Abstract
Bone health screening is crucial before and during androgen deprivation therapy (ADT) for prostate cancer, yet changes in bone mineral density during ADT are often overlooked. To improve surveillance rates, we developed an auto-recruit path integrated into the outpatient system, where a pop-up reminder prompts physicians to arrange bone health screenings when ADT is prescribed without a dual-energy x-ray absorptiometry (DXA) screening in the past year. If selected, the system orders DXA and related examinations automatically. We retrospectively reviewed DXA screening rates from 2000 to 2018. During that period, only 286 out of 3,019 patients (9.5%) received DXA screenings. After implementing the auto-recruit system, 251 out of 747 eligible patients (33.6%) were screened from March 2021 to February 2022. Participants using ADT for over a year had worse T-scores and higher osteoporosis rates (34.5% vs. 23.2%) compared to those using ADT for less than a year. Post-screening, there was a significant increase in calcium supplement and bone protective agent use, highlighting improved patient awareness and proactive bone health management. In conclusion, bone health screening for prostate cancer patients on ADT remains an unmet need. The auto-recruit path in the outpatient system effectively increases screening rates and enhances bone health management.
Similar content being viewed by others
Introduction
Prostate cancer is the second most commonly diagnosed cancer in men worldwide and a leading cause of cancer-related deaths among men1. Androgen deprivation therapy (ADT) is a cornerstone of treatment for advanced and metastatic prostate cancer, effectively suppressing androgens to inhibit tumor growth2,3. However, ADT disrupts the balance of bone remodeling by suppressing testosterone levels, leading to accelerated bone loss and an increased risk of osteoporosis4.
Osteoporosis, characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, is a common consequence of long-term ADT use in prostate cancer patients5. The imbalance between bone formation and resorption induced by ADT results in decreased BMD, making bones more susceptible to fractures and skeletal complications. Dual-energy x-ray absorptiometry (DXA) examination is the major method to define BMD, allowing for the identification of osteoporosis and assessment of fracture risk6. Consequently, osteoporosis significantly impacts the quality of life and overall prognosis of prostate cancer patients7,8.
Given the detrimental effects of ADT on bone health, the importance of BMD screening cannot be overstated. BMD screening plays a crucial role in identifying individuals at risk of osteoporosis and fractures, allowing for early intervention and preventive measures. Consequently, guidelines from organizations such as NCCN and ESMO recommend routine BMD screening and the use of the Fracture Risk Assessment Tool (FRAX) to assess fracture risk in patients undergoing ADT9,10.
Despite the significance of BMD screening, it remains underutilized in clinical practice, particularly among prostate cancer patients undergoing ADT. Studies, such as those utilizing the Quebec public health care insurance database and the SEER-Medicare database, have reported alarmingly low rates of DXA testing in this population11,12. For instance, the Quebec study observed a modest increase in DXA testing rates from 4.1% in 2000 to 23.4% in 201511, while the SEER-Medicare database revealed only a slight rise from 6.8% in 2005 to 8.4% in 201512. These findings underscore the persistent challenges in ensuring adequate BMD screening among prostate cancer patients on ADT and highlight the urgent need for enhanced awareness and proactive management of bone health in this population.
To amplify screening rates and enhance patient willingness, we streamlined the screening process by developing an auto-recruit path within the outpatient system. This initiative aimed to improve surveillance rates by making the process more accessible and efficient. We also reviewed historical BMD screening rates over the past decades to evaluate improvements following the implementation of this electronic system. Furthermore, we analyzed factors influencing patients’ willingness to undergo screening and pursue subsequent related treatments, aiming to identify and address potential barriers.
Results
Impact of the auto-recruit system on bone health screening rates
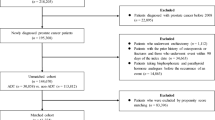
From 2000 to 2018, a retrospective analysis of 3,019 patients found that only 286 (9.5%) underwent DXA screening, with just 51 (1.9%) having follow-up DXA exams before and after starting ADT. After implementing the auto-recruit system from March 2021 to February 2022, 747 patients met the inclusion criteria, with 251 (33.6%) undergoing bone health screening, significantly increasing the screening rate (p < 0.001).
Baseline characteristics of study participants and non-participants
Table 1 presents the baseline characteristics of participants and non-participants in the Auto-Recruit Outpatient System. A total of 747 patients were included in the study, with 251 participants and 496 non-participants. Participants were younger, with a median age of 73.0 years compared to 77.0 years for non-participants (p < 0.001). Participants were also significantly taller and heavier. The median BMI was higher in participants at 25.5 compared to 24.8 in non-participants (p = 0.028). The duration of ADT use was significantly shorter among participants, with a median of 0.8 years compared to 2.1 years in non-participants (p < 0.001). There was no significant difference in the proportion of patients diagnosed with CRPC between participants (32.3%) and non-participants (33.5%) (p = 0.743). However, the usage of BMAs was significantly lower among participants (7.2%) compared to non-participants (20.4%) (p < 0.001).
Analysis of participants by duration of ADT usage
We further analyzed the impact of ADT duration on bone health by dividing participants into two groups: ADT usage ≤ 1 year (138 participants) and > 1 year (113 participants) (Table 2). There were no significant differences in age, height, weight, or BMI between the groups. However, the minimal T-score was significantly lower in the group with ADT usage > 1 year (-2.1 vs. -1.7, p = 0.009), and a higher proportion met the osteoporosis criteria (T-scores ≤ -2.5) (34.5% vs. 23.2%, p = 0.039). The FRAX scores for major and hip fractures, as well as the percentage of participants with a history of fractures, did not differ significantly between the groups. Calcium supplement use before screening was similar between the groups. However, the use of BMAs before screening was significantly higher in the group with ADT usage > 1 year (14.2% vs. 1.4%, p < 0.001).
Impact of screening protocol on calcium supplement and bone health agent use
Table 3 illustrates the changes in calcium supplement and bone health agent use before and after bone health screening, categorized by ADT usage duration (≤ 1 year vs. >1 year). Overall, calcium supplement use increased significantly from 16.3 to 44.2% post-screening (p < 0.001), and bone-protective agent use rose from 7.2 to 25.5% (p < 0.001). Subgroup analysis showed that the duration of ADT usage did not alter this trend, with both groups exhibiting significant increases in calcium supplement and bone-protective agent use after screening.
Discussion
Addressing the unmet need for BMD screening in prostate cancer patients undergoing ADT requires a multifaceted approach13. Healthcare providers must prioritize bone health assessments and integrate BMD screening into routine clinical practice. This project, utilizing the Auto-recruit path for bone health screening in the outpatient management system, effectively increased the overall screening rate from 9.5% between 2000 and 2018 to 33.6% in 2021–2022. Additionally, patients’ willingness to accept osteoporosis-related prevention and treatment significantly improved after undergoing the screening process. To our knowledge, this study is the first to demonstrate a significant increase in prostate cancer osteoporosis screening rates through a simple program setup, proving that electronic system support can effectively enhance healthcare efficiency.
Despite a significant improvement in the screening rate, the actual rate of 33.6% remains below expectations. Several factors may explain this outcome. First, the system’s mandatory pop-up reminder is designed to comprehensively ensure that all eligible patients are considered. However, the inclusion criteria did not account for factors such as age, race, comorbidities, performance status, or life expectancy, which may make patients unsuitable for screening. These factors could potentially impact the final enrollment results and the overall screening rate. Second, some physicians may have considered BMD screening unnecessary for specific patients, as indicated by the higher age and longer ADT duration among non-participants, which could correlate with a poorer prognosis. Third, 20.4% of non-participants were already receiving BMAs without prior DXA screening, likely impacting the physicians’ decision to forgo additional screening. Furthermore, some non-participants may have undergone DXA screening over 12 months prior, or had bone metastases, where BMAs were prescribed to prevent skeletal-related events rather than to address low BMD.
Bone-modifying agents (BMAs) are effective in preventing fractures and reducing skeletal-related events (SREs) in patients with CRPC with bone metastasis14. In contrast, previous clinical trials have shown that there is no significant survival benefit from using BMAs in patients with metastatic castration-sensitive prostate cancer (mCSPC), which is why routine use of BMAs is not recommended in this population15,16. However, in recent years, this recommendation against BMA use has often been interpreted more broadly as an indication of “unnecessary use.”17 Nonetheless, providing BMAs selectively to patients who truly need it can still yield substantial benefits, as supported by the post hoc analysis of the LATITUDE trial18. A crucial component of this approach is BMD screening and the prevention of osteoporotic complications. Thus, implementing comprehensive BMD screenings for prostate cancer patients on ADT could help identify those at high risk for fractures. These high-risk patients could then receive BMAs, effectively preventing fractures while avoiding unnecessary side effects and reducing medical waste19,20.
ADT can accelerate bone loss, leading to osteoporosis and an increased risk of fractures. A 24-month prospective observational study of prostate cancer patients undergoing ADT found that the prevalence of osteoporosis and osteopenia significantly increased from 10% and 58–22% and 70%, respectively21. In our study, patients who had been on ADT for more than a year showed a significant decrease in their minimal T-scores and a higher proportion meeting the criteria for osteoporosis, echoing these findings. Although older patients on long-term ADT might initially overlook bone health issues, our study revealed that this group showed a significant increase in willingness to accept calcium supplements and bone-modifying agents after BMD screening. This indicates that these patients are not unwilling to take action to protect themselves, but rather they are unaware of their bone health status. Therefore, patient education and awareness initiatives are crucial to empower individuals to advocate for their bone health and pursue appropriate screening and preventive interventions22.
This study has several limitations. First, although it is a prospective cohort study, most patients lacked baseline BMD data prior to initiating ADT, which may affect the accuracy of osteoporosis data analysis. To address this issue, we simultaneously established a bone-health database aimed at facilitating long-term and comprehensive data analysis regarding the impact of ADT on bone health. Second, although the auto-recruit path in the outpatient system was associated with a significant increase in screening rates, other factors—such as heightened clinician awareness, patient education initiatives, and evolving clinical practices over time—may also have contributed to this rise. Our current study design does not allow us to isolate the impact of each of these factors. Future research will focus on examining the effects of different clinical policies over time to better assess the specific contribution of the auto-recruit system. Third, while many patients took preventive and therapeutic measures for bone health after BMD screening, there is still no direct evidence that these measures effectively reduce SREs. Long-term follow-up and comparison of cohort differences are necessary to establish this evidence. Furthermore, in this study, we referred to ‘patient willingness’ regarding the increased uptake of calcium supplementation and BMA use. However, without direct patient-reported outcomes, we lack definitive evidence to attribute the increase solely to patient willingness. Other factors, such as increased clinician awareness or prioritization, may have also played a role. Therefore, it is challenging to fully distinguish the contributions of each factor in this context. Lastly, this study was conducted at a single medical center. Although the implementation relied on simple diagnostic and medication codes, not all healthcare electronic systems may support such configurations, potentially limiting the generalizability of the system. Further validation in diverse healthcare settings is needed to assess its broader applicability.
Conclusion
Bone health screening for patients with prostate cancer undergoing ADT remains an unmet need. The convenience of the system plays a crucial role in influencing physicians’ decisions to screen for this issue. The auto-recruit path within the outpatient management system can effectively remind physicians of the appropriate inclusion criteria, significantly increasing the bone health screening rate. Additionally, it enhances patient education and awareness initiatives. Such programs allow us to effectively identify and treat high-risk patients for fractures while avoiding unnecessary side effects and medical waste.
Materials and methods
Auto-recruit path and study design
To enhance the BMD screening rate and assist physicians in arranging the necessary examinations, we implemented a mandatory pop-up reminder within the outpatient management system, launched in March 2021. This reminder activates whenever ADT is prescribed for a prostate cancer patient who has not undergone a DXA screening within the past year.
If the physician opts to proceed with the screening, the system prompts for DXA, spinal X-rays, serum testosterone levels, other relevant laboratory tests, and the Fracture Risk Assessment Tool (FRAX) score. Additionally, we collaborated with the Osteoporosis Prevention and Treatment Center, osteoporosis specialists, and the dental department, which play crucial roles in preventing complications associated with bone-modifying agents (BMAs) (Fig. 1).
Study cohort and bone-health database
The system identifies prostate cancer patients (ICD10: C61) in the outpatient system who are receiving ADT (Drug ID) and have not undergone a DXA exam within the past year. This includes patients receiving ADT-based therapy for metastatic prostate cancer and those undergoing combination therapy of ADT and radiotherapy for localized prostate cancer.
We established a prostate cancer-related bone health database to collect and analyze data. The study protocol was approved by the Institutional Review Board (IRB) of Taichung Veterans General Hospital (IRB No. CG20068A). All research was conducted in accordance with relevant guidelines and regulations, and all participants provided written informed consent.
Factors influencing screening and outcomes
We assessed all eligible patients and divided them into participants and non-participants to identify factors affecting the overall screening rate, including age, height, weight, body mass index (BMI), duration of ADT use, percentage of castration-resistant prostate cancer (CRPC), and usage of BMAs.
Within the participant group, we analyzed the impact of ADT duration on bone health outcomes. Additionally, we evaluated changes in the usage rates of calcium supplements and BMAs before and after the screening protocol. This analysis aimed to understand factors influencing participation in screening and the effectiveness of the intervention in improving bone health management.
To assess the impact of the auto-recruit system, we retrospectively reviewed prostate cancer patients who underwent ADT from 2000 to 2018, focusing on the number and results of DXA tests to evaluate the BMD screening rate. This review was conducted under the same IRB approval from Taichung Veterans General Hospital (IRB No. CE21454B).
Statistical analysis
Descriptive statistics were presented as medians with interquartile ranges (IQR) for continuous variables and as frequencies with percentages for categorical variables. The Chi-square test was used for comparing categorical variables, while the Mann-Whitney U test was applied to continuous variables. Additionally, changes in the usage rates of calcium supplements and BMAs before and after the implementation of the screening protocol were analyzed using the McNemar test for paired nominal data. All statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA), with statistical significance determined by a p-value of less than 0.05.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. Cancer statistics CA: A Cancer J. Clin. 71, 7–33, https://doi.org/10.3322/caac.21654 (2021).
Teo, M. Y., Rathkopf, D. E. & Kantoff, P. Treatment of advanced prostate Cancer. Annu. Rev. Med. 70, 479–499. https://doi.org/10.1146/annurev-med-051517-011947 (2019).
Sekhoacha, M. et al. Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules. 27 https://doi.org/10.3390/molecules27175730 (2022).
Bienz, M. & Saad, F. Androgen-deprivation therapy and bone loss in prostate cancer patients: A clinical review. Bonekey Rep. 4, 716. https://doi.org/10.1038/bonekey.2015.85 (2015).
Kim, T. J. & Koo, K. C. Pathophysiology of bone loss in patients with prostate Cancer receiving androgen-deprivation therapy and lifestyle modifications for the management of Bone Health: a Comprehensive Review. Cancers (Basel). 12. https://doi.org/10.3390/cancers12061529 (2020).
Sawicki, P., Tałałaj, M., Życińska, K., Zgliczyński, W. S. & Wierzba, W. Current applications and selected technical details of dual-energy X-ray absorptiometry. Med. Sci. Monit. 27, e930839. https://doi.org/10.12659/msm.930839 (2021).
Oefelein, M. G., Ricchiuti, V., Conrad, W. & Resnick, M. I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 168, 1005–1007. https://doi.org/10.1016/s0022-5347(05)64561-2 (2002).
Baldessari, C. et al. Bone metastases and health in prostate cancer: From pathophysiology to clinical implications. Cancers (Basel). 15 https://doi.org/10.3390/cancers15051518 (2023).
Schaeffer, E. M. et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 21, 1067–1096. https://doi.org/10.6004/jnccn.2023.0050 (2023).
Parker, C. et al. Prostate cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Annals Oncol.: Off. J. Eur. Soc. Med. Oncol. 31, 1119–1134. https://doi.org/10.1016/j.annonc.2020.06.011 (2020).
Hu, J., Aprikian, A. G., Vanhuyse, M. & Dragomir, A. Contemporary Population-based analysis of bone mineral density testing in men initiating androgen deprivation therapy for prostate Cancer. J. Natl. Compr. Canc Netw. 18, 1374–1381. https://doi.org/10.6004/jnccn.2020.7576 (2020).
Suarez-Almazor, M. E. et al. Association of Bone Mineral Density Testing with Risk of Major Osteoporotic fractures among older men receiving androgen deprivation therapy to treat localized or Regional prostate Cancer. JAMA Netw. Open. 5, e225432. https://doi.org/10.1001/jamanetworkopen.2022.5432 (2022).
Smit, A. E., Meijer, O. C. & Winter, E. M. The multi-faceted nature of age-associated osteoporosis. Bone Rep. 20, 101750. https://doi.org/10.1016/j.bonr.2024.101750 (2024).
So, A., Chin, J., Fleshner, N. & Saad, F. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: incorporating new agents into clinical practice. Can. Urol. Assoc. J. 6, 465–470. https://doi.org/10.5489/cuaj.12149 (2012).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 387, 1163–1177. https://doi.org/10.1016/s0140-6736(15)01037-5 (2016).
Smith, M. R. et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J. Clin. Oncol. Offi. J. Am. Soc. Clin. Oncol. 32, 1143–1150. https://doi.org/10.1200/jco.2013.51.6500 (2014).
Mitchell, A. P. et al. Costs to Medicare of Nonrecommended Bone-Modifying Agent use for castration-sensitive prostate Cancer. JCO Oncol. Pract. 20, 393–400. https://doi.org/10.1200/op.23.00602 (2024).
Fukuokaya, W. et al. Bone-modifying agents in patients with high-risk metastatic castration-sensitive prostate Cancer treated with abiraterone acetate. JAMA Netw. Open. 7, e242467. https://doi.org/10.1001/jamanetworkopen.2024.2467 (2024).
Mitchell, A. P. et al. Cost to Medicare from bone modifying agent overuse in castration-sensitive prostate cancer. JCO Oncol. Pract. 19, 12–12. https://doi.org/10.1200/OP.2023.19.11_suppl.12 (2023).
Mitchell, A. P. et al. Real-world use of bone-modifying agents in metastatic castration-sensitive prostate Cancer. J. Natl. Cancer Inst. 114, 419–426. https://doi.org/10.1093/jnci/djab196 (2022).
Poulsen, M. H. et al. Osteoporosis and prostate cancer; a 24-month prospective observational study during androgen deprivation therapy. Scand. J. Urol. 53, 34–39. https://doi.org/10.1080/21681805.2019.1570328 (2019).
Ribeiro, E. M. et al. Bone health education programs for older people: An integrative review. Cien Saude Colet. 28, 2025–2034. https://doi.org/10.1590/1413-81232023287.10602022 (2023).
Acknowledgements
I would like to extend my gratitude to the Biostatistics Group at the Department of Medical Research, Taichung Veterans General Hospital, for their valuable assistance in statistical analysis. Their expertise greatly contributed to the accuracy of our study. I appreciate their collaboration and support throughout this research.
Author information
Authors and Affiliations
Contributions
Conception and design: C.Y.L., C.L.W.; Acquisition of data: C.L.W., J.R.L., K.Y.C.; Analysis and interpretation of data: C.C.C., C.K.Y Drafting of the manuscript: C.L.W.; Critical revision of the manuscript:. S.S.W., C.S.C.; Statistical analysis: S.C.H, C.C.C ; Administrative, technical support: C.S.C., S.C.H; Supervision: C.Y.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, CL., Wang, SS., Chen, CS. et al. Enhancing screening rates for bone health management in prostate cancer patients on androgen deprivation therapy with an automated outpatient system. Sci Rep 14, 28460 (2024). https://doi.org/10.1038/s41598-024-79888-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79888-w