Abstract
Objective: To describe the implementation of a multi-step antimicrobial stewardship program in a haemato-oncology and stem cell transplantation program unit. Methods: Pre-post quasi-experimental study with two interrupted time-series analyses, conducted between 01/01/2019 and 31/12/2022 in the Paediatric Haemato-Oncology Unit of the Padua Paediatric Hospital. The interventions were: (1) 02/2020: dissemination of febrile neutropenia clinical pathways, (2) April 2021: provision of the clinical pathways via a customized App (Firstline.org) and implementation of a twice-a-week prospective audit and feedback. The main outcome was antibiotic consumption measured by days of administered therapy (DOTs)/1000 patients’ days for all antibiotics and most used molecules. Results: The first intervention (clinical pathways) resulted in a decrease in the overall antibiotic use by the haemato-oncology unit, with an abrupt reduction of 3-gen cephalosporins in favor of piperacillin-tazobactam, as indicated by the clinical pathways. Meropenem and glycopeptide use did not vary. The second intervention (antimicrobial stewardship) further decreased total antibiotic consumption, and a significant decline in meropenem, amikacin, and glycopeptides was achieved. Conclusions: Multi-step stewardship based on guidelines dissemination, multidisciplinary team intervention and collaboration (“handshake” stewardship) was highly effective in optimizing guidelines adherence and reducing overprescriptions in a fragile patient cohort.
Similar content being viewed by others
Background
Severe bacterial infections are among the most critical complications in paediatric cancer patients, and prompt broad-spectrum antibiotic treatment is required in patients with fever and neutropenia (FN)1. The overuse of antimicrobial agents leads to a higher risk of antimicrobial resistance, adverse events, Clostridium difficile infections1,2. Those risks are higher in particular with broad-spectrum antibiotics such as meropenem and glycopeptides, which utilization is often inappropriate2. In this view, antimicrobial stewardship programs (ASP) could reduce unnecessary treatments by optimizing durations and promoting de-escalation strategies. In general paediatric settings ASPs have already been demonstrated to improve patient outcomes while reducing antibiotic use, inappropriate prescriptions, and antimicrobial resistance (AMR)3,4. Antibiotic stewardship programs have also been implemented in high-risk settings, such as neonatal and pediatric intensive care units and haemato/oncology units5. Most studies focused on the implementation of protocols for the management of FN, with only a few describing effective antimicrobial stewardship interventions5,7,8. This results in the lack of guidelines and recommendations for ASP in paediatric haemato/oncology, right where the infectious risk is greater and antibiotic prescribing is more controversial.
Guidelines for the management of FN provide general information that needs to be adapted to the local practice9,10. Studies addressing this gap are thus greatly needed. In line with this need, our study that was conducted within the ENSURE Project (Enforcing Surveillance of Antimicrobial Resistance and antibiotic usE to drive Stewardship), aimed at assessing the impact of the implementation of an ASP program in the paediatric haemato/oncology (HO) unit and the associated haematopoietic stem cell transplantation (HSCT) program in a tertiary pediatric hub for haematological/oncological patients within the Padova Hospital. The ASP was implemented in different phases. First, we revised the internal clinical pathways for febrile neutropenia based on local susceptibility data and evaluated antimicrobial consumption, identifying the most commonly used agents in the wards. Second, we disseminated the internal guidelines (clinical pathways - CP) through a mobile app. Finally, the third phase involved formal stewardship with an active intervention, using a handshake approach that included a twice-weekly prospective audit and feedback.
Results
Results on antibiotic consumption are reported in Table 1 for the entire Unit, in Table 2; Fig. 1 for the HO ward, and in Table 3; Fig. 2 for the HSCT Unit.
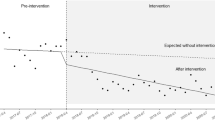
Interrupted time series of monthly antimicrobial consumption for the Haemato-Oncology ward (blue line) with reference monthly value and 95%CI (grey lines) for meropenem (A), piperacillin/tazobactam (B), third-generation cephalosporins (C), amikacin (D), glycopeptides (E) and quinolones (F). Orange box refers to the period from the first intervention (febrile neutropenia protocol dissemination) and the second intervention (Antimicrobial Stewardship program, ASP). The green box refers to the post-ASP intervention.
Interrupted time series of monthly antimicrobial consumption for the Haematopoietic Stem Cell Transplantation (HSCT) Unit (blue line) with reference monthly value and 95%CI (grey lines) for meropenem (A), piperacillin/tazobactam (B), third-generation cephalosporins (C), amikacin (D), glycopeptid€(E) and quinolones (F). Orange box refers to the period from the first intervention (febrile neutropenia protocol dissemination) and the second intervention (Antimicrobial Stewardship program, ASP). The green box refers to the post-ASP intervention.
The total antimicrobial consumption decreased after the first (CP dissemination) and second intervention (ASP) in the HO ward, while decreased only after the ASP intervention in the HSCT unit.
Meropenem consumption decreased in the entire unit after the first intervention and further decreased after the ASP. Glycopeptides consumption improved significantly in the HO ward, with a significant level change after CP and a decreasing trend after ASP. After both interventions the consumption of amikacin immediately increased, followed by a reduction trend in the HO ward but not in the HSCT Unit.
During the summer, we noted a rise in the overall consumption of antibiotics. The measures implemented since March 2020 to reduce the spread of SARS-CoV-2 did not influence the total antibiotic consumption (S1, S2).
The surveillance of antimicrobial resistance (AMR) on blood cultures did not show a significant change in susceptibility patterns during the study period (S3).
Mortality within wards did not change in the study period, with 4 deaths in the pre-intervention period, 2 deaths in the intervention 1 period and 3 deaths in the intervention 2 period (p = 0.415).
Admission rates in paediatric intensive care units were: 2,5/1000 patient days in the pre-intervention period, 5,1/1000 patient days in the intervention 1 period and 3,5/1000 patient days in the intervention 2 period (p = 0.129).
Discussion
Recommendations for the empiric treatment of FN in pediatrics and subsequent de-escalation or escalation strategies mainly come from low-moderate quality evidence and leave a wide space of choice to prescribers10.
This has led in the last years to the establishment of local practice habits, that are deep-settled in the Haemato-Oncology wards and, thus, difficult to change.
In our study, the focuses of CP were the carbapenem-sparing policy and the early withdrawal of amikacin. In fact, the empiric therapy in our setting, due to high incidence of MDR bacteria in our hospital, includes double coverage for gram-negative bacteria with piperacillin-tazobactam plus amikacin. This issue was addressed in a previous study conducted in our center which demonstrated that the empiric combination of piperacillin-tazobactam, amikacin and glycopeptides covered 97% of bacteria causing bloodstream infections in haemato-oncological paediatric patients with bloodstream infections in our setting11. Goals of carbapenem reduction and amikacin early withdrawal were partially reached in the HO ward with a monthly trend reduction for both meropenem and amikacin. In the HSCT section meropenem use abruptly declined after the CP. On the other hand, amikacin increased immediately and continued to increase after that. This is probably due to fewer escalations to meropenem, resulting in the prolonged use of amikacin in association with piperacillin-tazobactam. Our study also highlighted an increased use of quinolones after the pathway dissemination, followed by a decrease after the second ASP intervention. This could be explained by the initial tendency to continue a double gram-negative coverage in front of persisting fever. The increase in other non-target molecules could be expected in ASP programs, and need to be monitored as well12,13,14.
While ASP interventions in general pediatric settings typically yield a substantial reduction in inappropriate prescriptions3, our intervention did not result in a sudden decrease in antibiotic consumption. This may be due to the strategy we adopted, not based on a restrictive pre-authorization approach, but on audits and feedback to enhance the collaboration between the ASP team and the prescribers. The heightened concern for the vulnerability of this population poses challenges in strictly adhering to decalage or suspension recommendations. In this view, the ASP team worked to broaden the clinical knowledge of every patient, not only for infectious occurrences, in order to propose a plausible and acceptable de-escalation strategy rather than providing punctual indications. This “handshake” approach enhanced bidirectional trust and peer education and has been shown also by others to give positive results15.
Other studies report experience of ASP focusing the efforts on gram-negative directed agents7,16. In the study by Henning, the FN protocol resulted in a decrease in the use of gentamycin both in empirical and targeted therapy16. Wattier et al. showed that the FN guidelines alone did not improve either the level or the trend of gentamycin, while ciprofloxacin use increased; ASP intervention resulted in significant gentamycin reduction, while ciprofloxacin decreased in trend but not in level7. These results are similar to ours, demonstrating that FN alone without an active ASP intervention may not be sufficient to reduce antibiotic consumption. Compared to the protocol dissemination alone, the ASP intervention in our study was successful in reducing the utilization of amikacin, and further reducing the meropenem use in the HO ward.
In our center, during the pre-implementation phase, glycopeptides emerged as the most frequently prescribed antimicrobials among anti-gram-positive molecules. The reduction of glycopeptides use was another important target of the stewardship intervention. Given the infrequent isolation of methicillin-resistant Staphylococcus aureus and the low morbidity associated with Coagulase-negative staphylococci bacteraemia, efforts were made to limit the ongoing use of glycopeptides only in cases where a gram-positive alert was detected in blood cultures. Following the second ASP intervention, we observed a modest yet statistically significant reduction trend across the entire unit.
Karandikar et al. showed that an ASP intervention, with analogue glycopeptide-criteria for FN, was successful in reducing the vancomycin DOT/1000 ppd and reducing the vancomycin-resistant Enterococcus incidence (any isolation)17. In our study, microbiological data were exclusively collected for invasive infections, a factor that could limit the observation of a substantial reduction in resistant microorganisms, due to their low occurrence.
In our study, we observed after both interventions an immediate decrease with a significant level change achieved in some cases, but the following months did not follow the same decreasing trend or even increased, as in other studies in the same setting8. This probably occurs because the highest impact of a novelty is reached at the beginning and, possibly, because of milder pressure from the ASP team as months passed from the start of the intervention.
Notably, we observed higher prescription rates during the summer months in both the HO ward and the HSCT unit. We postulate that the climatic changes taking place in summer might contribute to an elevated airborne spore count, resulting in an increased incidence of fungal infections18. These infections typically necessitate prolonged antimicrobial therapies before diagnosis, as previously outlined.
The SARS-CoV-2 pandemic did not impact on the results. The viral pandemic may have increased the FN episodes, which were treated with empirical antibiotics until defervescence and 72-hour negativity of blood cultures. However, independently from the pandemic, immunocompromised patients and their caregivers already adopted restrictive measures; this could have contributed to the low impact of the SARS-CoV-2 spread in our analysis.
This study provides insights into the challenging realm of antimicrobial stewardship in high-risk immunocompromised children. The favorable outcomes emerged through a systematic process rather than a standalone intervention. Following the adaptation of local guidelines to the ward’s epidemiology, we systematically disseminated the pathway, concurrently fostering a robust collaboration with the HO medical team through frequent, rigorous audits (conducted up to three times a week). This approach benefited from a comprehensive understanding of clinical cases, extending beyond infectious aspects, and providing more informed guidance on choices and strategies.
The second structured ASP intervention further enhanced prescriptions through increased cooperation and trust, alongside collaborative efforts with the Microbiology unit for early diagnosis and the Hospital Pharmacy for efficient drug dispensation.
This study presents some limitations. First, we could not measure the impact of the ASP on colonization by MDR organisms and C. difficileinfections, because of changing policies and modalities of screening during the study period. Many pediatric studies demonstrated a decrease in extended-spectrum beta-lactamase (ESBL) producers E. coli and K. pneumoniae192021, and reduction in P. aeruginosa carbapenem resistance2122 following ASP.
Second, we did not systematically collect data about empiric and targeted therapy appropriateness.
Third, we did not dispose of molecular diagnostic tools for blood cultures, that would accelerate the removal of glycopeptides or amikacin in the case of gram-negative or positive bacterial identification and would increase the sensibility in the case of low-charge bacteremia.
Materials and methods
Study design
This is a pre-post quasi-experimental study conducted within the ENSURE project.
The study was granted approval by the local ethic committee on April 7th, 2022 (N. AOP2612).
Setting
The ENSURE project was started in early 2021 and was developed as a joint initiative between the Infectious Diseases teams of University of Verona and University of Padua. In this work we present data from Padova Center.
The AS intervention was conducted in the Haemato-Oncology Unit of the Paediatric Department of the Padua University Hospital in the North-Est of Italy, which is composed by a general Haemato-Oncology ward (21-bed structure) and the HSCT (6-bed structure).
Antimicrobial stewardship intervention
Before 2019, infectious diseases (ID) consultations were already available upon request from the HO physicians.
In January 2020, using the microbiological data on bacterial resistance, an internal clinical pathway for managing febrile neutropenia (FN) was introduced and disseminated including all the recommendations from the 8th European Conference on Infections in Leukaemia (ECIL-8) guidelines9. This clinical pathway (CP) mainly focused on replacing ceftazidime with piperacillin-tazobactam and introducing the carbapenem-sparing strategy, strengthening the recommendation to not escalate empiric therapy in the presence of persisting fever without clinic derangement.
Between November 2020 and March 2021, an initial surveillance report was elaborated, entailing the retrospective gathering of antimicrobial consumption data within the ward and updated information on invasive isolates along with their corresponding susceptibility patterns. Second, a focused meeting was conducted within the AS team, comprising ID clinicians, the HO team dedicated to infection management, a pharmacist, and a microbiologist, to identify gaps and critical issues related to antimicrobial prescribing. Antimicrobials characterized by a higher likelihood of prescribing inadequacies were defined as “high-risk”, and were pinpointed for special monitoring. Third, internal guidelines in the HO ward were updated based on current literature and local epidemiology (FN, sepsis, catheter-related infections, pneumonia, skin and soft tissue infections, and C.difficile infections).
Starting from April 2021, the updated guidelines were disseminated and made available through the hospital intranet. Subsequently, they were made available on a mobile app (Firstline.org ®), ensuring easy accessibility for all clinicians in the wards. The app also featured updated information on antimicrobial dosages, side effects, drug monitoring, and dose adjustments based on renal excretion. The handshake ASP intervention was then initiated. This approach comprised conducting three weekly audits by ID team with the collaboration of a hospital pharmacist, on every antimicrobial prescription within the HO unit. These audits entailed a thorough examination of the prescribed antimicrobials for both empiric and targeted treatments. Indeed, this “handshake” approach, involves no constraints or preauthorization requirements for antimicrobial prescribing, instead emphasizing surveillance and discussions on antimicrobial therapies.
Main goals of the recommendations were: avoiding antimicrobial escalation in the presence of persistent fever without clinical deterioration; discontinuing broad-spectrum antimicrobials when not warranted (e.g., meropenem, glycopeptides); stopping double coverage for gram-negative bacteria in cases where blood cultures are non-informative; and tailoring therapies when bacteria are isolated from blood cultures.
All recommendations were delivered orally during ward rounds, with some provided as written consultations in the hospital’s electronic health system.
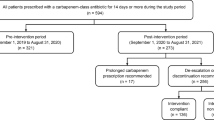
In delineating distinct temporal phases, the FN protocol dissemination was considered as a first intervention, as it already included some stewardship targets and might be considered a preliminary step. The study, thus, was carried out in: (i) period 1 from January 2019 to January 2020 (pre-intervention), (ii) period 2 from February 2020 to March 2021, when the FN protocol was implemented and disseminated (intervention 1) and (iii) period 3 from April 2021 to December 2022 when the structured ASP was implemented (intervention 2).
Study endpoints
The primary endpoint was antibiotic consumption, described as days of therapy (DOTs) per 1000 patient days (PPD) for the entire HO, including both the general ward and the HSCT section, and then for single units, encompassing overall antibiotics usage and further divided by classes.
The secondary endpoint were mortality (number of deaths), number of admission to PICUs (admissions/1000 patient days), the surveillance of antimicrobial resistance of invasive infections, specifically blood cultures.
In the original protocol of the ENSURE study, secondary outcomes included trend of antimicrobial prescription appropriateness (for empiric and targeted therapies). However, due to the lack of data in the two-year pre-intervention phase and the complexity of systematic collection of those data in the intervention phase, this outcome was excluded from the current study.
Data collection
DOTs were computed from doses of administered antibiotics by specific antibiotic classes retrieved from the pharmacy’s electronic prescriptions software database.
All blood cultures of patients admitted to the unit during the study period were retrieved from the database of the microbiology laboratory. Positive samples were de-duplicated keeping only the first positive sample per pathogen per patient in a timeframe of 20 days.
Statistical analysis
Results were summarized as numbers and percentages (categorical variables). Categorical variables were compared with χ2 or Fisher’s 2-tailed exact test in a contingency table r x c; a Fisher test was used when the value in any of the cells of the contingency table was below five.
Two interrupted time series analyses supposing an abrupt step change and a trend change in monthly antibiotic consumption expressed as DOT/1000 patients’ days using a Poisson regression models were used to determine the effect of the two interventions. A variable representing the summer months (from May to September when usually ID services are disrupted more often because of the annual vacation season) and a variable indicating the period before and after the SARS-CoV-2 pandemic were considered. Autocorrelation was assessed, examining the plot for residuals and the partial autocorrelation function. The corresponding relative risk and 95% confidence interval (95% CI) according to normal approximation were calculated.
Data were analyzed using R statistical software (version 4.2.3., Vienna, Austria)23. Statistical significance was set at the 0.05 level and p values were two-sided.
Conclusions
Compared to protocols’ dissemination alone, the ASP intervention in the HO and the HSCT section of the HO unit was useful in reducing and optimizing antimicrobial use.
However, it is important to emphasize how the production and dissemination of clinical pathways have provided an excellent background for undertaking a subsequent conscious intervention of handshake ASP and collaboration. Therefore, these two approaches must be viewed as interdependent, each fundamental to the other, to ensure an effective and enduring improvement in antibiotic prescriptions for these patients.
Greater efforts and strategy optimizations are needed to strengthen the intervention toward further decrease and responsibilities of antimicrobial usage. The implementation of regular electronic updates on antimicrobial guidelines, including the introduction of new antimicrobials or updated recommendations, targeted at ward prescribers, could help standardize education and increase adherence to prescribing practices, thereby sustaining the impact of the stewardship intervention.
Data availability
The data used in this study cannot be made publicly available due to Italian data protection laws. The datasets generated during the current study can be provided on request, from the corresponding author.
Abbreviations
- AMR:
-
Antimicrobial Resistance
- ASP:
-
Antimicrobial Stewardship Programs
- CP:
-
Clinical Pathway
- DOTs :
-
Days Of Therapy
- ENSURE:
-
Enforcing Surveillance of Antimicrobial Resistance and antibiotic usE to drive Stewardship
- FN:
-
Febrile Neutropenia
- HO:
-
Haemato/Oncology
- HSCT:
-
Haematopoietic Stem Cell Transplantation
- ID:
-
Infectious Diseases
- PPD:
-
Patient days
References
Lehrnbecher, T. et al. 8th European conference on infections in leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2045 (20), 1–11. https://doi.org/10.1016/s1470-2045(20)30725-7 (2021).
Papan, C. et al. Antimicrobial use in pediatric oncology and hematology in Germany and Austria, 2020/2021: a cross-sectional, multi-center point-prevalence study with a multi-step qualitative adjudication process. Lancet Reg. Heal - Eur. 28, 1–12. https://doi.org/10.1016/j.lanepe.2023.100599 (2023).
Donà, D. et al. Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. https://doi.org/10.1186/s13756-019-0659-3
Smith, M. J., Gerber, J. S. & Hersh, A. L. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J. Pediatr. Infect. Dis. Soc. 4 (4), e127–e135. https://doi.org/10.1093/jpids/piu141 (2015).
Muratore, E. et al. Antimicrobial stewardship interventions in Pediatric Oncology: a systematic review. J. Clin. Med. 11 (15). https://doi.org/10.3390/jcm11154545 (2022).
JL, G. & JG, N. Clinical impact of an antimicrobial stewardship program on high-risk pediatric patients. Infect. Control Hosp. Epidemiol. 40 (9), 968–973. https://doi.org/10.1017/ICE.2019.198 (2019).
Wattier, R. L., Levy, E. R., Sabnis, A. J., Dvorak, C. C. & Auerbach, A. D. Reducing second gram-negative antibiotic therapy on Pediatric Oncology and hematopoietic stem cell transplantation services. Infect. Control Hosp. Epidemiol. 38 (9), 1039–1047. https://doi.org/10.1017/ICE.2017.118 (2017).
Horikoshi, Y. et al. The north wind and the sun: Pediatric antimicrobial stewardship program combining restrictive and persuasive approaches in hematology-oncology ward and hematopoietic stem cell transplant unit. Pediatr. Infect. Dis. J. 37 (2), 164–168. https://doi.org/10.1097/INF.0000000000001746 (2018).
Andreas, H. et al. Adilia Warris TL. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. Published online 2021. https://doi.org/10.1016/S1470-2045(14)70017-8
Lehrnbecher, T. et al. Guideline for the management of Fever and Neutropenia in Pediatric patients with Cancer and hematopoietic cell transplantation recipients: 2023 update. J. Clin. Oncol. 41 (9), 1774–1785. https://doi.org/10.1200/jco.22.02224 (2023).
Liberati, C. et al. Application of the weighted-incidence Syndromic Combination Antibiogram (WISCA) to guide the empiric antibiotic treatment of febrile neutropenia in oncological paediatric patients: experience from two paediatric hospitals in Northern Italy. Ann. Clin. Microbiol. Antimicrob. 23 (1), 1–9. https://doi.org/10.1186/s12941-024-00673-8 (2024).
Leardini, D. et al. Effectiveness of Quinolone Prophylaxis in Pediatric Acute Leukemia and hematopoietic stem cell transplantation: a systematic review and Meta-analysis. Published Online. https://doi.org/10.1093/ofid/ofac594 (2022).
Use of fluoroquinolones for empirical management of febrile… The Pediatric Infectious Disease Journal. Accessed August 29. (2023). https://journals.lww.com/pidj/fulltext/1997/01000/use_of_fluoroquinolones_for_empirical_management.39.aspx
Olson, J. et al. Journal of the Pediatric Infectious diseases Society oral step-down Therapy with Levofloxacin • jpids 2021:10 (January) • 27. J. Pediatr. Infect. Dis. Soc. 10 (1), 27–33. https://doi.org/10.1093/jpids/piaa015 (2021).
Hurst, A. L. et al. Handshake stewardship: a highly effective rounding-based antimicrobial optimization service. Pediatr. Infect. Dis. J. 35 (10), 1104–1110. https://doi.org/10.1097/INF.0000000000001245 (2016).
Hennig, S. et al. Pediatric Blood & Cancer Antimicrobial stewardship in paediatric oncology: impact on optimising gentamicin use in febrile neutropenia. https://doi.org/10.1002/pbc.26810
Karandikar, M. et al. Limiting Vancomycin exposure in Pediatric Oncology patients with Febrile Neutropenia May be Associated with decreased vancomycin-resistant Enterococcus incidence. J. Pediatr. Infect. Dis. Soc. 9 (4), 428–464. https://doi.org/10.1093/jpids/piz064 (2020).
Panackal, A. A. et al. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 50 (12), 1588–1597. https://doi.org/10.1086/652761 (2010).
Murki, S., Jonnala, S., Mohammed, F. & Reddy, A. Restriction of cephalosporins and control of extended spectrum β-lactamase producing gram negative bacteria in a neonatal intensive care unit. Indian Pediatr. 47 (9), 785–788. https://doi.org/10.1007/S13312-010-0118-Y (2010).
Lee, K. R., Bagga, B. & Arnold, S. R. Reduction of broad-spectrum antimicrobial use in a tertiary children s hospital post antimicrobial stewardship program guideline implementation. Pediatr. Crit. Care Med. 17 (3), 187–193. https://doi.org/10.1097/PCC.0000000000000615 (2016).
Ding, H. et al. Influencing the use of antibiotics in a Chinese pediatric intensive care unit. Pharm. World Sci. 30 (6), 787–793. https://doi.org/10.1007/S11096-008-9220-9/TABLES/3 (2008).
Horikoshi, Y. et al. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int. J. Infect. Dis. 64, 69–73. https://doi.org/10.1016/J.IJID.2017.09.012 (2017).
R: The R Project for Statistical Computing. Accessed May 22, (2023). https://www.r-project.org/
Acknowledgements
Elisa Barbieri position is currently supported by the European Union - Next Generation EU under the National Recovery and Resilience Plan (NRRP) (project INF-ACT - One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases).
Funding
The ENSURE study was funded by the COMBACTE-MAGNET EPI-Net project. EPI-Net receives financial support from Innovative Medicines Initiative Joint Undertaking under grant agreement No. 115737, resources of which are composed of financial contributions from the European Union Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution.
Author information
Authors and Affiliations
Contributions
Contribution: Conceptualization D.D., A.B., E.C., E.T., C.G.Data curation C.L., F.C., MG.P, G.R., M.DP., M.P., A.M., M.G., G.B. S.T., D.M., F.V., E.DC., C.DVMethodology D.D M.D.P., D.M., E.B. Formal analysis E.B.: Funding acquisition C.G. E.T. Original article draft C.L., E.B., F.C.Revision of the article E.C., E.T., F.O., E.R., A.M., M.G.Final approval of the version to be submitted D.D., A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
This study was conducted in accordance with the tenets of the Declaration of Helsinki. The study was granted approval by the ethical committee for clinical trials of the province of Padova on April 7th, 2022 (N. AOP2612).
Informed consent
Patient informed consent was waived due to the retrospective nature of the study by the ethical committee for clinical trials of the province of Padova, and the use of wards aggregated data provided by thehospital pharmacy, in accordance with the ethical committee for clinical trials of the province of Padova
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liberati, C., Barbieri, E., Cavagnero, F. et al. Impact of a two step antimicrobial stewardship program in a paediatric haematology and oncology unit. Sci Rep 14, 29296 (2024). https://doi.org/10.1038/s41598-024-80163-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80163-1