Abstract
Beneficial associations between higher fruit and vegetable intakes and risk of depression appear to exist but few studies have focused on adults aged 45 + years and the potential that associations are due to residual confounding has not been tested. This longitudinal study of twins (n = 3483, age 45–90 years) from Australia, Denmark, Sweden and USA, assessed the associations between baseline fruit/vegetable intake and depressive symptoms over 5–11 years using linear mixed effects models. Intakes from food frequency questionnaires were trichotomized. Depressive symptoms were assessed using validated measures. The co-twin method was used to examine familial confounding. Compared with low intakes, both high fruit and high vegetable intakes were associated with lower depressive symptoms (fruit: β -.007 [95%CI − .014, < − .001], p = .040; vegetables: β − .006 [95%CI -.011, -.002], p = .002); whereas only moderate vegetable intakes, were associated with lower depressive symptoms (vegetables: β − .005 [95%CI − .009, − .001], p = .014). No familial confounding was found for vegetables, while the results for fruit were inconclusive, likely due to smaller sample size and the marginal significance of the main result. Higher fruit and vegetable intakes may protect against depressive symptoms, presenting another argument for increasing intakes in adults aged 45 + years.
Similar content being viewed by others
Introduction
Depressive disorders significantly contribute to disease burden in older adults, constituting approximately 4% of years lived with disability in individuals aged 55 years and older1. Prevalence of depressive disorders appears to peak between the ages of 55 – 75 years, with a rate of around 8% in women and 5.5% in men2. The disorders vary from mild depression that does not reach the threshold of a clinical diagnosis but impacts quality of life3, to severe major depressive disorder.
A growing body of evidence suggests diet plays a role in depression. Meta-analyses of results from observational population-based studies have reported a reduced risk of incident depression with higher adherence to a Mediterranean diet and a diet with a low Dietary Inflammatory Index (33% and 24% respectively)4. When examining middle-aged to older adults only, beneficial relationships have been reported between diet quality and depressive symptoms5. Our meta-analyses of four observational studies in adults 45 + years found higher intakes of both fruit and vegetables were associated with lower risk of incident depression (15% and 9% respectively)6. There are, however, limitations to this evidence; for example, few studies to date have focused on older adults, and the associations detected may be due to residual confounding.
Both fruit and vegetable intake and depression appear to be influenced by a combination of genetics and the environment. In an examination of twins aged 65 + years, we found both vegetable intakes and depressive symptoms to be moderately heritable (vegetable intake 39%, depressive symptoms 30%)7. Twin studies provide a unique opportunity to reduce the influence of a range of genetic and environmental confounders. Monozygotic twins are matched for genetic material and dizygotic twins on average share 50% genetic material. Monozygotic and dizygotic twins are matched for age and early shared environment in twins reared together. Also, the variability of education, body mass index (BMI), socio-economic status and a variety of non-communicable health conditions are reduced by studying twin pairs. In this study we employed an Inference about Causation from Examination of FAmiliaL CONfounding (ICE FALCON) regression model8 to examine familial confounding as a possible explanation for associations detected between fruit and vegetable intake and depressive symptoms.
The aim of this study is to examine the longitudinal associations between baseline fruit and vegetable intake and depressive symptoms over a period of up to 11 years in twins aged 45 + years from four international cohorts using linear mixed effect models. We hypothesise that higher intakes of fruit and/or vegetables are associated with lower symptoms of depression over time, and that associations detected are not due to familial confounding.
Methods
Contributing cohorts
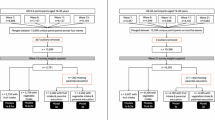
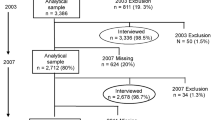
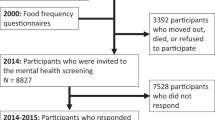
Data were collected from four longitudinal twin cohorts from four countries (Australia, Denmark, Sweden, and the USA). All cohorts are members of the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium9,10. Cohorts were eligible for inclusion if they collected i) baseline dietary data to enable calculation of daily intake serves of both fruits and vegetables, ii) baseline and at least one follow-up depression assessment. Baseline for each study was defined as the first period where both diet and depression were assessed at the same time. The number of follow-up periods used for each cohort was chosen to ensure comparability across studies. Brief descriptions of the four cohorts are provided.
Minnesota Twin Study of Adult Development and Aging (MTSADA)
The MTSADA is a study of same-sex twin pairs identified from state birth records11. MTSADA commenced in 1989 with 1153 twins aged 45 + years. Zygosity was based on a physical similarity questionnaire, where results were equivocal, a blood test was employed.
Middle Age Danish Twins study (MADT)
The MADT is a study of Caucasian twins identified from the Danish Twin Registry12,13. MADT commenced in 1998 with 4308 participants. Zygosity was assessed based on a physical similarity questionnaire, which has been validated against genetic testing14.
Older Australian Twins Study (OATS)
The OATS is a study of twins aged 65 + years residing in New South Wales, Victoria or Queensland identified from the Australian Twin Registry15. OATS commenced in 2006 with 599 participants. Zygosity was based on available genome-wide genotyping data and self-report data16.
The Swedish Adoption/Twin Study of Aging (SATSA)
The SATSA is a study of Caucasian twins identified from the Swedish Twin Registry17. All twins listed in the registry who were reared apart (having been separated before the age of 10 years) were chosen. A sample of twins reared together was selected to match the twins reared apart for age, sex and country of birth. The SATSA began in 1984 with 2019 participants. For the current study, baseline is treated as 1987, this being the first time diet and depressive symptoms were measured at the same period. In 1987, 1421 twins aged 45 + years participated in the assessment. Zygosity was assessed using a combination of a physical similarity questionnaire, blood typing and genotyping.
Participant inclusion criteria for the current study were (a) baseline age 45 + years, (b) baseline fruit and vegetable intake data, (c) baseline depression data, and d) follow-up depression data. A summary of cohorts and follow-up periods is contained in Table 1. This study adheres to the reporting guidelines of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). IGEMS has been approved by the Internal Review Board of the University of Southern California and the research was conducted in accordance with the Helsinki Declaration. All participants provided informed consent.
Measurements
Fruit and vegetable intake
Fruit and vegetable intake were collected at baseline by self-report. Details of assessment methods are contained in Supplementary Tables S1-2. Briefly, all studies collected intakes using food frequency questionnaires (FFQs). MADT and MTSADA collected frequency for intake of “fruit”, SATSA frequency for (i) bananas, (ii) oranges, mandarins, grapefruit, and other citrus fruit, (iii) apples and pears, and for OATS frequency of intake for 13 different types of fruit. Each study separately captured juice consumption which was not included in total fruit intake as the percentage of juice and whether the juice contained added sugar was not available. Similarly for vegetables, MADT collected frequency for intake of (i) boiled vegetables and (ii) raw vegetables, MTSADA frequency for intake of (i) salads, and (ii) cooked vegetables, SATSA frequency for intake of eight types of vegetables and for OATS frequency for intake of 22 types of vegetables. All studies collected potato intake separately. Potato intake was not included in total vegetables for the main analysis as the type of potato (e.g. potato chips, mash, crisps) was not available across all cohorts. As potatoes may provide health benefits (depending on how they are cooked) we included potatoes in vegetable intake for sensitivity analysis.
Intakes for each cohort were trichotomized into three categories (“low”, “moderate”, “high”) for both fruit and vegetables. Cut-offs for each cohort were selected to achieve as close as possible to equal numbers of participants in each category, taking into consideration the distribution of intakes (for example in the MADT cohort, 1210 of the 2298 participants reported consuming one serve of fruit/day therefore the 3 categories used were < 1 serve/day; 1 serve/day and > 1 serve/day) (Supplementary Table S3). Intakes were used as categorical variables as the majority of studies used brief dietary tools, which are more applicable to categorising consumption rather than determining absolute intakes18.
Depressive symptoms
Depressive symptoms were assessed at baseline and follow-up using validated depression assessment measures. In MTSADA and SATSA the 20-item Center for Epidemiologic Studies Depression Scale (CES-D)19 was used. The CES-D has been validated for use in older adults20. Participants were asked how frequently they had experienced 20 different feelings over the past week. Responses ranged from “rarely or never” to “most of the time or all of the time”, with the responses then coded from 0 to 3 resulting in a total possible score ranging from 0 to 60. OATS used the 15-item Geriatric Depression Scale (GDS-15)21. The version used included Question 9 as described by Brink22. The GDS-15 has shown to be a reliable and valid measure of depressive symptoms in older adults21,23. A summary score of 0–15 was calculated based on completed answers. MADT used a subset of 17-items from the depression scale of the Cambridge Mental Disorders of the Elderly Examination (CAMDEX) schedule24,25. A summary score from 17 to 49 was calculated based on provided answers. To create a common depression measure, CES-D and GDS scores were converted to CAMDEX scores using existing crosswalk tables. Crosswalks were developed based on a separate sample in which CAMDEX, GDS and CES-D were administered. Item response theory was used to create a conversion of results between depression measures with CAMDEX used as the reference measure26. Possible scores range from 17 to 49 with higher scores indicating a higher level of depressive symptoms. Depressive symptoms were used as a continuous rather than categorical variable to increase the statistical power of our study, and as there was no harmonised cut-off for depression across the measures used.
Confounders
Baseline data on age, sex, education, living alone status, BMI, physical health, and cognitive ability were collected. Educational attainment was categorised using the International Standard Classification of Education (ISCED)27. Living alone status (yes/no) was based on self-report of being the only person living in the household. BMI was calculated as weight in kilograms divided by height in metres squared. Weight and height were either collected by self-report or measurement. Where BMI data were not available at baseline, data at the next follow-up were imputed to baseline. Physical health was assessed using a modified version of the Cumulative Illness Rating Scale (CIRS)28,29. Participants self-reported their illnesses and medical conditions across 13 domains (e.g. cardiac, hypertension, vascular), and a score was assigned based on the presence or absence of health conditions in each ___domain. An overall physical health summary score ranging from 0 to 13 was calculated. Cognitive health was assessed using the Mini-Mental State Examination (MMSE)30for MTSADA, OATS and SATSA. In MADT, cognitive health was assessed using a composite score derived from six basic tests (fluency, digits forward, digits backwards, immediate recall, delayed recall and speed) as previously described31. Both MMSE scores and cognitive composite scores were converted to z-scores for comparability, and the result used as a continuous variable.
Statistical analysis
Participant baseline characteristics were presented as mean and standard deviation (SD) for normally distributed continuous variables, median and interquartile range (IQR) for non-normally distributed variables, and number and percentage for categorical variables. Continuous variables were assessed for normality using skewness and kurtosis statistics together with visual inspection of histograms. Depressive symptom data were found to be positively skewed and therefore log10 transformed.
A comparison of included versus excluded participants was performed using t-tests for normally distributed continuous variables, Mann Whitney U tests for non-normally distributed continuous variables, and chi-square tests for categorical variables.
Covariate data were assessed for missingness by cohort to determine the pattern and percentage of missing data. Data were missing for education, living alone status, BMI, physical health, and cognitive ability. Covariate data were assessed to be missing at random with cognitive ability having the highest rate of missingness at 4%. Missing covariate data were imputed by cohort using multiple imputation (20 imputations). All confounders, as well as the co-twin’s confounder data, were used as predictors in the imputation model. Imputation was performed using the mice package version 3.15.0 in R32.
The associations between baseline fruit and vegetable intake and depressive symptoms over time (change in depressive symptoms between baseline and follow-ups) were assessed utilising between-within analysis33employing linear mixed effects regression using the lme4 package version 1.1.31 in R34. Analysis was based on log10transformed depressive symptom data. Regression coefficients (β) were calculated as the average of the imputed regression coefficients. 95% confidence intervals (CIs) were based on the sum of the average standard error of the imputations plus the variability of the regression coefficients from the imputations35. Random effects were used to account for the clustering of twin pairs and for the time in study. Analysis was performed by cohort and then results were combined using a random-effects meta-analysis model using the metafor package in R36. Heterogeneity between studies was assessed using I2.
Possible confounders were selected based on their likely relationship with both fruit/vegetable intake and depressive symptoms as determined by a review of the literature. A Directed Acyclic Graph (DAG)37 was used to assist in determining which potential confounders should be adjusted based on the direction of their likely relationships with the exposure and outcome (Supplementary Figure S1). The partially adjusted model included age, sex, education and living alone status. The fully adjusted model included these confounders together with BMI, physical health, and cognitive ability. Baseline physical activity data were not available in two of the four cohorts and hence could not be included as a potential confounder. The interactions between fruit and vegetable intake and age and sex were assessed and found not to be significant and therefore were omitted from the model. Sensitivity analyses were performed firstly including potatoes in total vegetable intake, and secondly, excluding MADT (which was much larger than the other cohorts) to determine the robustness of the results.
Where an association was found between fruit and/or vegetable intake and depressive symptoms, this relationship was examined using an ICE FALCON model with the sample restricted to complete twin pairs8. Intakes and depressive symptoms at each wave were residualised based on age, sex, education, living alone status, BMI, physical health, and cognitive ability. Depressive symptoms were additionally residualised based on time in study. In model 1, the relationship between each twin’s intake (intake self) and their depressive symptoms (depressive symptoms self) over time was analysed using Generalised Estimating Equations. In model 2 the relationship between intake self and their co-twin’s depressive symptoms (depressive symptoms co-twin) over time was analysed. If a relationship was found in model 1 and 2, the change in the relationship was examined in model 3 by including intake self in model 2 (with the standard error calculated using 1,000 bootstrap samples). All models were run by cohort and results combined by meta-analysis.
Results
There were 7481 individuals aged 45 + years across the four cohorts at baseline. Of these 1691 did not provide baseline fruit and vegetable intake or depression data and were excluded from this study. A further 2307 did not provide follow-up depression data, leaving 3483 as the final participant number for the current study.
Baseline characteristics of participants were presented in Table 2. The mean age of participants was 58.5 ± 8.2 years (range 45–90 years) and 51.4% were females. Participants were generally healthy with a mean physical health score of 1.4 ± 1.5 (out of a maximum 13), indicating a relatively low prevalence of medical conditions across different domains and a cognitive health z-score of 0.2 ± 1.0. Mean daily servings of fruit and vegetable intake were 1.0 ± 0.8 and 1.2 ± 0.8, respectively, and baseline depressive symptoms were low (median 18.0 IQR 4 out of a possible range of 17–49). Characteristics by category of fruit and vegetable intake are included in Table 3. A comparison of included versus excluded participants revealed that included participants were younger, healthier, more educated, and less likely to be female or living alone (Supplemental Table S4).
Associations between fruit and vegetable intake and depressive symptoms over time
Across all cohorts in the partially adjusted model, there were no differences in associations between a moderate, or high intake of fruit, and depressive symptoms, versus a low fruit intake (moderate: β = − 0.006 [95%CI − 0.016, 0.004], I2 = 77%; high: β = − 0.006 [95%CI − 0.013, 0.001], I2 = 42%]). In the fully adjusted model, there was no difference in associations between a moderate intake of fruit and depressive symptoms compared with a low intake (β = − 0.006 [95%CI − 0.015, 0.003], I2 = 69%), while there was a relationship between high intake of fruit and lower depressive symptoms when compared with low intake (β = − 0.007 [95%CI − 0.014, < − 0.001], I2 = 43%) (Table 4).
For vegetables, across all cohorts using the partially adjusted model, both moderate and high intakes were associated with lower depressive symptoms over time, compared with low vegetable intakes (moderate: β = − 0.005 [95%CI − 0.009, < − 0.001], I2 = 8%; high: β = − 0.007 [95%CI − 0.011, − 0.003], I2 = 0%). A similar result was found in the fully adjusted model (moderate: β = − 0.005 [95%CI − 0.009, − 0.001], I2 = 0%; high: β = − 0.006 [95%CI − 0.011, − 0.002], I2 = 0%) (Table 4).
Sensitivity analysis
In the first sensitivity analysis, when potatoes were included in vegetable intake, there was no longer a relationship for either moderate or high vegetable intakes, compared with low intakes (moderate: β = − 0.005 [95%CI − 0.012, 0.001], I2 = 55%; high: β = − 0.004 [95%CI − 0.008, 0.001], I2 = 10%) (Supplementary Table S5). In the second sensitivity analysis, when MADT was excluded from the fully adjusted analysis, results for fruit were in line with the main analysis, but results for vegetable were no longer significant (fruit—moderate: β = − 0.007 [95%CI − 0.020, 0.007]; high: β = − 0.010 [95%CI − 0.017, − 0.003]; vegetables—moderate: β = − 0.006 [95%CI − 0.013, 0.002]; high: β = − 0.006 [95%CI − 0.014, 0.001]).
Test for familial confounding
In ICE FALCON analysis using paired twin data, for fruit intake, no association was found in either Model 1 or Model 2 (no association between either a twin’s intake and their depressive symptoms or a twin’s intake and their co-twin’s depressive symptoms). For vegetable intake, an association was found in Model 1 and not Model 2 (an association found between a twin’s intake and their depressive symptoms but no association between a twin’s intake and their co-twin’s depressive symptoms) (Supplementary Table S6).
Discussion
In this study the associations between baseline fruit and vegetable intakes and depressive symptoms over a follow-up period of up to 11 years were examined in twins aged 45 + years. Both a high fruit and a high vegetable intake (mean serves/day 2.1 and 2.0 respectively) were associated with lower depressive symptoms over time versus low intakes (mean serves/day 0.3 and 0.5 respectively). A moderate vegetable, but not fruit, intake was also associated with lower depressive symptoms versus low intakes. Familial confounding was not found to be responsible for the association between vegetable intake and depressive symptoms, with the result for fruit intake being uncertain.
This study’s finding of a protective association between higher fruit and vegetable intake and depressive symptoms is consistent with most prior evidence. Our previously published meta-analyses of four population-based studies of adults aged 45 + years found higher fruit intakes were associated with a 15% reduced odds of incident depression, and higher vegetable intakes with 9% reduced odds6. Similarly, meta-analyses of cohort studies (fruit n = 6, vegetables n = 7) in the general adult population has reported a reduced risk of depression associated with higher fruit and vegetable intake (fruit 17% decreased risk, vegetables 14% decreased risk)38. Three of these studies overlap with our published meta-analyses. Conversely, Elstgeest et al. reported no association between fruit or vegetable intakes and depressive symptoms over a 3-year period in their analysis of 1058 Italian adults with a mean age of 66 years (fruit β − 0.08, [95%CI − 0.84, 0.68], vegetables β − 0.49 [95%CI − 1.28, 0.31])39. There are a number of possible reasons for these divergent results, Elstgeest et al. utilised depressive symptoms as a continuous measure (as in the current study) whereas the previously mentioned meta-analyses6 used depression as a categorical measure (incident depression). Additionally, their sample size was smaller (n = 1058) than in the current study (n = 3483).
The beneficial associations detected in our study are modest. Our results indicate a high versus low intake of fruit (average of 1.8 more serves/day) is associated with a reduction in depressive symptoms of 0.29. For vegetables a high versus low intake (average of 1.5 more serves/day) is associated with a reduction in depressive symptoms of 0.27. However, total fruit and vegetable consumption in the “high” category still falls notably below the dietary recommendations of most countries40. Intakes in the two large Scandinavian cohorts were particularly low, with the average for both cohorts being less than half the World Health Organization recommended intake of at least 5 serves per day41. It remains unclear what the reduction in depression scores would be if intakes were increased to recommended levels.
The result of our sensitivity analysis suggest higher potato intake does not have a beneficial association with depressive symptoms. We detected a beneficial relationship between vegetable intake (excluding potatoes) and depressive symptoms, but when potatoes were included in vegetable intake, the relationship was no longer detected. It is unclear what is driving this difference in relationship as details of cooking methods are not available. The consumption of deep-fried potatoes is a possible explanation as high intakes of Western diets have been associated with higher risk of depression in older adults6. Further research is required to understand the relationship between potato intake and depression.
The beneficial relationship between fruit and vegetable intake and depression appears most likely to be due to the high levels of dietary fibre, vitamins and micronutrients contained in fruit and vegetables42. The importance of the gut microbiome and its potential influence on depression via inflammation, both systemic and neuroinflammation, is becoming increasingly well understood43. Higher levels of pro-inflammatory cytokines, oxidative stress markers and diminished levels of antioxidant markers have all been observed in individuals with major depression compared to healthy controls44,45. Consumption of fruit and vegetables appears to beneficially impact the composition of the gut microbiome and protect against oxidative damage to the brain46,47. This is supported by prior research, which has demonstrated that higher intakes of dietary fibre48and flavonoids49 are associated with a reduced incidence of depression in older adults.
Our study has a number of strengths, in particular its twin design. A limitation of observational evidence is that relationships detected may be due to residual confounding. Residual confounding may be due to either unmeasured, inaccurately measured, or unknown confounders. By utilising twin data, we are able to reduce the influence of a range of potential confounders such as socio-economic status, physical activity and chronic health conditions as the variability of these confounders is lower in twins than in unrelated individuals50,51,52. Additionally, employing ICE FALCON methodology allowed us to test whether the associations detected were due to familial confounding. Childhood socio-economic status is an example of a possible familial confounder as it may influence fruit and vegetable intake and depression in adulthood. We found that no relationship existed between a twin’s vegetable intakes and depressive symptoms in their co-twin, this suggests our results were not due to familial confounding. Results for fruit intake were unclear. ICE FALCON analysis can only be performed using complete twin pairs, whereas the between-within twin analysis, which we used for our main analysis utilised complete and incomplete sets of twins. As a result, the sample size in the ICE FALCON analysis is smaller than in the main analysis (n = 3483 versus n = 2506) which may have impacted our ability to detect an association. Additionally, as ICE FALCON analysis involves the exposure variable being residualised for the covariates, we have used fruit and vegetable intakes as continuous rather than categorical variables. These differences, together with the result for fruit in our main analyses only being marginally significant, appear to have resulted in no observed relationships for fruit in the ICE FALCON analysis. We therefore cannot draw a conclusion as to whether the relationship detected between fruit intake and depressive symptoms in the main analysis is due to familial confounding. Other strengths of our study in addition to its twin design are the inclusion of participants from three continents, providing insight into the relationship between fruit and vegetable intake and depression in a range of high-income countries (HICs). Moreover, we have been able to harmonise categories of fruit and vegetable intake, depressive symptoms, and covariates, in addition to controlling for the same covariates across all cohorts, which is a limitation of previous meta-analyses.
The results of our study should be considered in light of the following limitations. We were unable to adjust for physical activity as it was not available across all studies at baseline. However, prior work has demonstrated physical activity to be moderate-to-highly heritable, (estimate 31% to 71%)50,53, which in part mitigates this limitation, as the variability of physical activity is reduced by using twin data. Another limitation of our study is the use of a variety of different self-report dietary questionnaires. In particular, two of these contained only one question for fruit intake, and two questions for vegetable intake. This has resulted in reduced variability in this data, with a large proportion of responses for fruit intake of one serve/day in these cohorts. Moreover, fruit and vegetable consumption were generally low, with mean daily servings of 1.0 ± 0.8 and 1.2 ± 0.8, respectively, compared to global recommendations for combined daily intake of least 5 serves40,41. Low overall intake and a lack of differentiation between intakes is likely to have reduced our ability to detect associations with depressive symptoms. Additionally, depressive symptoms were assessed based on self-report and participants in our study had low baseline depressive symptoms. Clinical diagnosis is the gold standard for depression assessment, and it is possible that future studies in clinically depressed participants may shed more light on the associations. Although we have examined cohorts from four different countries, all were HICs. Dietary intakes54and rates of depression55 differ between low- and middle-income countries (LMICs) and HICs, therefore our results may not be generalisable to LMICs. Additionally, the MADT cohort was much larger than the other cohorts and is potentially driving the associations observed for vegetables, although a similar trend was observed when MADT was excluded from the analysis. Moreover, it is possible that our results are biased by the exclusion of participants not providing data. Excluded participants were older, more likely to be female, less educated, and less healthy than included participants. However, we found no interaction between either age or sex and the relationship between intakes and depressive symptoms. Furthermore, the addition of BMI, physical health and cognitive ability to the model had minimal impact on results. This suggests the exclusion of participants is unlikely to have impacted the results. Lastly, despite utilising a twin model and adjusting for a range of potential confounders based on a review of the literature and use of a DAG, we cannot rule out the possibility that associations detected are due to residual confounding.
Conclusion
In our analysis of four international twin cohorts, modest beneficial associations were found between higher fruit and vegetable intakes and depressive symptoms over time, presenting another argument for increasing intakes. Consideration should be given to the monitoring of fruit and vegetable intakes and behavioural counselling in primary care settings. Future research should utilise comprehensive and standardised tools for fruit and vegetable data collection, and clinical assessment for depressive symptoms, while incorporating cohorts from LMICs.
Data availability
IGEMS data are not publicly available given the variety of data agreements and regulations governing the different studies and countries. However, many of the individual studies participating in IGEMS do have ways to access their data, some by direct request to the participating study, and many of the datasets may be accessed through National Archive of Computerized Data on Aging (NACDA). See https://doi.org/https://doi.org/10.3886/ICPSR03843.v2 (SATSA). For access to data from the Danish Twin Registry, see https://www.sdu.dk/en/om_sdu/institutter_centre/ist_sundhedstjenesteforsk/centre/dtr/researcher. To request OATS data please contact the CHeBA Research Bank via email on [email protected] for a current application form.
References
Institute for Health Metrics and Evaluation. Global Health Data Exchange, <https://www.healthdata.org/> (2019).
World Health Organisation. Depression and other common mental disorders: global health estimates. (WHO, 2017).
Judd, L. L. & Akiskal, H. S. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. Am J Geriatr Psychiatry 10, 233–238 (2002).
Lassale, C. et al. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 24, 965–986. https://doi.org/10.1038/s41380-018-0237-8 (2019).
Lee, M., Bradbury, J., Yoxall, J. & Sargeant, S. Is dietary quality associated with depression? An analysis of the Australian Longitudinal Study on Women’s Health data. Br J Nutr 129, 1380–1387. https://doi.org/10.1017/S0007114522002410 (2023).
Matison, A. P., Mather, K. A., Flood, V. M. & Reppermund, S. Associations between nutrition and the incidence of depression in middle-aged and older adults: a systematic review and meta-analysis of prospective observational population-based studies. Ageing Res Rev 70, 101403. https://doi.org/10.1016/j.arr.2021.101403 (2021).
Matison, A. P. et al. Genetic and environmental influences on fruit and vegetable consumption and depression in older adults. BMC Geriatr 23, 67. https://doi.org/10.1186/s12877-023-03745-0 (2023).
Li, S., Bui, M. & Hopper, J. L. Inference about causation from examination of familial confounding (ICE FALCON): a model for assessing causation analogous to Mendelian randomization. Int J Epidemiol 49, 1259–1269. https://doi.org/10.1093/ije/dyaa065 (2020).
Pedersen, N. L. et al. IGEMS: the consortium on interplay of genes and environment across multiple studies. Twin Res Hum Genet 16, 481–489. https://doi.org/10.1017/thg.2012.110 (2013).
Pedersen, N. L. et al. IGEMS: The consortium on interplay of genes and environment across multiple studies - An update. Twin Res Hum Genet 22, 809–816. https://doi.org/10.1017/thg.2019.76 (2019).
Finkel, D. & McGue, M. The origins of individual differences in memory among the elderly: a behavior genetic analysis. Psychol Aging 8, 527–537. https://doi.org/10.1037/0882-7974.8.4.527 (1993).
Gaist, D. et al. Strength and anthropometric measures in identical and fraternal twins: no evidence of masculinization of females with male co-twins. Epidemiology 11, 340–343. https://doi.org/10.1097/00001648-200005000-00020 (2000).
Pedersen, D. A. et al. The Danish twin registry: an updated overview. Twin Res Hum Genet 22, 499–507. https://doi.org/10.1017/thg.2019.72 (2019).
Christiansen, L. et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res 6, 275–278. https://doi.org/10.1375/136905203322296610 (2003).
Sachdev, P. S. et al. A comprehensive neuropsychiatric study of elderly twins: the Older Australian Twins Study. Twin Res Hum Genet 12, 573–582. https://doi.org/10.1375/twin.12.6.573 (2009).
Batouli, S. A. et al. The heritability of brain metabolites on proton magnetic resonance spectroscopy in older individuals. Neuroimage 62, 281–289. https://doi.org/10.1016/j.neuroimage.2012.04.043 (2012).
Pedersen, N. L. et al. The Swedish adoption twin study of aging: an update. Acta Genet Med Gemellol (Roma) 40, 7–20. https://doi.org/10.1017/s0001566000006681 (1991).
Perez Rodrigo, C., Moran Fagundez, L. J., Riobo Servan, P. & Aranceta Bartrina, J. Screeners and brief assessment methods. Nutr Hosp 31(3), 91–98. https://doi.org/10.3305/nh.2015.31.sup3.8757 (2015).
Radloff, L. S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1, 385–401. https://doi.org/10.1177/014662167700100306 (1977).
Beekman, A. T. et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med 27, 231–235. https://doi.org/10.1017/s0033291796003510 (1997).
Yesavage, J. A. et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17, 37–49. https://doi.org/10.1016/0022-3956(82)90033-4 (1982).
Brink, T. L. Geriatric depression and hypochondriasis: Incidence, interaction, assessment and treatment. Psychother: Theory Res. Pract. 19, 506–511. https://doi.org/10.1037/h0088464 (1982).
Krishnamoorthy, Y., Rajaa, S. & Rehman, T. Diagnostic accuracy of various forms of geriatric depression scale for screening of depression among older adults: Systematic review and meta-analysis. Arch Gerontol Geriatr 87, 104002. https://doi.org/10.1016/j.archger.2019.104002 (2020).
Estabrook, R., Sadler, M. E. & McGue, M. Differential item functioning in the Cambridge Mental Disorders in the Elderly (CAMDEX) Depression Scale across middle age and late life. Psychol Assess 27, 1219–1233. https://doi.org/10.1037/pas0000114 (2015).
Roth, M. et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 149, 698–709. https://doi.org/10.1192/bjp.149.6.698 (1986).
Gatz, M. et al. Data harmonization in aging research: not so fast. Exp Aging Res 41, 475–495. https://doi.org/10.1080/0361073X.2015.1085748 (2015).
United Nations Educational Scientific and Cultural Organization (UNESCO). International Standard Classification of Education. (UNESCO Institute for Statistics Canada, 2011).
Linn, B. S., Linn, M. W. & Gurel, L. Cumulative illness rating scale. J Am Geriatr Soc 16, 622–626. https://doi.org/10.1111/j.1532-5415.1968.tb02103.x (1968).
Gatz, M. et al. Age moderation of individual differences in chronic medical illness burden. J Behavior Genetics 45, 657 (2015).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. https://doi.org/10.1016/0022-3956(75)90026-6 (1975).
McGue, M. & Christensen, K. The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 years and older. Psychol Aging 16, 272–280. https://doi.org/10.1037/0882-7974.16.2.272 (2001).
van Buuren, S. & Groothuis-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw 45, 1–67. https://doi.org/10.18637/jss.v045.i03 (2011).
Begg, M. D. & Parides, M. K. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Statistics in medicine 22, 2591–2602. https://doi.org/10.1002/sim.1524 (2003).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
Rubin, D. B. Multiple imputation for nonresponse in surveys 1st edn. (John Wiley, Hoboken, 1987).
Viechtbauer, W. Conducting meta-analyses in R with the metafor Package. J. Stat. Softw 36, 1–48. https://doi.org/10.18637/jss.v036.i03 (2010).
Textor, J., van der Zander, B., Gilthorpe, M. S., Liskiewicz, M. & Ellison, G. T. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol 45, 1887–1894. https://doi.org/10.1093/ije/dyw341 (2016).
Saghafian, F. et al. Fruit and vegetable consumption and risk of depression: accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br J Nutr 119, 1087–1101. https://doi.org/10.1017/S0007114518000697 (2018).
Elstgeest, L. E. M. et al. Bidirectional associations between food groups and depressive symptoms: longitudinal findings from the Invecchiare in Chianti (InCHIANTI) study. Br. J. Nutr 121(4), 439–450. https://doi.org/10.1017/S0007114518003203 (2019).
Herforth, A. et al. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 10, 590–605. https://doi.org/10.1093/advances/nmy130 (2019).
World Health Organization. Healthy Diet, <https://www.who.int/news-room/fact-sheets/detail/healthy-diet#:~:text=A%20healthy%20diet%20includes%20the%20following%3A%201%20Fruit%2C,potatoes%2C%20cassava%20and%20other%20starchy%20roots.%20More%20items> (2020).
Marx, W. et al. Diet and depression: exploring the biological mechanisms of action. Mol. Psychiatry 26, 134–150. https://doi.org/10.1038/s41380-020-00925-x (2021).
Medina-Rodriguez, E. M., Cruz, A. A., De Abreu, J. C. & Beurel, E. Stress, inflammation, microbiome and depression. Pharmacol https://doi.org/10.1016/j.pbb.2023.173561 (2023).
Wang, A. K. & Miller, B. J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44, 75–83 (2017).
Liu, T. et al. A meta-analysis of oxidative stress markers in depression. PLoS One 10, e0138904. https://doi.org/10.1371/journal.pone.0138904 (2015).
Smaga, I. et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 67, 569–580. https://doi.org/10.1016/j.pharep.2014.12.015 (2015).
Xiong, R. G. et al. The role of gut microbiota in anxiety, depression, and other mental disorders as well as the protective effects of dietary components. Nutrients https://doi.org/10.3390/nu15143258 (2023).
Gangwisch, J. E. et al. High glycemic index diet as a risk factor for depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 102, 454–463. https://doi.org/10.3945/ajcn.114.103846 (2015).
Chang, S. C. et al. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 104, 704–714. https://doi.org/10.3945/ajcn.115.124545 (2016).
Hoed, Md. et al. Heritability of objectively assessed daily physical activity and sedentary behavior1234. Am. J. Clin. Nutr 98, 1317–1325. https://doi.org/10.3945/ajcn.113.069849 (2013).
Zdravkovic, S. et al. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J. Intern. Med. 252, 247–254. https://doi.org/10.1046/j.1365-2796.2002.01029.x (2002).
Poulsen, P., Ohm Kyvik, K., Vaag, A. & Beck-Nielsen, H. Heritability of Type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance – a population-based twin study. Diabetologia 42, 139–145. https://doi.org/10.1007/s001250051131 (1999).
Stubbe, J. H. et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS One 1, e22. https://doi.org/10.1371/journal.pone.0000022 (2006).
Yusuf, S. et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395, 795–808. https://doi.org/10.1016/S0140-6736(19)32008-2 (2020).
Zenebe, Y., Akele, B., W/Selassie, M. & Necho, M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry 20, 55. https://doi.org/10.1186/s12991-021-00375- (2021).
Acknowledgements
IGEMS is supported by the National Institutes of Health Grants No. R01 AG059329, R01 AG060470, RF1 AG058068, and previously by R01 AG037985 and R56 AG037985. SATSA was supported by grants R01 AG04563, R01 AG10175, the John D. and Catherine T. MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141). The Danish Twin Registry has been supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the US National Institute of Health (P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by NIA grant R01 AG06886. We acknowledge the contribution of the OATS research team (https://cheba.unsw.edu.au/project/older-australian-twins-study) to this study. The OATS study has been funded by a National Health & Medical Research Council (NHMRC) and Australian Research Council (ARC) Strategic Award Grant of the Ageing Well, Ageing Productively Program (ID No. 401162) and NHMRC Project Grants (ID 1045325 and 1085606). OATS participant recruitment was facilitated through Twins Research Australia, a national resource in part supported by a Centre for Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council. APM is supported through an Australian Government Research Training Program Scholarship. We thank the participants for their time and generosity in contributing to this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA. The authors thank Professor Roger Milne of the Cancer Epidemiology Division, Cancer Council Victoria, for permission to use the Dietary Questionnaire for Epidemiological Studies, Melbourne: Cancer Council Victoria, 1996.
Author information
Authors and Affiliations
Consortia
Contributions
Study conception and methodology—APM, AT, VMF, SR and KAM; formal analysis—APM and AT; data curation—VSC, KC, MN, NLP and PSS; writing—original draft preparation—APM; writing—reviewing and editing APM, AT, VMF, SR and KAM; supervision—KAM, SR, VMF. All authors read, provided feedback, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
PSS was on the Advisory Committees for Biogen Australia and Roche Australia in 2020 and 2021. The authors declare no other competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matison, A.P., Thalamuthu, A., Flood, V.M. et al. Longitudinal associations between fruit and vegetable intakes and depressive symptoms in middle-aged and older adults from four international twin cohorts. Sci Rep 14, 29711 (2024). https://doi.org/10.1038/s41598-024-79963-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79963-2