Abstract
Natural honey is enriched with essential and beneficial nutrients. This study aimed to investigate the melliferous flora microscopic techniques and assess the biochemical properties of honey. Flavonoid and phenolic contents in honey samples were analyzed via colorimetric and Folin-Ciocalteu methods and the alpha-amylase, reducing power, and minerals using Pull’s and spectroscopy methods. HPLC determined the Sucrose, fructose, and glucose content in the honey samples. Four different classes of moisture content were used to assist the honey quality, including A + < 17, A = 17–18, B = 18–19, and C > 19. This study identified the Eucalyptus globulus, Trifolium pratense, Neltuma juliflora, Ziziphus mauritiana, Asphodelus tenuifolius, Cynodon dactylon, Saccharum spontaneum, and Vachellia nilotica as the predominant plant species honey samples. The total range of moisture was observed from 16.5 to 21.1% in the samples studied. The honey class within the range of 16.5–17.5% was identified as the optimal class. Four classes of sucrose level were used to evaluate the honey quality, including: (2.50–2.60 = A +); (2.61–2.75 = A); (2.76–3.00 = B); (3.00 + = C). The total variation of sucrose levels in the analyzed ranged from 2.50 to 3.89%. Overall, the findings of these studies implemented various aspects of honey production, quality, and potential health benefits, benefiting consumers, beekeepers, and researchers.
Similar content being viewed by others
Introduction
Honey is a natural sweet substance prepared by Apis florea F. and Apis mellifera L. by collecting pollen, nectaries, and excrement of insects living on the plants1. The nectar composition of honey depends upon the pollinators2. The pollen and nectar collected by Apis mellifera L. and Apis florea F. during the foraging activities for honey production3,4,5. It has been used as a sweetener substance for millions of years ago for different diseases6. The biochemical composition of honey is mostly influenced by various environmental factors specific to its geographic origin. Climatic variations, including humidity and temperature, impact the diversity and availability of floral sources, thereby affecting the amino acids, sugars, sucrose, antioxidants, moisture, and secondary metabolites present in the nectar collected by honeybees. Nectar and pollen are used as a primary nutrition source for honeybees7. These honeybees collect and gather all the appropriate nutrients surrounding bee apiaries for individuals residing in beehives, like nurse bees, queens, and developing larvae8. The pollen load contributes to producing proteinaceous royal jelly to feed queen larvae and mature queens9.
The word melissopalynology is derived from the Greek word Melissa, which means honeybees, while palanin means scattered10. The interrelationship of pollen and honey is covered by applied melissopalynology. Honey types, origin, and quality can be determined using melissopalynolgical techniques11. Honey also contains pollen from plants foraged by honeybees, indicating its origin and source of the melliferous flora involved in the bees’ diet12. The quantification and identification of pollen grains in different honey samples is an advanced method for identifying the origin of botanical sources in honey13. Typically, monofloral honey comprises pollen and nectar sourced solely from a single flowering plant, constituting at least 45% of its composition. Morphological features of pollen grains make it possible to determine and identify the relationship between bee floral sources and the origin of honey14,15,16. The floral and botanical origin is investigated by the pollen percentage of different melliferous plants pollen using melissopalynology methods17.
Pollen’s systematic exploration holds paramount significance due to different aspects. Firstly, it serves as an identifying marker for quality and authentication control, allowing producers to verify the honey’s floral and botanical origin and detect mislabeling or adulteration. Accurate quantification of pollen aids in assessing purity, quality, and adherence to regulatory standards, ensuring consumer safety and confidence. Moreover, identifying pollen of melliferous plants is helpful for seasonal and geographical sources of melliferous plants, enabling producers to label their products with precise botanical origins, enhancing market value and differentiation. Additionally, the analysis of pollen acts as an important tool in scientific research, aiding fields related to floral ecology, bee foraging behavior, and different environmental monitoring. Overall, pollen systematic exploration not only ensures product integrity and consumer trust but also contributes significantly to the scientific understanding of bee foraging and sustainable beekeeping practices.
Honey quality assessment comprises different features, including flavonoid, phenol, alpha-amylase, reducing power, moisture content, sugar content, sucrose, fructose, glucose quantification, flavor, color, aroma, and absence of contaminants such as pesticides and antibiotics. The biochemical composition of honey depends upon the botanical source, bee species, physiological activities, climatic conditions, maturity of the honey, flowering periods in the study area, and beekeeping methodology during harvesting periods18,19. The biochemical features of honey are used as parameters to distinguish between adulterated and pure honey20. In Brazil, the legislation about honey is implemented for the classification of different kinds of honey from Apis mellifera L. and does not deal with the features of the stingless bee’s honey product. The biochemical properties of honey phenol, and flavonoids indicated health-promoting like anti-inflammatory, antiviral, and antioxidant37. Minerals in honey also exploit the environmental condition of melliferous plants which were noted from 0.04 to 0.2%21,22. Some compounds are used as a chemical marker to detect the floral origin of honey23.
Currently, the description of the honey melissopalynological analysis of pollen, rheological, and biochemical features has received much attention. It has described that honey samples collected from different geographical and environmental locations significantly varied in melissopalynological, rheological (viscosity and sugar), and biochemical characteristics. The findings showed the monofloral, polyfloral, rheological, non-Newtonian, and Newtonian liquid behavior of honey24,25. The rheology of honey obtained from Pine species, Helianthus, Brassica, and Trifolium, was found to be related to the sensitivity of viscosity due to temperature changes. Therefore, it can be concluded that honey is a temperature-dependent substance26.
In Pakistan, the Bannu division is famous for honey production in terms of quantity and quality. The Bannu Division is in the Khyber Pakhtunkhwa province of Pakistan and consists of four districts: Bannu, Lakki Marwat, FR Bannu, and FR Lakki and North Waziristan. The Bannu division has a long history due to its strategic ___location along the Tochi and Kurram routes which lead into the Indus Valley. The Bannu division is 9975 km2 in total area. The study area is bounded by North Waziristan on its north side, east side Karak, south side is Mianwali, and west side Dera-Ismail Khan and South Waziristan. Topographically surrounded by dried and hard mountain ranges of Koh-e-Sulaiman and Koh-e-Safed. The mountainous ranges have low temperatures while they are increasing toward plain areas of the concerned division27. Bannu has a semi-arid hot climate with extremely hot summers season and mild winters.
In this study, the floral source of honey, rheological (viscosity & sugar), and composed profiling like mineral concentration, phenolics and flavonoid content, reducing power, and alpha-amylase activity were determined. This study aimed to identify the melliferous flora from wild honey samples using melissopalynological techniques and to analyze the biochemical profiling of honey samples collected from the Bannu division.
Methods
Honey sample collection
The area of collection showed the Apis florea F. and Apis mellifera L. frequently around bee apiaries. Eleven samples were collected from Apis florea F. and twenty-four samples of Apis mellifera L. The squeezing and centrifugation methods were used to collect the honey samples, for example, Apiaries honey was harvested by centrifugation while wild honey was by squeezing beehives. A collection of samples was taken from thirty-five various areas of the Bannu division. The selection criteria for the honey samples from different localities involve considerations such as geographic diversity to encompass variation in flora, climate, and practices of agriculture to account for differences in farming methods, pesticide usage, and rural–urban gradient to capture the impact potential of human activities, botanical diversity, and environmental factors to reflect the range of pollen and nectars sources available to honey bees to identify the region of interest for beekeepers to ensure beekeeping profession with full potential. All the collected honey samples were named according to the collected areas. For biochemical, rheological (viscosity & sugar), and melissopalynological analysis, the samples in Eppendorf were brought to the Taxonomy Laboratory, Botany Department, PMAS Arid Agriculture University Rawalpindi (Fig. 1).
Study areas
For the present study, various localities were selected in division Bannu with aspects of different botanical and geographical diversity. Globally the study area lies from 32.17 to 33.07 N latitude & 70.20–70.60 E longitude. The study area is bounded by North Waziristan on its north side, east side Karak, south side is Mianwali, and the west side includes Dera- Ismail Khan and South Waziristan (Fig. 2). Maps (Figs. 1 and 2) were constructed with the help of the Arc GIS10.3.zip (https://mega.nz/file/hU83iCBY#Whg68giZ6XRtwyEbUyxeY1CZq5tDX_sFHOFUqL6SpU).
Melissopalynology and Identification of melliferous pollen
Systematic exploration of pollen was done under the rules of the plant commission27. The melissopalynological pollen was prepared through the acetolysis method28. Pollen was qualitative and quantitative features were observed by a Light microscope29. For melissopalynological studies only 0.5gm of honey and 7 ml centrifuge filled with distilled water heated from 43 to 46 °C of solution. The centrifuge machine ran for 5 min at 3500 rpm speed. The supernatant removal and centrifugation process was repeated several times and in the last drop of the pellet solution was deposited on the slide, stained by glycerin jelly, and then turned by coverslips. Therefore, various methods of light microscopy were applied to examine the palynomorphs of botanical sources and their identification. However, observable pollen was identified by comparing it with previously published literature and some field observations on melliferous plants. The Nikon Fx-35 equipped camera of Light microscope was used to capture identified plant taxa pollen. Using the procedure30,31 the pollen of honey was processed for light microscopy and scanning electron microscopy.
Assessment of pollen percentage
The pollen number of each honey sample (slide) was counted using the Adenipekunss standard technique32. Further, the pollen grains were counted according to systematic way from species, genus, and family. Moreover, we used a parameter for the dominancy of pollen in each slide i.e. predominant pollen if > 45%, secondary pollen to 16–45%, and minor pollen is 3–15%. Hence, in pollen count the honey could be considered as monofloral if the relative pollen frequency of the taxon exceeds 45%. This percentage of pollen (> 45%) was considered as the best quality of Honey. The percentage (%) of pollen for a particular species was calculated as;
Light microscopy (LM)
Using the method of Erdtman14 pollen grains were analyzed for the process of acetolysis. Pollen grains were put on the slide, 1–2 drops of acetic acid were added and then crushed with a metal rod. With the help of a camel hairbrush, the debris was removed, and then one drop of glycerin jelly was added for staining purposes. A cover slip was placed on a slide and cleaned through tissue paper. Exine thickness, polar and equatorial diameters, number of colpi, pollen shapes, and number of fertile and sterile pollen were investigated with the help of a light microscope Meiji Techno MT4300H. The investigated slides were then stored vertically in a wooden box.
Scanning electron microscopy
SEM techniques were used for confirmation and correct identification. For SEM studies the prepared slide of melissopalynology honey was utilized with acetic acid with a concentration of 45%. This honey slide was pasted on the stubs (prepared)33,34. The pollen samples were coated with gold–palladium after mounting and then observed using SEM to capture high-quality pollen images, enabling the differentiation of closely related melliferous taxa and pollen types. SEM images of these pollen were captured using an SEM (Model JEOL JSM5910) at the Central Resource Laboratory (CRL), Department of Physics, and University of Peshawar.
Biochemical analysis of honey
Chemical and reagents
Aluminum chloride, diammonium salt, phenazine methosulphate, Thiobarbituric acid, trichloroacetic, ferric chloride, and potassium persulphate were purchased from the National Laboratory of Pakistan Atomic Energy. Chemicals were used in the analysis such as potassium ferricyanide, sodium hydroxide, deoxyribose, sodium carbonate, sodium dihydrogen phosphate, and HCL.
Total phenolic content (TPC)
In honey samples, the phenolic amount was determined by using the Oddo et al. method35. 50 ml water and 5gm honey were mixed through the filtration process. A solution of 500 µL was mixed with 2.5 ml of Folin–Ciocalteu reagent (0.2 N) for 5 min and added a solution of sodium carbonate (75 g/l). Incubation was done at room temperature for two hours with 760 nm absorbance, the ethanol mixture indicated a blank mixture. The Gallic acid in ethanol (1 mg /ml) of stock solution is represented by a curve.
Flavonoid quantification
Colorimetric adopted method of honey was utilized35. Every sample of honey a mixture of 20 µL was taken in a 96-well plate in the presence of aluminum chloride, potassium acetate, and distilled water of 160 µL. After that solution was incubated for 30 min. Then 20 µL solvent was used as negative control and 2.5, 5, 10, 20, and 40 µg/mL quercetin solution as a standard. The microplate reader was used to record the TFC at 405 nm wavelength and represented as QE/mL (quercetin equivalents).
Total reducing power (TRP)
In the Eppendorf tube, 100 µL of honey was taken with 0.2 Molar phosphate buffer of 200 µL (pH 6.6). After that 1% potassium ferricyanide of 250 µL was added and incubated at 50 °C for 20 min. Then 10% trichloroacetic acid solution of 200 µL was added to the mixture and incubated at 3000 rpm for 10 min. 50 µL of ferric chloride was added and a supernatant of 150 µL repeatedly mixed. Ascorbic acid (stock solution) of 100 μL of 1 mg/mL was used as a positive control (100 μg/mL final concentration) and the solvent for control (negative). The reading was taken at 630 nm.
Alpha-amylase determination
The alpha-amylase activity was determined following the technique33. This experiment was done in a microplate of 96. Alpha amylase of 25 µL and 15 µL of PBS were added to all the wells. Test samples of 10 µL and 1% starch solution of 40 µL were added and incubated at 50 °C temperature for 30 min. 1 M HCL of 20 µL of aliquot and iodine solution of 90 µL were also added and mixed well.
Determination of minerals
2.5 g of honey and 0.1 M nitric acid solution were mixed in a flask. Atomic absorption spectroscopy was used to determine the mineral content in each honey sample. For each mineral, a specific rod was used to determine its quantity. The following minerals like nickel, chromium, magnesium, cadmium, calcium, and copper, etc.
Quality assessment of honey
Moisture (%) determination
To determine the moisture of each honey, sample the Refractometer (HHR.2N) was used. 5 g of each honey sample was taken on the lens of the refractometer. With the help of light, readings were taken according to the correction factor. The distilled water was used to clean the lens of the refractometer. Every sample was practiced for three replicates. In the end, the Mean was calculated for each sample replicate. The common temperature at which the viscosity of honey was determined is room temperature, i.e. is measured around 25 °C (77°F).
Total soluble sugar quantification (%)
To determine the total soluble sugar of each honey, sample the Refractometer (MA871) was used. 4 g of each honey sample was taken over the lenses of the refractometer. The reading was noted from zero to till counting of total soluble sugar. The distilled water was used to clean the lens of the refractometer. Every sample was practiced with three replicates. In the end, the Mean was calculated for each sample replicate. The refractometer (MA871) is used to determine the total soluble sugar and is a practical and common method in the field of analysis of honey. It should be noted that the term "total soluble sugar" as measured by refractometer (MA871) primarily refers to the sugar present in honey, specifically glucose and fructose. The refractive index of a solution is measured by a refractometer (MA871), but in the case of honey, the refractive index is influenced by dissolved sugar concentration. Honey is also composed of water but in honey, the sugar (glucose and fructose) significantly contributes to its refractive index.
Sucrose quantification (%)
To analyze glucose, fructose, and sucrose using HPLC with a Diode Array Detector (DAD), a specific method is employed. Typically, the carbohydrates column was used with suitable selectivity standards to separate the sub-classes of sugar. A mobile phase, often a mixture of acetonitrile and water, is pumped through the column at a constant rate. The separation of sucrose, sucrose, and fructose is based on their different affinities for the stationary phase. The elution order is usually fructose, glucose, and then sucrose. The DAD detector continuously monitors the eluent, measuring absorbance at multiple wavelengths. Each sugar has a characteristic UV–visible absorption spectrum, allowing for quantification and identification. By comparing retention times and spectral characteristics with known standards, the presence and concentration of fructose, glucose, and sucrose in the sample can be determined. Additionally, calibration curves can be constructed using standards of known concentration to quantify the sugar accurately. The standard quantity of sucrose for the best quality is about 2.5 percent, which is used worldwide for honey authentication. The most applied method for sucrose quantification is high-performance liquid chromatography (HPLC).
Fructose and glucose (%) quantification
The most applied method for quantification (Fructose + Glucose) is high-performance liquid chromatography (HPLC). In this quantification method of (Fructose and Glucose), the Agilent 1200 series of liquid chromatographs were used. This liquid chromatograph was equipped with a temperature-regulated Diode-array Detector SL, temperature-regulated column oven, autosampler, and quaternary pump. For sugar separation the following chromatographic condition used: analytical column 4.6 mm in diameter and 250 mm in length, containing lichrospher 100 RP 18, temperature detector and column of 350 C, mobile phase -acetonitrile: water (75:25, v/v), flow rate 1.3 mL/min and sample volume of 10 μL. In 40 mL of water, 5 gm honeys were dissolved in a beaker and then transferred into a 100 mL volumetric flask were 25 mL of methanol. After filling the volumetric flask with water, it is filtrated to obtain a solution sample through a membrane filter and collected in a sample. The honey (Fructose + Glucose) was identified by comparison of the retention times and quantified by the peak area of the honey (Fructose + Glucose) level with those of the standard (Fructose + Glucose) concentrations. Then the mass percentage of the (Fructose + Glucose) was calculated.
Statistical analysis
The Origin software was used to analyze and determine the relationship between (Fructose + Glucose) and sucrose and mentioned an error bar to indicate quality assessment. R-Software was used to analyze and determine the relationship among the flavonoids phenolic, alpha-amylase, and reducing power in honey to determine the biochemicals profile.
Results
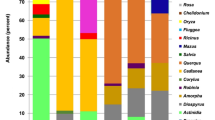
The honey samples of both Apis mellifera L. & Apis florea F. collected from thirty-five different locations in the Bannu Division Demonstrated that the variety of pollen may be associated with distinct Melliferous plant sources (Table 1). The honey samples examined varied in geographical origin and hue, spanning from shades of brown to yellow. The collected honey was also observed using SEM & LM (Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12).
LM (a) Anethum graveolense, (b) Senegalia catechu, (c) Vachellia nilotica, (d) Albizia lebbeck, (e) Alhagi maurorum, (f) Anagalis arvensis, (g) Arnebia linearifolia, (h) Asphodelus tenuifolius, (i) Aster subulatus, (j) Brassica rapa, (k) Brssica spp., (l) Melaleuca citrina, (m) Centaurea cyanus, (n) Centaurea cyanus, (o) Centaurium pulchellum, (p) Dysphania ambrosioides, (q) Ismelia carinata, (r) Cicer arietinum, (s) Cichorium intybus, (t) Cirsium arvense, (u) Citrullus colocynthis, (v) Volkameria inermis, (w) Conocarpus erectus, (x) Erigeron bonariensis, (y) Covalvulaus arvensis.
LM (a) Cymbopogon jwarancusa, (b) Cynodon dactylon, (c) Cyperus rotundus, (d) Datura innoxia, (e) Dalbergia sissoo, (f) Diplotaxis griffithii, (g) Eucalyptus camaldulensis, (h) Eruca vesicaria, (i) Syzygium cumini, (j) Eucalyptus globulus, (k) Euphorbia helioscopia, (l) Fumaria indica, (m) Galium aparin, (n) Helianthus annus, (o) Heliotropium currassavicum, (p) Imperata cylindrica, (q) Ipomoea cairica, (r) Lantana camara, (s) Lepidium draba, (t) Leucaena leucocephala, (u) Luffa cylindrica, (v) Melia azadirachta, (w) Melilotus indicus , (x) Mentha longifolia, (y) Morus alba.
LM (a) Nerium oleander, (b) Parkinsonia aculeata, (c) Parthenium hysterophorus, (d) Peganum harmala, (e) Cenchrus americanus, (f) Pheonix dactylifera, (g) Physalis minima, (h) Plantago lanceolata, (i) Prosopis cineraria, (j) Neltuma juliflora, (k) Pulicaria undulata, (l) Punica granatum, (m) Rumex dentatus, (n) Saccharum spontaneum, (o) Salix babylonica, (p) Salvia moocroftiana, (q) Sesbania herbacea, (r) Solanum virginianum, (s) Sonchus asper, (L.), (t) Sorghum halepense, (u) Spergula arvensis, (v) Tamarix dioica, (w) Taraxacum campylodes, (x) Trifolium constantinopolitanum, (y) Trifolium pratense.
LM (a) Tripleurospermum caucasicum, (b) Verbesina encelioides, (c) Vicia sativa, (d) Zea mays, (e) Ziziphus mauritiana, (f) Vachellia spp., (g) Anagallis spp., (h) Anethum spp., (i) Asteraceae, (j) Brassica spp., (k) Brassicaceae, (l) Convolvulus spp., (m) Cynodon spp., (n) Cyperus spp., (o) Datura spp., (p) Eruca spp., (q) Eucalyptus spp., (r) Euphorbia spp., (s) Euphorbiaceae, (t) Fabaceae, (u) Galium spp., (v) Ipomoea spp., (w) Lamiaceae, (x) Mentha spp., (y) Moraceae, (z) Myrtaceae, (a1) Poaceae, (b1) Prosopis spp., (c1) Solanaceae, (d1) Solanum spp., (e1) Tamarix spp., (f1) Trifolium spp.
SEM (a) Senegalia catechu, (b) Vachellia nilotica, (c) Albizia lebbeck, (d) Alhagi maurorum, (e) Lysimachia arvensis, (f) Anethum graveolens, (g) Arnebia linearifolia, (h) Asphodelus tenuifolius, (i) Aster subulatus, (j) Brassica rapa, (k) Brassica rapa, (l) Melaleuca citrina, (m) Centaurea cyanus, (n) Centaurea iberica, (o) Centaurium pulchellum, (p) Dysphania ambrosioides, (q) Ismelia carinata, (r) Cicer arietinum, (s) Cichorium intybus, (t) Cirsium arvense.
SEM (a) Citrullus colocynthis, (b) Volkameria inermis, (c) Conocarpus erectus, (d) Convolvulus arvensis, (e) Conyza boranensis, (f) Cymbopogon jwarancusa, (g) Cynodon dactylon, (h) Cyperus rotundus, (i) Datura innoxia, (j) Dalbergia sissoo, (k) Diplotaxis griffithii, (l) Eruca vesicaria, (m) Eucalyptus camaldulensis, (n) Eucalyptus globulus, (o) Syzygium cumini , (p) Euphorbia helioscopia, (q) Fumaria indica, (r) Galium aparin, (s) Helianthus annus, (t) Heliotropium currassavicum.
SEM (a) Imperata cylindrica, (b) Ipomoea cairica, (c) Lantana camara, (d) Lepidium draba, (e) Leucaena leucocephala, (f) Luffa cylindrica, (g) Melia azadirachta, (h) Melilotus indicus, (i) Mentha longifolia, (j) Morus alba, (k) Nerium oleander, (l) Parkinsonia aculeata, (m) Parthenium hysterophorus, (n) Peganum harmala, (o) Cenchrus americanus, (p) Pheonix dactylifera, (q) Physalus minima, (r) Plantago lanceolata, (s) Prosopis cineraria, (t) Neltuma juliflora.
SEM (a) Pulicaria undulata, (b) Punica granatum, (c) Rumex dentatus, (d) Saccharum spontaneum, (e) Salix babylonica, (f) Salvia moorcroftiana, (g) Sesbania herbacea, (h) Solanum virginianum, (i) Sonchus asper, (j) Sorghum halepense, (k) Spergula arvensis, (l) Tamarix dioica, (m) Taraxacum campylodes, (n) Trifolium constantinopolitanum, (o) Trifolium pratense, (p) Tripleurospermum caucasicum, (q) Verbesina encelioides, (r) Vicia sativa, (s) Zea mays, (t) Ziziphus mauritiana.
SEM (a) Vachellia spp., (b) Anagallis spp., (c) Anethum spp., (d) Asteraceae, (e) Brassica spp.(f) Brassicaceae, (g) Convolvulus spp., (h) Cynodon spp., (i) Cyperus spp., (j) Datura spp., (k) Eruca spp., (l) Eucalyptus spp., (m) Euphorbia spp., (n) Euphorbiaceae, (o) Fabaceae, (p) Galium spp., (q) Ipomoea spp., (r) Lamiaceae, (s) Mentha spp., (t) Moraceae.
Pollen count
The main results of this study were to extract the pollen grains from the honey samples and determine the biochemical composition of honey. The highest bee pollen was observed in Mamash Khel (honey) of Apis florea F., with 29,686 pollens. Pollen diversity was observed in eight diversified bee plants in which Ziziphus mauritiana shows 47.9% of grain indicated as a monofloral characteristic while Zea mays L. shows minimum pollen with 2.7%.
The least number of pollens was observed as (20,113) in the Ghaza khel of Apis mellifera L. honey among Salix babylonica representing dominancy with 17.9% of the melliferous pollen, and Zea mays L., Asteraceae family showing 1.5% pollen. Monofloral characteristics were observed in eight samples, Jando khel honey with Eucalyptus globulus (47.3%), Khajori honey dominating Trifolium pratense L. pollen (46.50%), Kakki sample with the presence of Neltuma juliflora (47.8%), Mamash khel honey with Ziziphus mauritiana pollen (47.9%), Spina Tangai honey with presences of Asphodelus tenuifolius Cav. (47.5%), Tajori samples with Cynodon dactylon pollen (47.3%), Wara tarai honey with the dominance of Saccharum spontaneum pollen (47.5%), and Tajbi khel samples with the presence of Vachellia nilotica pollen (46.9%).
The lowest pollen count per slide was observed among the taxa recorded in the honey samples were Fabaceae, Brassicaceae, Eruca vesicaria, Cyperus rotundus, Centaurium pulchellum, Eucalyptus spp, Euphorbia spp. and Prosopis spp (1%), and the highest pollen grain for Ziziphus mauritiana (47.9%). In the Landiwa sample, the highest diversity of pollen grains was observed encompassing pollen from 12 distinct Melliferous plants. These Melliferous species such as Saccharum spontaneum (16.5%), Sesbania herbacea (17.5%), Cenchrus americanus (16%), Poaceae (17.5%), Convolvulus arvensis L. (6.3%), Cynodon dactylon (6.2%), Moraceae (6%), Melilotus indicus (6%), Fabaceae (2%), Ipomoea spp. (3%), Euphorbia helioscopia (2%) and Brassica spp. (1%). In the Wara Tarai sample, the lowest diversity of pollen grains was observed, consisting of pollen from seven honeybees of different taxa. These melliferous plants such as Saccharum spontaneum (47.5%), Cynodon dactylon (16.5%), Cymbopogon jwarancusa (18%), Cicer arietinum (6%), Spergula arvensis (7.5%), Poaceae (2.5%) and Fabaceae (2%). The Jando khel, Khajori, Kakki, Mamash khel, Spina Tangai, Tajori, Wara tarai, and Tajbi khel samples were deemed significant, primarily because of their monofloral nature. Overall, the analysis of pollen grains identified 80 honeybee plant species, which included representatives from thirty-two families, with some categorized at the genus or family level where species identification was not feasible (Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12).
The dominant plant families were observed as Fabaceae (15 species); Asteraceae (14 species); Poaceae (8 species of bee plants); Brassicaceae (5 bee plants species); Myrtaceae (4 honeybee plant species); Solanaceae (3 species); Convolvulaceae, Verbenaceae, Lamiaceae, Cucurbetaceae, and Boraginaceae (2 species) and the other 21 families contain only one species. The identified taxa are composed by bees visited species with blooming variation with an aspect of the season and significance for good quality of honey production, which is also affected by honey melissopalynological, nutritional properties, biochemical, and quality assessment tests. Among the twenty-seven honey samples, no honeybee pollen was found to have a prevalence of 45% in quantity compared to others (Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12).
Phenolic quantity in honey
The phenolic quantity variations were observed in different samples of the area studied. For phenolic quantification, the Gallic acid chemical is a standard used. The maximum phenolic content was examined in the Spina Tangai honey of 1.51 ± 0.31 μg GE/mL, followed by Jando khel, Kakki, and Mamash khel with quantity of 1.47 ± 0.31 μg GE/mL, 1.37 ± 0.21 μg GE/mL, and 1.36 ± 0.21 μg GE/mL, respectively. The minimum quantity observed in Ghaza khel honey is 0.38 ± 0.01 μg GE/mL (Table 2).
Flavonoid quantity in honey
The maximum quantity of flavonoid was observed in the Kakki sample of 1.31 ± 0.41 μg QE/mL, followed by Mamash khel, Tajbi khel, and Shamsi khel samples with respective quantities of 1.30 ± 0.41 μg QE/mL, 1.24 ± 0.24 μg QE/mL and 1.20 ± 0.21 μg QE/mL. The minimum quantity of flavonoid was observed in the Ghaza khel honey with 0.36 ± 0.42 μg QE/mL (Table 2).
Alpha-amylase quantification
The maximum quantity of alpha-amylase was examined in the Mamash khel honey of 0.83 ± 0.02 µg/mL, followed by Kakki, Shamsi khel, and Spina Tangai honey, with the quantity of respectively 0.81 ± 0.01 µg/mL, 0.80 ± 0.21 µg/mL and 0.78 ± 0.12 µg/mL, respectively. The minimum value of alpha-amylase was observed in the Hussain khel honey, containing 0.38 ± 0.13 µg/mL. The total variation observed from 0.38 to 0.83 µg/mL observed in honey samples (Table 2).
Reducing power of honey
The range of reducing power was noted from 0.21 ± 0.11 to 0.53 ± 0.04 µg/mL in different honey samples. The maximum reducing power was recorded in the Khajori honey, with 0.53 ± 0.04 µg/mL followed by Jando khel, Kakki, and Mamash khel honey with the quantities of respectively 0.52 ± 0.08 µg/mL, 0.51 ± 0.60 µg/mL and 0.50 ± 0.60 µg/mL. The lowest amount of reducing power was observed in the Ghaza khel honey, with 0.21 ± 0.11 µg/mL (Table 2). The biochemical profiling of honey is presented in Fig. 13.
Total mineral content
Natural products, including honey, can exhibit variation in their biochemical composition based on factors like geographical origin, botanical source, seasonal variations, sample diversity, processing and handling of honey, anthropogenic effect, and bee activities. The mineral concentration was recorded in various samples. The cadmium ranges from 0.010 to 0.056 mg/g. The maximum cadmium was observed in Ghaza khel (0.056 mg/g) while the minimum was in Khajori (0.010 mg/g). The chromium range was observed from 0.014 mg/g (Khajori) to 0.059 mg/g (Ghaza Khel). Nickel was observed in a range varying 0.009 mg/g (Link Road) to 0.058 mg/g (Azad Khel). The maximum manganese was observed in Tajbi khel (0.590 mg/g) while the minimum was in Gambila (0.197 mg/g). Magnesium variation was observed from 0.077 mg/g (Nar Jafar) to 1.090 mg/g (Kakki). The highest calcium level was observed in Spina Tangai (1.010 mg/g) while the lowest was in Ghaza Khel (0.179 mg/g). The copper range was observed from Jando khel (0.010 mg/g) to Mamash khel (0.080 mg/g). (Table 3). The quantification of minerals and their relation to each other is presented in Fig. 14.
Moisture of honey
The quantifications of solid matter and moisture were not enough to reflect overall honey quality. In these studies, different parameters required for overall quality assessment were analyzed like fructose, reducing power, galactose, phenolic, alpha-amylase, minerals and flavonoid, etc. The moisture content of honey was observed to evaluate and improve the honey’s quality. Thirty-five honey samples were classified into four categories based on moisture content (%) including A + < 17, A = 17–18, B = 18–19, and C > 19. Among the thirty-five honey samples, eight samples were observed as ‘A + ’ class, which indicates good quality honey, six samples were studied as ‘A’ class honey, thirteen honey samples were evaluated as ‘B’ class, and eight samples were observed as a ‘C’ class which represented low-quality honey. The maximum quantity of moisture was observed in the Landiwa sample (21.1%) while the minimum was in Mamash Khel (16.5%). The good-quality honey samples’ moisture content was observed as Jando Khel (16.9%), Khajori (16.8%), Kakki (16.9%), Mamash Khel (16.5%), Spina Tangai (16.9%), Tajori (16.6%), Wara tarai (16.9%) and Tajbi Khel (16.9%). The low-quality honey moisture content was observed as Serki Khel (20.03%), Landiwa (21.1%), Sarain Naurang (19.33%), Gambila (19.2%), Derra Pezu (20.8%), Aba Khel (19.11%), Azad Khel (19.4%) and Ghaza Khel (20.5%). The most repeated class was observed as the B class (18–19%) in thirteen honey samples (Table 4).
Total soluble sugar
In honey samples, the soluble sugar is inversely proportional to the moisture content. The maximum soluble sugar was observed in the Wara tarai honey samples (82.57%), followed by Mamash Khel (82.56%), Spina tangai (82.55%), Khajori (82.46%), and Tajbi Khel (82.28%), respectively. The lowest amount was observed in the Serki Khel (78.23%). Fourteen honey among studied samples had more than 82% soluble sugar, which reflects their good quality. This sample includes: Jando Khel (82.3%), Khajori (82.46%), Hakim Barat (82.2%), Kakki (82.4%), Mamash Khel (82.56%), Ghani Khel (82.26%), Spina Tangai (82.55%), Saiydi Khel (82.03%), Tajori (82.3%), Ghazni Khel (82.2%), Wara Tarai (82.57%), Masti Khel (82.2%), Tajbi Khel (82.27%), and Hussain Khel (82.27%). In seventeen samples, 80–82% of soluble sugar was observed. The four honey samples have less than 80% soluble sugar, reflecting the low quality of honey (Table 4).
Fructose and glucose content
The fructose and glucose contents are counted in total soluble sugar by HPLC (Fig. 5). The maximum fructose and glucose content was observed in Mamash Khel (80.06%), followed by Spina Tangai (80.02%), Wara tarai (80.01%), and Khajori (79.96%), the minimum was observed in Landiwa (74.94%). The total variations observed ranged from 74.94 to 80.06% (Table 4).
Sucrose content
The sucrose contents are counted in the total soluble sugar by HPLC (Fig. 6). The optimal standard quantity of sucrose for ensuring the best quality of honey is approximately 2.5%, which is worldwide used for the authentication of honey. The 2.5% sucrose standard is supported by different international standards and regulations set by different organizations such as the Codex Alimentarius Commission & the European Union36. These standards are established to reliable measure for the quality assessment of industrial honey and safeguard consumers. The sucrose content of total soluble sugar was observed to evaluate the honey quality. Thirty-five honey samples were classified into four categories based on sucrose content (%): (2.50–2.60 = A +); (2.61–2.75 = A); (2.76–3.00 = B); (3.00 + = C). In thirty-five honey samples eleven were observed as ‘A + ’ class, indicating good quality honey, four samples were examined as ‘A’ class honey, twelve honey samples were evaluated as ‘B’ class, and eight samples were observed as a ‘C’ class which represented the low-quality honey.
The maximum quantity of sucrose was observed in the Ghaza Khel sample (3.79%) while the minimum was in Mamash Khel (2.50%). The good quality honey sample’s sucrose content was observed as Jando Khel (2.51%), Khajori (2.50%), Hakim Barat (2.59%), Kakki (2.53%), Mamash Khel (2.50%), Ghani Khel (2.58%), Spina Tangai (2.54%), Dodi Khel (2.91%), Tajori (2.53%), Wara tarai (2.55%) and Tajbi Khel (2.59%). The low-quality honey, sucrose content was observed as Ghaza Khel (3.79%), Azad Khel (3.71%), Aba Khel (3.89%), Derra Pezu (3.68%), Gambila (3.32%), Sarain Naurang (3.12%), Landiwa (3.56%) and Serki Khel (3.43%). The total differences noted in the honey samples studied ranged from 2.50 to 3.89%. (Table 4). The relationship between moisture contents and sugar contents is presented in Fig. 15.
Discussion
The results confirm the significance and importance of the melissopalynological technique to evaluate nectar secretion, pollen production, pollination of plants, and their effect on the composition of honey in the study area. In the current research work, 35 honey samples were investigated in terms of melissopalynology and biochemical composition. Fabaceae was observed as a dominant family in terms of species number in the analyzed samples. The results identified 107 pollen types from the samples in the Bannu Division, of which eighty were identified genus-wise, eighteen species-wise, and nine family-wise. The pollen grains found in the current research quite vary with each other in morphological characters. The dominant taxa of Asteraceae, Poaceae, and Fabaceae were seen to be consistent with the previous data that investigated that most of these families were foraged by honeybees to collect nectars37. Poaceae was recorded as the dominant family visited by honeybees in the district Lakki Marwat in the study2.
The melissopalynological study helps to find out the accurate identification of the geographical and botanical origin of species38. The techniques of melissopalynology are frequently used for the systematic identification of the analyzed taxa of honey with an aspect of pollen and nectars39. Every sample of honey has a distinguished diversity of bee pollen that indicates evidence of its floral source, which is fed by honeybees. The samples of honey not only disclose the origin of nectar but also offer insights into the botanical origin and variety of plants across various locations within the studied areas. A honey sample containing 45% or more of pollen grains from a single plant species is classified as monofloral40,41. The honey’s aspect of botanical origin can be determined by the percentage quantification of melliferous pollen using melissopalynological techniques42,43. The results showed the origin of 107 different pollen shapes, of which eighty were identified genus-wise, eighteen species-wise, and nine family-wise. The study aimed to determine the botanical origin and biochemical profiling of honey which was discussed in detail in the last paragraph of the discussion section.
The pollen morphological features observed in honey samples represent tropical melliferous plants44. The pollen dominancy was counted in four different classes with aspects of pollen concentration of different taxa, i.e., minor pollen of melliferous (1–3%), minor important of Melliferous pollen (3–15%), secondary pollen of melliferous (15–45%), and dominant pollen of honeybees (> 45%). Most of the taxa were frequently observed in the secondary class of pollen (15–45%), while only eight plants observed in the dominant class of pollen (> 45%), can be defined as monofloral honey. These four classes of pollen concentration with the help of melissopalynology, were supported by45.
The quality of honey, in terms of its biochemical profile and viscosity, depends upon the floral origin and distribution of pollen, fed by honeybees46,47. The dominant and secondary pollen of the concerned bee flora plays an important role in determining whether the honey is monofloral. All eight monofloral honey samples were observed as good quality honey in terms of biochemical composition, total soluble sugar, and viscosity. Each region has its unique combination of flora which is preserved in honey samples in the form of pollen. Biochemical composition of honey depends upon the plants visited, environmental factors, and soil composition. Analysis of these chemicals such as minerals, flavonoids, phenol, phenolic, alpha-amylase, reducing power, sugar, and moisture content. Some minerals (Cd, Cr, Ni, and Cu) in a maximum amount in honey indicate pollution of the concerned geographic origin while Mn, Ca, and Mg indicate good quality of honey. The flavonoid, phenol, alpha-amylase, and reducing power presence indicate the antioxidant potential and good geographic environment for honey.
The phenolic quantification is a significant character in investigating the quality of honey44. The Spina tangai sample was declared the highest quantity of phenolic, due to monofloral characteristics determined by pollen grain analysis. Previous literature also showed a variation in the total phenolic content48. The maximum TPC was frequently recorded in monofloral honey as compared to polyfloral honey. Most of the earlier literature examined the phenolic and flavonoid presence in honey and attributed their specific medicinal properties, which supported the present study49. A high level of TFC was observed in monofloral honey, while it was minimal in polyfloral honey, as supported by50. A colorimetric method was used to find out the total flavonoid content (TFC) of honey, following the protocol of51. The honey color was higher due to phenol, flavonoid, and phenolic content. The range of alpha-amylase was noted from Mamash khel (0.83 ± 0.01 µg/mL) to Hussain khel (0.38 ± 0.13 µg/mL). The mineral concentrations reported in different samples of the study area, like Ca, Ni, Mn, Mg, Cd, Cr, and Cu, are comparable to the values recorded in the previously published literature52. Our results show similarity with the previous research of53, in which magnesium, nickel, calcium, chromium, cadmium, manganese, and copper were investigated.
The palyno-morphological features of melliferous taxa were carried out in the previous study. The wide variations in pollen sculpture and exine wall thickness are the distinguishing features of bee-foraged species54. With the help of melissopalynological studies, the pollen grains were extracted from the honey samples, and identifications of these pollen grains led to the botanical origin of taxa in the reported study.
The findings of the design project offer valuable scientific knowledge with broader implications for food quality and safety, biodiversity conservation, and sustainable management of beekeeping practices. This research also contributes to exploring the intricate relationship between melliferous plants, honeybees, and human beings, highlighting the significance of ecological preserving balance and the potential of biological resources for societal benefits.
Conclusion
In the current melissopalynological studies, 107 pollen grains belonging to 32 families were recorded from 35 honey samples. Fabaceae, Asteraceae, Poaceae, Brassicaceae, and Myrtaceae were considered important families with abundant taxa foraged by honeybees for honey production. Temperature was examined as a key factor that influenced the variations in the honey viscosity and moisture contents. This study is not just limited to floral diversity, but it provides knowledge about botanical origin, geographical locations, honey quality, and its medicinal uses. In the future, this project will explore antimicrobial and specific enzymatic properties of honey from these species could unveil novel therapeutic applications. Secondly exploring how landscape heterogeneity influences honey composition and nutritional value could inform sustainable beekeeping practices. Additionally investigating product development, market strategies, and scientific inquiry could add cultural significance and ecosystem services provided by these bee species, fostering holistic approaches to honey production and conservation.
Data availability
The data is available on the request from the corresponding author.
Change history
13 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-89637-2
References
Mosti, S., Ross Friedman, C., Pacini, E., Brighigna, L. & Papini, A. Nectary ultrastructure and secretory modes in three species of Tillandsia L. (Bromeliaceae) that have different pollinators. Botany 91, 786–798. https://doi.org/10.1139/cjb-2013-0126 (2013).
Rosdi, I. N., Selvaraju, K., Vikram, P., Thevan, K. & Arifullah, M. Melissopalynological analysis of forest honey from north Malaysia. J. Trop. Resour. Sustain. Sci. 4(2), 128–132. https://doi.org/10.47253/jtrss.v4i2.621 (2016).
Ahmad, S. et al. Palynological studies of winter weeds melliferous flora of District Bannu, Khyber Pakhtunkhwa, Pakistan. Annali di Botanica 10, 77–86. https://doi.org/10.13133/2239-3129/14863 (2020).
Coelho, M. S., da Silva, J. H. V., Oliveira, E. R., Araújo, J. A. & Lima, M. R. Alimentos convencionais e alternativos para abelhas. R Caatin. 21(1), 403–429 (2008).
Ullah, S. A. et al. Microscopic implication and evaluation of herbaceous melliferous plants of southern Khyber Pakhtunkhwa-Pakistan using light and scanning electron microscope. Microsc. Res. Tech. 84, 1750–1764. https://doi.org/10.1002/jemt.23732 (2021).
Bänsch, S., Scharntke, T., Ratnieks, T., Härtel, F. L. & Westphal, S. C. Foraging of honey bees in agricultural landscapes with changing patterns of flower resources. Agric. Ecosyst. Environ. 291, 106792. https://doi.org/10.1016/j.agee.2019.106792 (2020).
Donkersley, P. et al. Nutritional composition of honey bee food stores vary with floral composition. Oecologia 185(4), 749–761. https://doi.org/10.1007/s00442-017-3968-3 (2017).
Brodschneider, R. & Crailsheim, K. Nutrition and health in honey bees. Apidologie. 41(3), 278–294. https://doi.org/10.1051/apido/2010012 (2010).
Winston Mark, L. Biology of the Honey Bee: Lessons from the Hive (Harvard University Press, 2016).
Van Nest, B. N. & Moore, D. energetically optimal foraging strategy is emergent property of time-keeping behavior in honey bees. Behav. Ecol. 23(3), 649–658. https://doi.org/10.1093/beheco/ars010 (2012).
Klein, S. et al. Honey bees increase their foraging performance and frequency of pollen trips through experience. Sci. Rep. 9(1), 1–10. https://doi.org/10.1038/s41598-019-42677-x (2019).
Dórea, M. D. C., Novais, J. S. D. & Santos, F. D. A. R. D. Botanical profile of bee pollen from the southern coastal region of Bahia, Brazil. Acta Bot. Bras. 24(3), 862–867. https://doi.org/10.1590/S0102-33062010000300028 (2010).
Alves, R. M. O., Carvalho, C. A. L., Souza, B. D. A., Sodré, G. D. S. & Marchini, L. C. Características físico-químicas de amostras de mel de Melipona mandacaia Smith (Hymenoptera: Apidae). Ciência. Tecnologia de Aliment 25(4), 644–650. https://doi.org/10.1590/S0101-20612005000400004 (2005).
Vit, P., Bogdanov, S. & Kilchenmann, V. Composition of venezuelan honeys from stingless bees (Apidae: Meliponinae) and Apis mellifera L. Apidologie 25(3), 278–288. https://doi.org/10.1051/apido:19940302 (1994).
Souza, B. et al. Composition of stingless bee honey: Setting quality standards. Interciencia 31(12), 867–875 (2006).
Seeley, T. D. Honeybee Ecology: A Study of Adaptation in Social Life (Princeton University Press, 1985).
Gilliam, M., Buchmann, S. L., Lorenz, B. J. & Roubik, D. W. Microbiology of the larval provisions of the stingless bee, Trigona hypogea, an obligate necrophage. Biotropica 17(1), 28–31. https://doi.org/10.2307/2388374 (1985).
Gilliam, M. & Roubik, D. W. Lorenz BJ Microorganisms associated with pollen, honey, and brood provisions in the nest of a stingless bee. Melipona fasciata Apidologie 21(2), 89–97. https://doi.org/10.1051/apido:19900201 (1990).
Menezes, C., Vollet-Neto, A., Contrera, F. A. F. L., Venturieri, G. C. & Imperatriz-Fonseca, V. L. The Role of Useful Microorganisms to Stingless Bees and Stingless Beekeeping. In Pot-Honey (eds Vit, P. et al.) (Springer, 2013). https://doi.org/10.1007/978-1-4614-4960-7_10.
Salonen, A. & Julkunen-Tiitto, R. Characterisation of two unique unifloral honeys from the boreal coniferous zone: Lingonberry and mire honeys. Agric. Food Sci. 21(2), 159–170. https://doi.org/10.23986/afsci.4931 (2012).
Noor, M. J., Khan, M. A. & Camphor, E. S. Palynological analysis of pollen loads from pollen sources of honeybees in Islamabad, Pakistan. Pak. J. Bot. 41(2), 495–501 (2009).
Ige, O. E. & Obasanmi, O. A palynological assessment of honey samples from Delta State, Nigeria. Am. Int. J. Biol. 2(2), 47–59 (2014).
Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 63, 549–562. https://doi.org/10.1016/S0308-8146(98)00057-0 (1998).
Russmann, H. Originalarbeit-Hefen und Glycerin in Blutenhonigen-Nachweis einer Garung oder einer abgestoppten Garung. Lebensmittelchem. 52(5), 116–117 (1998).
Felsner, M. L. et al. Characterization of monofloral honeys by ash contents through a hierarchical design. J. Food Compos. Anal. 17, 737–747. https://doi.org/10.1016/j.jfca.2003.11.001 (2004).
Ahmad, S., Zafar, M., Ahmad, M., Lubna Yaseen, G. & Sultana, S. Microscopic investigation of palyno-morphological features of melliferous flora of Lakki Marwat district, Khyber Pakhtunkhwa, Pakistan. Microscopy Res.Tech. 82(6), 720–730. https://doi.org/10.1002/jemt.23218 (2019).
Noor, M., Ahmad, M., Zafar, M. & Ashraf, M. A. Palynological studies of melliferous and allergenic flora of Pakistan: A key to pollen and spore identification. J. Apic. Res. 56(3), 300–309. https://doi.org/10.1080/00218839.2017.1306373 (2017).
Barth, O. M. Melissopalynology in Brazil: A review of pollen analysis of honeys, propolis and pollen loads of bees. Sci. Agricola 61(3), 342–350. https://doi.org/10.1590/S0103-90162004000300018 (2004).
Der, Von et al. Harmonized methods of melissopalynology. Apidologie 35(Suppl. 1), S18–S25. https://doi.org/10.1051/apido:2004050 (2004).
Arrigoni, E., Kast, C. & Walther, B. Effects of dietary sugars from natural sources on health outcomes. Diet. Sugars Health https://doi.org/10.1016/j.lwt.2016.09.021 (2014).
Campos, G. et al. Classificação do mel em floral ou mel de melato. Ciênc. Tecnol. Aliment. 23(1), 1–5. https://doi.org/10.1590/S0101-20612003000100002 (2003).
Sahinler, N., Sahinler, S. & Gul, A. Biochemical composition of honeys produced in Turkey. J. Apic. Res. 43(2), 53–56. https://doi.org/10.1080/00218839.2004.11101110 (2004).
Duarte, A. W. F., Vasconcelos, M. R. S., Oda-Souza, M., Oliveira, F. F. & López, A. M. Q. Honey and bee pollen produced by Meliponini (Apidae) in Alagoas, Brazil: Multivariate analysis of physicochemical and antioxidant profiles. J. Food Sci. Technol. 38(3), 493–503. https://doi.org/10.1590/fst.09317 (2018).
Ahmed, J., Prabhu, S. T., Raghavan, G. S. V. & Ngadi, M. Physico-chemical, rheological, calorimetric and dielectric behavior of selected Indian honey. J. Food Eng. 79(4), 1207–1213. https://doi.org/10.1016/j.jfoodeng.2006.04.048 (2007).
Oddo, L. P. et al. Main European unifloral honeys: Descriptive sheets. Apidologie 35(Suppl. 1), S38–S81. https://doi.org/10.1051/apido:2004049 (2004).
Juszczak, L. & Fortuna, T. Rheology of selected Polish honeys. J. Food Eng. 75(1), 43–49. https://doi.org/10.1016/j.jfoodeng.2005.03.049 (2006).
Witczak, M., Juszczak, L. & Gałkowska, D. Non-Newtonian behaviour of heather honey. J. Food Eng. 104(4), 532–537. https://doi.org/10.1016/j.jfoodeng.2011.01.013 (2011).
Oddo, L. P. & Bogdanov, S. Determination of honey botanical origin: Problems and issues. Apidologie 35(Suppl. 1), S2–S3. https://doi.org/10.1051/apido:2004044 (2004).
Barth, O. M. Melissopalynology in Brazil: A review of pollen analysis of honeys, propolis and pollen loads of bees. Sci. Agricola. 61(3), 342–350. https://doi.org/10.1590/S0103-90162004000300018 (2004).
Punt, W., Hoen, P., Blackmore, S., Nilsson, S. & Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 143(1–2), 1–81. https://doi.org/10.1016/j.revpalbo.2006.06.008 (2007).
Orijemie, E. A. Comparative pollen analysis of honeys from apiary and open Markets in Nigeria and Bénin Republic. Ife J, Sci. 19(2), 217–225. https://doi.org/10.4314/ijs.v19i2.2 (2017).
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods enzymol. 299(14), 152–178 (1999).
Sahreen, S., Khan, M. R., Khan, R. A. & Shah, N. A. Estimation of flavoniods, antimicrobial, antitumor and anticancer activity of Carissa opaca fruits. BMC Comp. Altern. Med. 13(1), 1–7. https://doi.org/10.1186/1472-6882-13-372 (2013).
Maurizio, A. & Hodges, F. Pollen analysis of honey. Bee World 32(1), 1–5. https://doi.org/10.1080/0005772X.1951.11094660 (1951).
Khan, K. et al. Melissopalynological and biochemical profile of honeybee (Apis mellifera L) flora in Southern Khyber Pakhtunkhwa, Pakistan. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 156(5), 1177–1186. https://doi.org/10.1080/11263504.2021.2024908 (2022).
Von Der Ohe, W., Oddo, L. P., Piana, M. L., Morlot, M. & Martin, P. Harmonized methods of melissopalynology. Apidologie 35(Suppl. 1), S18–S25. https://doi.org/10.1051/apido:2004050 (2004).
Noor, M. J., Ahmad, M., Ashraf, M. A., Zafar, M. & Sultana, S. A review of the pollen analysis of South Asian honey to identify the bee floras of the region. Palynology 40(01), 54–65. https://doi.org/10.1080/01916122.2014.988383 (2016).
Sharma, C. M., Baduni, N. P., Gairola, S., Ghildiyal, S. K. & Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 260(12), 2170–2179. https://doi.org/10.1016/j.foreco.2010.09.014 (2010).
Sahinler, N., Sahinler, S. & Gul, A. Biochemical composition of honeys produced in Turkey. J. Apic. Res. 43(2), 53–56. https://doi.org/10.1080/00218839.2004.11101110 (2004).
Oroian, M. & Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 138, 148–156. https://doi.org/10.1016/j.compag.2017.04.020 (2017).
Iucure, C. T. & Geană, E. I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 30(4), 481–492. https://doi.org/10.1002/pca.2831 (2019).
Martos, I., Ferreres, F., Yao, L., D’Arcy, B. & Caffin, N. Flavonoids in monospecific Eucalyptus honeys from Australia. J. Agric. Food Chem. 48(10), 4744–4748. https://doi.org/10.1021/jf000277i (2000).
Al-Mamary, M., Al-Meeri, A. & Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 22(9), 1041–1047. https://doi.org/10.1016/S0271-5317(02)00406-2 (2002).
Aljadi, A. & Kamaruddin, M. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 85(4), 513–518. https://doi.org/10.1016/S0308-8146(02)00596-4 (2004).
Acknowledgements
The authors extend their appreciation to the Researchers supporting Project number (RSP2024/95), King Saud University, Riyadh, Saudi Arabia.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KK: Writing—original draft, Writing—review & editing; KM, MA, MTN: Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, Visualization, Project administration, Supervision; MN and AK: Methodology, Formal analysis; GA, SA, MN, and MNIR: Visualization, Resources, Data curation; AK: Validation, Resources, Methodology, Formal analysis, Data curation; GA, SA and MNIR: Investigation, Resources; MN, KM, MA, MTN and AK: Resources, Writing—review & editing. GA: Resources, Writing—review & editing. All authors have read and approved the final manuscript and declare that they have no competitive interest.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Shabir Ahmad which was incorrectly given as Shabir Ahmed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, K., Malik, K., Ahmad, M. et al. Exploring the nutritional composition and quality parameters of natural honey from diverse melliferous flora. Sci Rep 14, 31626 (2024). https://doi.org/10.1038/s41598-024-79672-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79672-w
Keywords
This article is cited by
-
Pollen morphology and taxonomic implications of bee-foraged plant taxa using microscopic techniques
Genetic Resources and Crop Evolution (2025)
-
Pollen as an insect interaction marker: palynological, chemical, and microscopic perspectives of selected medicinal plants
Genetic Resources and Crop Evolution (2025)
-
Integration of multivariate indices to correlate pollen morphology and evolutionary adaptations in halophytes
Genetic Resources and Crop Evolution (2025)