/height2 (kg/m2).
(2) Ca correction (mmol/L) = serum Ca (mmol/L) + 0.02 × [40—ALB (g/L)].
(3) Ca-P product (mg/dl)2 = Ca correction × serum P.
Serum Ca: 1 mmol/L = 4 mg/dL, serum P: 1 mmol/L = 3.1 mg/dL.
(4) The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula15.
Statistical analyses
Statistical analyses were carried out using the IBM SPSS 25.0 software (Chicago, IL, USA). The Shapiro–Wilk test was used to check normality of data distribution. Normally and non-normally distributed continuous variables were expressed as the mean \(\pm\) standard deviation and median (interquartile range), respectively. Differences among groups were established using the t-test or ANOVA analysis for normally distributed data, and using the Wilcoxon Mann–Whitney test or non-parametric Kruskal–Wallis H test for skewed distributed data. The categorical variables were expressed as frequency with percentages, and the differences among groups were examined by chi-square test. The patients were divided into four groups according to the quartile of the HIF-1α levels, and post hoc Bonferroni analysis was carried out for variables with P < 0.05, adjusted for P < 0.008. Spearman rank correlation analysis was carried out to analyze the correlation between the calcification degree and HIF-1α levels. Variables with statistical differences in univariate analysis were included in binary logistic regression analysis to explore the independent influencing factors of CAC, and the results were expressed as odds ratio (OR). The receiver operating characteristic curve (ROC) was plotted to analyze the diagnostic value of HIF-1α level for CAC in non-dialysis CKD patients. All the analyses were 2-tailed, with P < 0.05 considered as a statistical difference.
Results
Comparison of CAC and non-CAC groups
The study finally enrolled 123 non-dialysis CKD patients, of which mean age was 56.19 ± 14.52 years, males 87 (70.7%), female 36 (29.3%), Smoking 30.1%, Alcoholism 17.1%, diabetes 36.6% and hypertension 76.4%. The mean BMI of the patients was 26.60 ± 4.00 kg/m2; the mean hemoglobin concentration was 116.89 ± 23.66 g/L; and the median HIF-1α level was 424.14 (173.48, 716.75) pg/mL. CKD1 stage 23 (18.7%), CKD2 stage 26 (21.1%), CKD3 stage 23 (18.7%), CKD4 stage 21 (17.1%) and CKD5 stage 30 (24.4%). There were 76 (61.8%) with CAC and 50 cases (40.7%) with VC, including 40 with only aortic valve calcification (AVC) and 10 with AVC combined with mitral valve calcification (MVC) (Table 1).
Compared to the non-CAC group, the CAC group had a higher proportion of males (77.6% vs. 59.6%), smokers (38.2% vs. 17.0%), with a history of hypertension (84.2% vs. 63.8%) and diabetes (46.1% vs. 21.3%), older age [(62.21 ± 11.11) vs. (46.45 ± 14.18)], higher SBP at admission [(159.36 ± 27.39) vs. (148.91 ± 24.51) mmHg], lower eGFR [(35.7 (14.62, 76.12) vs. 62.06 (21.52, 94.23) ml/min/1.73m2], lower high-density lipoprotein cholesterol [1.17 (0.98, 1.50) vs. 1.31 (1.16, 1.80) mmol/L] (all P < 0.05), and higher overall HIF-1α levels [545.34 (336.43, 948.23) vs. 256.85 (100.00, 421.26) pg/mL] (P < 0.001). However, there were no significant differences in HGB, ALB, ALP, Ca, P, FPG, BUN, Scr, UA, TC, TG, LDL-C, and Lp(a) between the two groups (P > 0.05) (Table 1).
Clinical data comparison of patients with different HIF-1α levels
According to the quartile of HIF-1α levels, patients were divided into four groups (Table 2). The results showed that as HIF-1α levels elevated, the proportion of patients with VC increased (12.9% vs. 35.5% vs. 43.3% vs. 71.0%), age increased [(49.32 ± 13.64) vs. (52.16 ± 14.87) vs. (57.27 ± 12.77) vs. (66.03 ± 11.22)], SBP increased [(145.71 ± 22.93) vs. (151.00 ± 27.16) vs. (155.1 ± 20.98) vs. (169.65 ± 29.77) mmHg], HGB decreased [(127.90 ± 24.61) vs. (117.74 ± 25.04) vs. (109.30 ± 22.60) vs. (112.35 ± 18.56) g·L-1] (all P < 0.05), and Agatston scores increased [0 (0, 18) vs. 0 (0, 32) vs. 43.5 (16.5, 99.5) vs. 321 (92, 669)] (P < 0.001). However, there were no significant differences in gender, BMI, DBP, ALP, FPG, ALB, Ca, P, BUN, Scr, UA, eGFR, TC, and TG among the four groups (P > 0.05).
Correlation of HIF-1α levels with CAC
Spearman rank correlation analysis showed that among non-dialysis CKD patients, HIF-1α levels were positively correlated with history of hypertension (r = 0.259, P = 0.004), history of diabetes (r = 0.166, P = 0.067), VC (r = 0.402, P < 0.001), age (r = 0.445, P < 0.001), CKD stage (r = 0.194, P = 0.032), and Agatston score (r = 0.631, P < 0.001), and negatively correlated with HGB concentration (r =—0.243, P = 0.007) and superoxide dismutase (r =—0.226, P = 0.012) (Table 3). Patients with CAC were divided into three groups according to the Agatston score, and the results showed a positive correlation between the Agatston score and the HIF-1α level, no matter in the mild calcification group, the moderate calcification group, the severe calcification group, or all patients with CAC (Fig. 2). In addition, HIF-1α levels increased with increasing calcification (Fig. 3).
Differences in HIF-1α levels between VC group and non-VC group
Based on the presence or absence of cardiac valve calcification, the patients were divided into valve calcification (VC) and non-valve calcification (non-VC) groups. The study showed that HIF-1α levels were higher in the VC group compared to the non-VC group, 615.87 (373.09, 1081.96) pg/mL vs. 288.33 (113.41, 554.62) pg/mL, (Z = -4.44, P < 0.001), respectively (Fig. 4).
Relationship between CKD stages and HIF-1α levels
HIF-1α levels were 316.53 (114.68, 624.60) pg/mL in patients with CKD stages 1 ~ 2, 524.61 (311.26, 851.13) pg/mL in patients with CKD stages 3 ~ 4, and 461 (248.89, 664.02) pg/mL in patients with CKD stage 5. Compared with patients with CKD stages 1 ~ 2, HIF-1α was higher in patients with CKD stages 3 ~ 4 (P = 0.046). However, there was no significant difference in HIF-1α between patients with CKD stages 1 ~ 2 versus CKD stage 5 and between patients with CKD stages 3 ~ 4 versus CKD stage 5 (P > 0.05) (Fig. 5).
Predictive value of HIF-1α levels for CAC
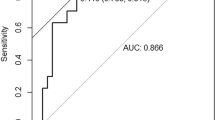
Variables that were statistically different in the univariate analysis were included in the binary logistic regression analysis after we checked for the absence of multicollinearity. The results showed that men had an increased risk of CAC relative to women (OR = 0.242, 95%CI 0.064–0.912, P = 0.036). The risk of CAC was higher with older age (OR = 1.903, 95%CI 1.043–1.146, P < 0.001) and higher HIF-1α levels (OR = 1.002, 95%CI 1.000–1.004, P = 0.025) (Table 4). Receiver-operating characteristic (ROC) curve analysis showed that the sum of sensitivity and specificity for predicting CAC was greatest at HIF-1α ≥ 451.177 pg/ml (sensitivity = 65.8%, specificity = 80.9%, AUC = 0.750, [95% CI 0.664 to 0.835]) (Fig. 6).
Discussion
The rate of cardiovascular events increases gradually as CKD progresses. Studies have confirmed that hypertension and diabetes, the most common causes of CKD, are risk factors for cardiovascular disease16,17. However, CKD was associated with an increased risk of coronary events even after adjusting for traditional cardiovascular risk factors, suggesting that CKD is an independent risk factor for cardiovascular disease181920. The CAC is an important indicator of future cardiovascular events in patients. CAC is more common in CKD patients compared to the general population. Accumulation of uremic toxins, chronic inflammation, disturbances in Ca and P metabolism, and oxidative stress are specific factors for the development of CAC in CKD patients212223. Recently, a prospective cohort study of 580 patients with non-dialysis CKD studied the predictive value of calcification at five major arterial sites on major adverse cardiovascular events (MACE) and all-cause mortality, with a mean follow-up time of 4.1 years, and found that CAC and carotid artery calcification were the best predictors of outcome2.
In 1990, Agatston et al14. first used computed tomography (CT) scanning technology to quantify CAC and found it to be highly sensitive and specific in recognizing CAC. In this study, all patients underwent CT scanning, and a total of 76 patients were found to have CAC. Arterial calcification is a manifestation of human aging. As the prevalence of diabetes increases with age, there is an increase in the deposition of lipids and calcium salts in the vessel walls, sclerosing of the vessel walls, narrowing of the lumen, and gradual degeneration and calcification of the arterial intima-media, which can lead to an elevation in blood pressure and an increase in cardiovascular events. Therefore, vascular calcification is more common in elderly patients. Consistent with the results of previous studies242526, the present study demonstrated that the CAC group had a higher proportion of patients with a history of hypertension and diabetes mellitus, was older, and had lower HDL-C. Female patients have lower smoking rates compared to men, and a study on sex hormone levels and CAC has found that the higher the androgen level, the greater the risk of CAC progression27. Post-hysterectomy female patients had significantly lower CAC loads in the estrogen treatment group than in the placebo group28. However, the mechanism by which estrogen inhibits vascular calcification is unknown, which may be related to the inhibition of the pro-calcification effects of HIF-1α29. In the present study, male patients had a higher proportion of CAC (Table 1), and this association persisted even after adjusting for other confounders (Table 4). Disturbance of Ca and P metabolism is a known independent risk factor for CAC in CKD patients. However, the study found no significant difference in Ca and P between the CAC group and the non-CAC group, potentially due to the following factors: (1) Dietary Ca and P intake may affect the study results to some extent, and we are unable to know the patients’ dietary Ca and P intake; (2) This study lacks data on drugs that affect Ca and P metabolism, and the results of the study may be biased.
Anemia is a common complication in CKD patients. Prolyl hydroxylase inhibitors (PHIs) can improve anemia by simulating human exposure to hypoxia, stabilizing HIF levels, and promoting EPO transcription. In addition, PHIs regulate iron homeostasis through direct and indirect mechanisms to meet the iron requirements of erythropoiesis30. There are a lot of advantages to PHIs, however, due to the large number of downstream genes regulated by HIF, we have to consider the safety issues of PHIs use, such as iron deficiency, malignancy, diabetic retinopathy, age-related macular degeneration, thromboembolism, pulmonary arterial hypertension, hyperkalemia, and polycystic kidney disease (PCKD), among others31. Ectopic calcification, as a pathological term, can occur in the arteries, heart valves, or soft tissues. Nowadays, increasing evidence supports that vascular calcification in CKD is an active process regulated by multiple pathophysiological mechanisms involving a variety of molecules, including promoters and inhibitors of vascular calcification, in addition to some traditional factors32.
In recent years, PHIs have played an increasingly important role in the treatment of renal anemia. However, the relationship between elevated HIF-1α levels caused by PHIs, and vascular calcification in patients with CKD is unclear. Balogh et al11. cultured human aortic VSMCs under normoxic (21% O2) and hypoxic (5% O2) conditions and found that hypoxia increased protein expression of HIF-1α and its target genes including glucose transporter protein 1 (GLUT1) and vascular endothelial growth factor A (VEGFA), and facilitated calcification of the extracellular matrix of VSMCs by increasing the reactive oxygen species (ROS) and subsequently inducing the mRNA and protein expression of bone formation markers RUNX2 (runt-related transcription factor 2), SOX9 (Sry-related HMG box-9), OCN (osteocalcin) and ALP. HIF-1α inhibitors abolished hypoxia-induced elevation of bone formation markers and inhibited calcification of the extracellular matrix. In addition, an in vivo study has also found increased levels of RUNX2 mRNA in the lungs and aorta of mouse models exposed to hypoxia (10% O2), suggesting that hypoxia promotes vascular calcification by inducing osteochondrogenic differentiation of VSMCs in a HIF-1-dependent and mitochondria-derived reactive oxygen species-dependent manner11. Ababneh et al33. reported that hyperglycemia promotes osteogenic differentiation of human lens epithelial cells (LECs) through activation of the HIF-1 signaling pathway. A retrospective study of 405 patients with asymptomatic type 2 diabetes found significantly elevated levels of HIF-1α in patients with high Agatston scores13. In the present study, we found that HIF-1α levels were higher in the CAC group compared with the non-CAC group, and the correlation between HIF-1α and CAC remained significant even after adjusting for confounders (OR = 1.002, P = 0.025) (Table 1, Table 4). Also, there was a positive correlation between the calcification score and HIF-1α levels, which gradually increased with increasing calcification (Fig. 2, Fig. 3). Csiki et al12. reported that hypoxia could stabilize HIF-1α and HIF-2α levels, increase ROS levels, and thus promote osteogenic transformation of valve interstitial cells (VICs) and accelerated aortic valve calcification (AVC). Similar to the above study, we found that HIF-1α was significantly higher in the CVC group compared with the non-CVC group (Z = -4.44, P < 0.001) (Fig. 4).
Wu et al29. reported that HIF-1α mRNA and protein were increased in the thoracic aorta of ovariectomy female rats, and calcification was significantly increased. HIF-1α inhibitor could reduce vascular calcification and the expression of bone morphogenetic protein-2 (BMP-2) and Runx2 protein levels in ovariectomy rats. BMP-2 siRNA treatment decreased the levels of p-Smad1/5/8 in calcification cells, suggesting that the pro-calcification effect of HIF-1α is achieved by regulating BMP-2 and downstream Smad1/5/8. In the present study, the gender ratios did not show significant differences between different HIF-1α levels, which may be related to the overall older age of the included subjects, with estrogen levels gradually decreasing in women as they age. Orikasa et al34. demonstrated in a study of dental pulp mineralization that hypoxia/HIF1α-induced osteo/odontoblast differentiation of human dental pulp stem cells (hDPSCs) was partially dependent on Wnt/β-catenin signaling, where B-cell CLL/lymphoma 9 (BCL9) was a key mediator between HIF1α and Wnt/β-catenin signaling. Additionally, Wnt/β-catenin signaling can promote osteogenic transdifferentiation and calcification of VSMCs by regulating Runx2 gene expression35. Therefore, we hypothesized that HIF-1α can promote osteogenic transdifferentiation of VSMCs through the Wnt/β-catenin signaling pathway, and thus promote vascular calcification.
Advanced glycation end products (AGEs), macromolecular products formed by non-enzymatic reactions of reducing sugars with proteins, lipids, and nucleic acids, have been implicated in the occurrence of vascular calcification in patients with CKD in recent years36. Zhu et al37. observed in animal models that AGEs elevated the expression of HIF-1α and PDK4 during VSMCs calcification in a dose-dependent manner. Two important HIF-1α-regulated genes, VEGFA and GLUT-1, were significantly increased after exposure to AGEs. Stabilization or nuclear translocation of HIF-1α increased PDK4 expression, and knockdown of PDK4 downregulated the level of RUNX2 and attenuated calcification in VSMCs, suggesting that AGEs accelerate vascular calcification partly by activating the HIF-1α/PDK4 signaling pathway.
In summary, the pro-calcification effects of HIF-1α may be related to the following mechanisms: (1) hypoxia promotes calcification by stabilizing HIF-1α levels to increase ROS production, which in turn induces RUNX2 expression and promotes activation of its downstream genes OC and ALP; (2) HIF-1α promotes BMP-2 expression and then activates the Smad1/5/8 signaling pathway to promote vascular calcification, which can be inhibited by estrogen; (3) HIF1α activates the Wnt/β-catenin signaling pathway by up-regulating the expression of BCL9, which then promotes the expression of RUNX2 and ALP, thereby promoting vascular calcification; (4) HIF-1α is involved in the pro-calcification mechanism of AGEs through the HIF-1α/PDK4/RUNX2 signaling pathway.
Previous literature has reported that HIF-1α levels increase with the severity of urinary protein in type 2 diabetes patients with different stages of diabetic kidney disease38. However, there has been no study on the relationship between HIF-1α levels and renal function so far. The present study demonstrated that HIF-1α was significantly correlated with the CKD stage (r = 0.194, P = 0.032) (Table 3). HIF-1α was higher in patients with CKD stages 3 ~ 4 compared to patients with CKD stages 1 ~ 2 (Fig. 5), indicating that HIF-1α levels increase with the progression of CKD. However, the exact mechanism involved needs to be further explored, and we believe that it may be related to the exacerbation of renal ischemia and hypoxia due to the aggravation of advanced renal fibrosis. Nevertheless, HIF-1α levels did not rise to higher levels in patients with CKD stage 5, which needs to be further explored by expanding the sample size in the future. Unlike previous study39, the univariate analysis in this study showed lower eGFR in the calcification group, but further binary logistic regression analyses did not show an association between eGFR and calcification, which may be related to the following factors: Firstly, the limitation of a small sample size study cannot represent the relationship between eGFR and CAC in overall CKD patients; Secondly, this study screened out a large proportion of patients with severe vascular lesions and others with definite chronic hypoxic disease in the initial stage of the study. We are unclear about the relationship between renal function and calcification in this group of patients, which needs to be explored in future studies with the inclusion of this group of patients.
Some limitations of our study should be noted. Firstly, the design of the cross-sectional, observational study could not infer a causal relationship between HIF-1α and CAC, and further prospective randomized studies are needed to confirm this in the future. Second, the study was conducted at a single center and included relatively small sample size, so the association between HIF-1α and CAC was not generalizable to all patients with CKD, and a larger sample size will be needed in the future to validate the results of this study. In addition, we screened out some patients with incomplete data, which may have caused some bias. Finally, we were unable to draw any definitive conclusions regarding the mechanisms behind these associations, which will need to be clarified through animal and cellular experiments in the future.
In summary, this study was the first to investigate the relationship between serum HIF-1α levels and CAC in non-dialysis CKD patients. We found a positive correlation between HIF-1α levels and CAC score in non-dialysis CKD patients, and elevated HIF-1α may be a risk factor for promoting CAC. PHIs have been widely used in the clinical treatment of renal anemia. However, in terms of mechanism of action, the relationship between PHIs and CAC remains to be further explored.
Conclusion
The prevalence of CAC is high in non-dialysis CKD patients, with age and male gender being traditional risk factors for CAC occurrence in this population. Levels of HIF-1α are positively correlated with both CAC and VC, which can provide certain diagnostic values for non-dialysis CAC.
Data availability
The data analyzed in this study is available upon reasonable request from the corresponding author.
References
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 395(10225), 709–733. https://https://doi.org/10.1016/s0140-6736(20)30045-3 (2020).
Sørensen, I. M. H. et al. Coronary and extra-coronary artery calcium scores as predictors of cardiovascular events and mortality in chronic kidney disease stages 1–5: a prospective cohort study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 38(5), 1227–1239. https://https://doi.org/10.1093/ndt/gfac252 (2023).
Wetscherek, M. T. A. et al. Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death. European radiology. 33(7), 4723–4733. https://https://doi.org/10.1007/s00330-023-09428-z (2023).
Chen, J. et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA cardiology. 2(6), 635–643. https://https://doi.org/10.1001/jamacardio.2017.0363 (2017).
Wang, X. R., Zhang, J. J., Xu, X. X. & Wu, Y. G. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Renal failure. 41(1), 244–256. https://https://doi.org/10.1080/0886022x.2019.1595646 (2019).
Phadwal, K., Vrahnas, C., Ganley, I. G. & MacRae, V. E. Mitochondrial Dysfunction: Cause or Consequence of Vascular Calcification? Frontiers in cell and developmental biology. 9, 611922. https://https://doi.org/10.3389/fcell.2021.611922 (2021).
Hénaut, L., Sanchez-Nino, M. D., Aldamiz-Echevarría Castillo, G., Sanz, A. B. & Ortiz, A. Targeting local vascular and systemic consequences of inflammation on vascular and cardiac valve calcification. Expert opinion on therapeutic targets. 20(1), 89–105. https://https://doi.org/10.1517/14728222.2015.1081685 (2016).
Ciceri, P. et al. Therapeutic effect of iron citrate in blocking calcium deposition in high Pi-Calcified VSMC: role of autophagy and apoptosis. International journal of molecular sciences. 20(23), 5925. https://https://doi.org/10.3390/ijms20235925 (2019).
Düsing, P. et al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. Journal of molecular medicine. 99(3), 335–348. https://https://doi.org/10.1007/s00109-021-02037-7 (2021).
Qin, Q. et al. Hypoxia-inducible factors signaling in osteogenesis and skeletal repair. International journal of molecular sciences. 23(19), 11201. https://https://doi.org/10.3390/ijms231911201 (2022).
Balogh, E. et al. Hypoxia triggers osteochondrogenic differentiation of vascular smooth muscle cells in an HIF-1 (hypoxia-inducible factor 1)-dependent and reactive oxygen species-dependent manner. Arteriosclerosis, thrombosis, and vascular biology. 39(6), 1088–1099. https://https://doi.org/10.1161/atvbaha.119.312509 (2019).
Csiki, D. M. et al. Hypoxia-inducible factor activation promotes osteogenic transition of valve interstitial cells and accelerates aortic valve calcification in a mice model of chronic kidney disease. Frontiers in cardiovascular medicine. 10, 1168339. https://https://doi.org/10.3389/fcvm.2023.1168339 (2023).
Li, G. et al. The relationship between serum hypoxia-inducible factor 1α and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovascular diabetology. 13(1), 52. https://https://doi.org/10.1186/1475-2840-13-52 (2014).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 15(4), 827–832. https://https://doi.org/10.1016/0735-1097(90)90282-t (1990).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 150(9), 604–612. https://https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Cao, Y., Li, W., Yang, G., Liu, Y. & Li, X. Diabetes and hypertension have become leading causes of CKD in Chinese elderly patients: a comparison between 1990–1991 and 2009–2010. International urology and nephrology. 44(4), 1269–1276. https://https://doi.org/10.1007/s11255-012-0194-0 (2012).
Zhang, Y. et al. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: the Strong Heart Study. Hypertension. 47(3), 410–414. https://https://doi.org/10.1161/01.Hyp.0000205119.19804.08 (2006).
Tonelli, M. et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 380(9844), 807–814. https://https://doi.org/10.1016/s0140-6736(12)60572-8 (2012).
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 108(17), 2154–2169. https://https://doi.org/10.1161/01.Cir.0000095676.90936.80 (2003).
Matsushita, K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 375(9731), 2073–2081. https://https://doi.org/10.1016/s0140-6736(10)60674-5 (2010).
Opdebeeck, B., D'Haese, P. C. & Verhulst, A. Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and p-cresyl sulfate. Toxins. 12(1), 58. https://https://doi.org/10.3390/toxins12010058 (2020).
Sarnak, M. J. et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. Journal of the American College of Cardiology. 74(14), 1823–1838. https://https://doi.org/10.1016/j.jacc.2019.08.1017 (2019).
Ogata, H., Sugawara, H., Yamamoto, M. & Ito, H. Phosphate and Coronary Artery Disease in Patients with Chronic Kidney Disease. Journal of atherosclerosis and thrombosis. 31(1), 1–14. https://https://doi.org/10.5551/jat.RV22012 (2024).
Kramer, H., Toto, R., Peshock, R., Cooper, R. & Victor, R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. Journal of the American Society of Nephrology : JASN. 16(2), 507–513. https://https://doi.org/10.1681/asn.2004070610 (2005).
Mallikethi-Reddy, S., Rubenfire, M., Jackson, L. A. & Brook, R. D. Coronary artery calcium in hypertension: a review. Journal of the American Society of Hypertension : JASH. 9(12), 993–1000. https://https://doi.org/10.1016/j.jash.2015.09.004 (2015).
Alonso, R. et al. PCSK9 and lipoprotein (a) levels are two predictors of coronary artery calcification in asymptomatic patients with familial hypercholesterolemia. Atherosclerosis. 254, 249–253. https://https://doi.org/10.1016/j.atherosclerosis.2016.08.038 (2016).
Subramanya, V. et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of cardiovascular computed tomography. 13(1), 41–47. https://https://doi.org/10.1016/j.jcct.2018.09.010 (2019).
Manson, J. E. et al. Estrogen therapy and coronary-artery calcification. The New England journal of medicine. 356(25), 2591–2602. https://https://doi.org/10.1056/NEJMoa071513 (2007).
Wu, X. et al. Estrogen inhibits vascular calcification in rats via hypoxia-induced factor-1α signaling. Vascular. 28(4), 465–474. https://https://doi.org/10.1177/1708538120904297 (2020).
Wu, H. H. L. & Chinnadurai, R. Erythropoietin-stimulating agent hyporesponsiveness in patients living with chronic kidney disease. Kidney diseases. 8(2), 103–114. https://https://doi.org/10.1159/000521162 (2022).
Hirota, K. HIF-α prolyl hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines. 9(5): 468. https://https://doi.org/10.3390/biomedicines9050468 (2021).
Roumeliotis, S., Roumeliotis, A., Dounousi, E., Eleftheriadis, T. & Liakopoulos, V. Biomarkers of vascular calcification in serum. Advances in clinical chemistry. 98, 91–147. https://https://doi.org/10.1016/bs.acc.2020.02.004 (2020).
Ababneh, H. et al. High glucose promotes osteogenic differentiation of human lens epithelial cells through hypoxia-inducible factor (HIF) activation. Journal of cellular physiology. https://https://doi.org/10.1002/jcp.31211 (2024).
Orikasa, S. et al. Hypoxia-inducible factor 1α induces osteo/odontoblast differentiation of human dental pulp stem cells via Wnt/β-catenin transcriptional cofactor BCL9. Scientific reports. 12(1), 682. https://https://doi.org/10.1038/s41598-021-04453-8 (2022).
Cai, T. et al. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Experimental cell research. 345(2), 206–217. https://https://doi.org/10.1016/j.yexcr.2016.06.007 (2016).
Taki, K., Takayama, F., Tsuruta, Y. & Niwa, T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney international. 70(1), 218–224. https://https://doi.org/10.1038/sj.ki.5000330 (2006).
Zhu, Y. et al. Advanced glycation end products accelerate calcification in VSMCs through HIF-1α/PDK4 activation and suppress glucose metabolism. Scientific reports. 8(1), 13730. https://https://doi.org/10.1038/s41598-018-31877-6 (2018).
Shao, Y. et al. Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine. 55(1), 130–138. https://https://doi.org/10.1007/s12020-016-1069-4 (2017).
Yun, H. R. et al. Coronary artery calcification score and the progression of chronic kidney disease. Journal of the American Society of Nephrology : JASN. 33(8), 1590–1601. https://https://doi.org/10.1681/asn.2022010080 (2022).
Acknowledgements
We thank all authors and patients who participated in the study.
Funding
This study was supported by grants from the Health Commission of Hebei Province (No. 20211515).
Author information
Authors and Affiliations
Contributions
CQ: Research idea, study design, collection and analysis of data, and manuscript drafting. ZH: Study design, critical revision of the manuscript. YS, HW: Experimental instruction, data collection, critical revision of the manuscript. JM, JL: Read and approved this final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, C., Hu, Z., Shi, Y. et al. Association of serum HIF-1α levels with coronary artery calcification in patients of non-dialysis chronic kidney disease. Sci Rep 14, 29031 (2024). https://doi.org/10.1038/s41598-024-79393-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79393-0
Keywords
This article is cited by
-
Sinomenine attenuates uremia vascular calcification by miR-143-5p
Scientific Reports (2025)