Abstract
India’s high tuberculosis (TB) burden is exacerbated by concurrent silicosis, which increases TB susceptibility and worsens treatment outcomes. Limited studies on TB patients with silicosis highlight the need to address this vulnerable group’s specific challenges, particularly to improve diagnosis and management. This retrospective cohort study analyzed survival data from 137 silico-tuberculosis and 2,605 TB-only patients in Khambhat, India, using Kaplan-Meier curves, log-rank tests, and comparisons between Cox proportional hazards and accelerated failure time (AFT) models. The lognormal AFT model, selected for its lowest Akaike Information Criterion (AIC), estimated survival times based on age, gender, HIV status, and prior TB treatment. Among the 2,742 patients, 309 (11%) died within 27 months. Median time from diagnosis to outcome was shorter for deceased patients (1.7 months) than for censored patients (5.6 months, p < 0.001, median test). Kaplan-Meier analysis showed a significantly steeper survival decline for silico-tuberculosis patients (p < 0.001, log-rank test). Silico-tuberculosis was associated with a two-fold increased mortality risk (HR = 2.0, 95% CI: 1.4-3.0, p < 0.001, Cox-proportional hazards regression). The lognormal AFT model indicated silico-tuberculosis patients had 36% of the median survival time compared to TB-only patients (16 vs. 44 months). These findings highlight significantly earlier mortality in silico-tuberculosis patients, underscoring the need for targeted, employer-led care models and TB-silicosis collaborative screening within India’s TB program for high-risk occupational groups.
Similar content being viewed by others
Introduction
In 2022, the global tuberculosis (TB) burden reached 10.6 million incident cases, causing 1.3 million deaths1. India, a major TB hotspot, accounted for 27% of cases and 25% of deaths1,2. In 2023, India reported a record 2.8 million TB cases, with 0.3 million deaths3. Simultaneously, India faces a substantial occupational silicosis risk, with 52 million at risk among 227 million globally, contributing to the 2.65 million prevalent cases of silicosis worldwide4,5,6,7,8. Silicosis prevalence varies in India from 3.5 to 79%, with silico-tuberculosis ranging from 5–25%.7
While extensive research has identified key predictors of TB mortality through survival analysis such as age, gender, sputum smear positivity, multi-drug resistance, diabetes, HIV status, undernourishment, and chronic obstructive pulmonary disease9,10,11,12,13,14,15,16,17,18,19, few studies have explored the impact of silicosis on TB outcomes. Workers exposed to silica dust are at increased risk of developing TB, independent of silicosis, yet silicosis itself is associated with greater TB susceptibility and poor treatment outcomes20,21. TB patients with concurrent silicosis experience a 2–3 times higher mortality rate22,23, along with increased risks of drug resistance, treatment failure, and retreatment22,24, all of which may contribute to poorer survival.
Although survival analysis studies on pneumoconiosis often include TB as a co-variate, they rarely focus specifically on silicosis, a prevalent occupational lung disease25,26,27,28,29,30,31. Research shows that pneumoconiosis patients with TB have significantly reduced survival compared to those without TB32,33. However, while TB is a known risk factor for silicosis mortality34, many survival analyses involving silicosis patients have not accounted for TB as a covariate35,36,37,38, underscoring the need for comprehensive survival estimation specifically for patients with silico-tuberculosis.
One key challenge in this area is the clinical and radiological difficulty of distinguishing silicosis from TB, which can lead to reduced suspicion and underdiagnosis of silico-tuberculosis7,39,40. The overlapping features of both diseases complicate diagnosis and management, contributing to the scarcity of focused survival studies on this dual condition. Our study addresses these gaps by examining whether TB patients with concurrent silicosis experience earlier mortality compared to those with TB alone, an underexplored but critical area. Additionally, we assess the suitability of Cox regression versus accelerated failure time (AFT) models to better understand survival outcomes in this high-risk cohort, offering new insights into a largely neglected intersection of TB and silicosis.
Methods
Study design, setting, and duration
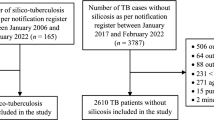
We conducted a survival analysis using data from a retrospective cohort, which included 138 patients with silico-tuberculosis and 2,610 patients with tuberculosis (TB) without silicosis, all from the Khambhat region in the state of Gujarat, located in western India21. The primary goal of the original cohort study was to examine the impact of silicosis on TB treatment outcomes including TB mortality. This current analysis builds on that study, focusing on estimating median survival times for both silico-tuberculosis and TB-only patients. The TB patients were registered in the Nikshay portal and TB treatment registers of the National TB Elimination Program (NTEP) from January 2006 to February 2022. Data for the retrospective cohort study were collected in February and March 2022. The Khambhat region is renowned for its agate-stone manufacturing, a sector that exposes workers to high levels of silica dust41,42. Historically, this industry employed about 50,000 workers43. Previous studies have reported the prevalence of silicosis among agate-stone workers to range from 18 to 69%, with the rate of silico-tuberculosis documented at around 5%.40
Study population
The study population consisted of two distinct patient groups: those diagnosed with silico-tuberculosis and those with TB without silicosis. To be included in the study, patients had to be aged ≥ 19 years and registered for TB treatment in the Nikshay portal or TB treatment registers from January 2006 to February 2022. Diagnosis of silico-tuberculosis was established by the Pneumoconiosis Board at Civil Hospital Ahmedabad, which includes a pulmonologist, radiologist, an official from the Directorate of Industrial Safety & Health (DISH), and a medical officer from the State TB Training and Demonstration Center (STDC). Diagnostic procedures involved evaluating chest X-rays of agate-stone workers referred by the Khambhat TB unit. In cases where chest X-ray results were inconclusive for TB, the Pneumoconiosis Board recommended further testing with sputum nucleic acid amplification testing (NAAT) to confirm TB retrospectively.
The TB without silicosis group comprised all TB patients aged ≥ 19 years, notified for treatment in the Nikshay portal from January 2017 to February 2022 in Khambhat. These patients had no diagnosis of silicosis, as verified through Pneumoconiosis Board records, ‘key population’ markers in the Nikshay portal, and exclusion of those with any occupational silica dust exposure (e.g., agate or mining industry workers). Age criteria followed the Indian TB program’s classification for adulthood (≥ 19 years).
Variables
The variables included in this study were carefully derived from the Nikshay portal dataset of TB patients in Khambhat21.
Outcome variable (event)
The primary outcome variable in our analysis was dichotomous, distinguishing between ‘death’ and ‘censored’ TB treatment outcomes44. ‘Death’ was assigned to patients who died during the treatment course44. In contrast, ‘censored’ encompassed patients experiencing alternative TB treatment outcomes, such as being cured (testing negative on sputum at treatment completion), completing treatment without clinical/radiological deterioration, facing treatment failure (testing positive on sputum at treatment completion), or being lost to follow-up (interrupting treatment for over a month)44.
Time-to-event variable
The time-to-event variable was calculated as the duration in months from the date of TB diagnosis to the date of TB treatment outcome assignment, with the starting point set at the date of diagnosis to minimize left-censored data.
Exposure variable
Our primary exposure variable of interest was whether patients with TB had co-existent silicosis, categorized as dichotomous (silico-tuberculosis vs. TB without silicosis)21,22.
Confounding variables
Confounding variables were categorized into three main groups for clarity: socio-demographic variables, comorbidities, and TB-related variables21,22. Socio-demographic variables included age, measured as a continuous variable, and gender, dichotomized as male or female. Comorbidities comprised HIV status and diabetes, which were included based on their established significance as predictors of TB outcomes21,22. TB-related variables included sputum-positive TB, previous TB treatment, multi-drug resistant TB, and pulmonary TB21,22. These variables were selected due to their demonstrated influence on mortality, treatment failure, and loss to follow-up in TB patients, as supported by prior studies12,21,22,23,45,46,47–49. In addition to their demonstrated relevance in the literature, we incorporated all available data from the Nikshay information portal on TB patients and TB treatment registers to ensure comprehensive variable selection.
Time of follow-up
Within the framework of the Indian TB program, the duration of drug-sensitive and drug-resistant TB regimens spans 6 and 6–20 months, respectively44,48. Patients on various regimens, such as isoniazid-mono resistant, isoniazid-poly resistant, shorter multi-drug resistant (MDR), and all-oral longer MDR regimens, undergo treatment for durations ranging from 6 to 20 months44,48. The follow-up period extended until the completion of treatment, with TB health workers relying on treatment supporters to relay information on changes in patient status. Data extraction from the Nikshay online portal and physical treatment registers, coupled with the recording of TB diagnosis and outcome assignment dates by TB program staff, formed the basis of our analysis.
Censoring
Censoring criteria encompassed patients who were lost to follow-up, those with positive sputum results at the completion of treatment, individuals who completed treatment without further clinical deterioration, and those who were declared successfully cured44. In our analysis, censoring was non-informative, meaning that the reasons for censoring were assumed to be unrelated to the likelihood of the primary outcome, which was death due to any cause. Patients who were lost to follow-up were treated as censored at their last known follow-up date. The same approach was applied to other censored cases, such as those who completed treatment or were declared cured. These cases were included in the survival models up to the time of their last known outcome and did not contribute to the risk set beyond that point. This ensures that censoring was appropriately accounted for in the Cox regression and accelerated failure time (AFT) models.
Statistical analysis
Our primary objective was to determine whether survival time in TB patients differs when comorbid with silicosis, and to identify other factors influencing survival. We used retrospective data from TB patients and conducted a comprehensive survival analysis. We began by constructing Kaplan-Meier survival curves for the entire cohort and stratified by co-existent silicosis. We assessed the proportional hazards (PH) assumption to validate the suitability of the Cox proportional hazards model for our data. For model selection, we compared the Akaike Information Criterion (AIC) across both PH and accelerated failure time (AFT) models, including Weibull, exponential, and lognormal distributions. The model with the lowest AIC was selected for further analysis.
Due to incomplete data, six cases were excluded—two due to zero time from diagnosis to outcome, and four due to loss to follow-up—resulting in an analysis on 2,742 participants (137 with silico-tuberculosis and 2,605 with TB without silicosis). We presented descriptive statistics for continuous variables as median (interquartile range, IQR) and categorical variables as number (percentage). We used the median test for continuous variables and Pearson’s chi-square test for categorical variables to compare death versus censored groups.
For survival times, we used life tables and Kaplan-Meier estimates. To test the difference in survival times between groups, we employed the Log Rank test. Since the survival curves did not cross the 50% survival threshold, we applied AFT models, particularly the Lognormal distribution, to estimate survival times beyond the median in the TB without silicosis group, facilitating comparison with the silico-tuberculosis group. We extracted 50%, 70%, and 90% survival times from the Lognormal distribution curves for both groups.
Next, we performed univariate Cox regressions for predictors such as age, gender, HIV status, diabetes, sputum positivity, previous TB treatment, multidrug-resistant TB (MDR-TB), and extrapulmonary TB. We then applied stepwise selection based on AIC to identify the most significant predictors for the final Cox model using the stepAIC function from the MASS package. All statistical analyses were conducted using R (https://www.r-project.org/),51 with primary packages including survminer (https://cran.r-project.org/package = survminer),52 MASS (https://cran.r-project.org/package = MASS),53 ggsurvfit (https://cran.r-project.org/package = ggsurvfit),54 and survival (https://cran.r-project.org/package = survival).55 Statistical significance was defined as p-values less than 0.05.
Results
Patient characteristics
Of 2742 participants, 309 (11%) died, 1247 (46%) completed treatment, 992 (36%) cured, 54 (2%) failed treatment, and 140 (5%) were lost to follow-up. The median age of patients who died was 50 years (IQR 40–60), significantly higher than those who were censored at 41 years (IQR 30–55) (p < 0.001, median test) (Table 1). Similarly, silico-tuberculosis was more prevalent among patients who died (10%) versus those who were censored (4.4%) (p < 0.001, Pearson’s chi-square test). Prior treatment for TB was also significantly higher among those who died (32%) compared to those who were censored (22%) (p < 0.001, Pearson’s chi-square test). The median time from diagnosis to outcome was shorter for patients who died (1.7 months; IQR 0.6–3.4) compared to those who were censored (5.6 months; IQR 5.5–6.2) (p < 0.001, median test).
Life tables
The median observation time was 6 months for both groups, with an interquartile range (IQR) of 4–7 months in the silico-tuberculosis group and 5–6 months in the TB without silicosis group. In the silico-tuberculosis group, survival rates declined from 76% at baseline to 66% at 6 months, remaining stable through 12 months (Table 2). However, by 18 months, the survival rate dropped sharply to 22%, with a standard error of 26%. In the TB without silicosis group, the initial survival rate was 86%, which declined gradually to 83% at 6 months and 76% at 12 months. By 18 months, the survival rate further declined to 66%, remaining steady through 24 and 30 months, with standard errors ranging from 1 to 9%.
Kaplan-Meier curves
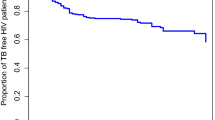
We constructed Kaplan-Meier curves for the overall sample (Fig. 1a) and for the silico-tuberculosis vs. TB without silicosis groups (Fig. 1b). The overall survival curve did not reach the median survival threshold. Visual inspection revealed that the survival curves began to diverge as early as two months, with the silico-tuberculosis group exhibiting a steeper decline in survival compared to the TB without silicosis group. By five months, 87% of survival events had occurred in the TB without silicosis group, whereas 81% had occurred in the silico-tuberculosis group. Also, the time to reach 75% survival probability was considerably shorter in the silico-tuberculosis group (around 7.5 months) than in the TB without silicosis group (approximately 17.5 months). The log-rank test indicated a significant difference in survival distributions between the two groups, χ²(1, N = 2742) = 18.5, p < 0.001 (see Supplementary Table S1 in Additional file 1). Thus, patients with silico-tuberculosis had significantly shorter survival times compared to those without silicosis.
Kaplan-Meier survival curves for patients with TB (n = 2742). The Kaplan-Meier survival curve in grey depicts overall survival probability for the study. X-axis represents time in months, and Y-axis represents the survival probability. The grey shaded area represents the 95% confidence interval. The shaded area represents 95% confidence intervals. Vertical ticks on the curve indicate censored observations. The median survival time was not reached by the end of follow-up, highlighting an extended survival period for the overall TB cohort.
Kaplan-Meier survival curves for silico-tuberculosis vs. TB without silicosis groups (n = 2742). The Kaplan-Meier survival curve shows the survival probability stratified by silico-tuberculosis status. The brown line represents the survival probability for the silico-tuberculosis group, and the green line represents the survival probability for the TB without silicosis group. X-axis represents time in months, and Y-axis represents the survival probability. The corresponding-colored shaded areas represent the 95% confidence intervals for each group. Vertical ticks on the curves indicate censored observations. The curves diverge early, around three months, with the silico-tuberculosis group crossing the median survival time significantly earlier. A log-rank test confirms a statistically significant survival difference between the two groups (p < 0.001).
Cox proportional hazards model
The Cox proportional hazards model was applied to assess the risk factors influencing survival among TB patients, considering the proportional hazards (PH) assumption. While silico-tuberculosis and age showed borderline results (see Supplementary Table S2 in Additional file 1), the overall model passed the global test for the PH assumption, indicating no significant violations. Model comparison using the Akaike Information Criterion (AIC) revealed that the lognormal accelerated failure time (AFT) model had the lowest AIC value (2901.8), suggesting a better fit for the dataset compared to other AFT models and the Cox model (see Supplementary Table S3 in Additional file 1).
To derive hazard ratios, both univariate and stepwise regression analyses using the Cox proportional hazards regression model were conducted (Table 3). The stepwise regression identified that patients with silico-tuberculosis had a significantly higher hazard of death compared to those without silicosis, with a hazard ratio (HR) of 2.0 (95% CI: 1.4-3.0, p < 0.001). This indicates that silico-tuberculosis patients have a 2-fold increased risk of mortality. Additional significant predictors from the stepwise analysis included male gender (HR = 1.4, 95% CI: 1.1–1.8, p = 0.023), positive HIV status (HR = 1.96, 95% CI: 1.01–3.82, p = 0.048), and previous TB treatment (HR = 1.32, 95% CI: 1.03–1.69, p = 0.029). These findings suggest that men, HIV-positive individuals, and those with prior TB treatment face higher mortality risks. Notably, multi-drug resistant TB (MDR-TB) was associated with a lower hazard of death compared to non-MDR TB, although this result was not statistically significant in the multivariate analysis (HR = 0.47, 95% CI: 0.19–1.15, p = 0.086).
Lognormal Accelerated failure time model
The Lognormal Accelerated Failure Time (AFT) model was utilized to estimate and compare survival times between the silico-tuberculosis group and the TB without silicosis group. The overall median survival time, predicted using the Lognormal distribution, was 245 months (Fig. 2a). Stratified analysis revealed that, according to unadjusted models, survival was significantly lower for the silico-tuberculosis group, with a median survival time of 78 months, compared to 264 months for the TB without silicosis group (Fig. 2b). After adjusting for covariates such as age, gender, HIV status, and previous TB treatment, the median survival times were further refined to 16 months for the silico-tuberculosis group and 44 months for the TB without silicosis group (see Supplementary Table S4 in Additional file 1 for 70% and 90% survival thresholds).
Lognormal AFT survival curves for patients with TB (n = 2742). The lognormal-based predicted survival curve based on a null model depicts the overall survival probability for the study cohort. The X-axis represents time in months, and the Y-axis represents the survival probability. The grey curve shows the predicted survival probability over time for all participants, with a median predicted survival time of 245 months, indicating a favorable long-term outlook for the cohort overall.
Lognormal AFT survival curves for silico-tuberculosis and TB without silicosis groups (n = 2742). The lognormal-based predicted survival curves with silico-tuberculosis as the predictor in the model depict survival probabilities for two groups: TB without silicosis and silico-tuberculosis. The X-axis represents time in months, and the Y-axis represents the survival probability. The green curve represents the survival probability for the TB without silicosis group, while the orange curve represents the silico-tuberculosis group. The unadjusted predicted median survival time was substantially lower for the silico-tuberculosis group (78 months) compared to the TB without silicosis group (264 months), underscoring the significant survival disadvantage faced by silico-tuberculosis patients.
We utilized a lognormal accelerated failure time (AFT) model to estimate survival times among tuberculosis (TB) patients, incorporating key covariates such as silico-tuberculosis status, age, gender, HIV status, and history of previous TB treatment (Table 4). The model provided exponentiated coefficients (derived from the lognormal AFT model) to assess the effects of these covariates on survival times. Silico-tuberculosis was associated with a significant reduction in median survival time, with an exponentiated coefficient of 0.36 (95% CI: 0.17–0.74, p = 0.005), indicating that patients with silico-tuberculosis had approximately 36% of the median survival time compared to those with TB alone. Age showed an exponentiated coefficient of 0.95 (95% CI: 0.94–0.97, p < 0.001), translating to a 5% decrease in survival time for each additional year of age. Male gender (exp(-0.496) = 0.61, 95% CI 0.40–0.92, p = 0.019), HIV positive status (exp(-1.25) = 0.29, 95% CI 0.08–0.96, p = 0.043), and previous TB treatment (exp(-0.527) = 0.59, 95% CI 0.39–0.90, p = 0.014) were also associated with reduced median survival times relative to their respective counterparts. The log(scale) parameter (exp(1.09) = 2.97, p < 0.001) highlighted considerable variability in survival times across the cohort, underscoring the diverse nature of survival outcomes observed.
Discussion
Summary of key findings
In our study setting, TB patients with silicosis had a significantly higher risk of earlier mortality compared to those with TB alone, exhibiting nearly twice the likelihood of dying prematurely. The cumulative survival curve for TB patients with silicosis showed a pronounced decline at six-month intervals, in contrast to their counterparts without silicosis. Male patients with silico-tuberculosis who were also HIV-positive had the poorest survival rates. We employed Accelerated Failure Time (AFT) models effectively to estimate survival times where non-parametric analysis did not report median survival. Adjusted for covariates, the AFT models revealed that the median survival time for silico-tuberculosis patients was 28 months shorter than that of TB patients without silicosis.
Explanation of key findings in local context
Our study was conducted in Khambhat, a region known for agate-stone polishing, leading to both occupational and non-occupational silica dust exposure41,43. Previous studies have reported silicosis prevalence rates between 18 and 69% among agate-stone workers in Khambhat, with silica dust levels exceeding permissible limits54,55. Although silica exposure in agate-stone work settings averages between 0.11 and 0.12 mg/m³,56,57 lower than levels in stone crushing (up to 2.29 mg/m³) and coal mining (up to 5.3 mg/m³),58–60 it still presents a significant risk of silicosis59. Silicosis has been shown to significantly increase the odds of mortality among TB patients22,23, and conversely, TB also increased mortality risk among those with silicosis29,34. Patients with silico-tuberculosis therefore face a compounded risk, resulting in higher mortality rates compared to those with either condition alone and explaining the earlier mortality observed in our study population.
Survival outcomes using accelerated failure time model
Our study, using the AFT model, found a median survival time of 16 months for silico-tuberculosis patients, significantly shorter than the 44 months observed for TB-only patients after adjusting for covariates. This aligns with prior studies that report significantly poorer survival rates for individuals with silico-tuberculosis across different contexts of silica exposure. For instance, a 1949 study from Sheffield, UK, indicated that while survival outcomes varied by occupation, those in high-silica occupations (e.g., grinders) had markedly poorer survival rates compared to coal miners, potentially due to differential toxicity levels of silica and metal dust mixtures60. Similar to our findings, a West Bengal study reported a median survival of 18 months among silicosis-affected jewelry workers, significantly shorter than the 54-month survival among those without silicosis, emphasizing the compounded risk of TB and occupational lung disease within high-silica industries25. In China, survival among pneumoconiosis patients varied by type and stage; mean survival times of 14 to 34 years were observed, with TB further exacerbating mortality risks26,32,33. For instance, Song et al. (2022) found a 20-year mean survival for TB-complicated pneumoconiosis cases versus 30 years for pneumoconiosis alone26. The consistently lower cumulative survival rates (e.g., 30% among TB-pneumoconiosis patients) and the impact of occupational dust exposure on survival also align with data from Zhejiang Province, where TB increased mortality risks by 17% among pneumoconiosis patients, underscoring the compounded effect of TB in high-risk industries27,29. Our findings are consistent with the trends observed in West Bengal25, highlighting the substantial mortality burden associated with coexisting silicosis and TB. Together with our previous research7,21,22, these results strongly support the need for integrated care strategies to improve outcomes for silica-exposed populations facing this dual disease burden61.
Survival outcomes using Cox proportional hazards model
Our Cox proportional hazards model reveals that silico-tuberculosis patients have a twofold higher mortality risk than TB-only patients. This aligns with global studies on pneumoconiosis and TB, with hazard ratios (HR) for mortality risk ranging from 1.17 in Taiwan to 1.75 in Changsha and 1.29 in Yueyang, China29,30,31. For example, in Changsha, pneumoconiosis patients with TB had a HR of 1.75, with life expectancy around 60 years and TB as the main cause of death31. By contrast, our cohort’s lower median age of death at 50 reflects the compounded mortality risk from high silica exposure and limited healthcare access in Khambhat, Gujarat. Similarly, a Shanghai study reported a HR of 1.47, showing TB’s role in decreasing survival time by nearly a decade for pneumoconiosis patients, with fatality rates sharply increasing with advancing disease stages32. In Yueyang, a HR of 1.29 was reported among pneumoconiosis patients with TB, where mortality risk was directly linked to disease severity30. The higher average age of death at 60.6, compared to our cohort, suggests that occupational differences and healthcare access can impact outcomes30. Finally, a Taiwanese cohort with occupational lung diseases and TB observed a HR of 1.17, with a TB mortality rate (24.8%) substantially exceeding that of the general population29. Comorbidities like cardiovascular disease intensified TB-related mortality risk, highlighting that silicosis patients face distinct comorbidity challenges29. These findings indicate that while hazard ratios are similar across studies, differences in occupational and healthcare contexts impact mortality and age of death. The younger mortality age in our silico-tuberculosis cohort points to the need for interventions addressing silica exposure and TB risk in high-exposure groups.
Secondary findings using Cox proportional hazards model
Our study identified age, HIV status, and prior TB treatment as significant mortality predictors, aligning with findings from other cohorts. Specifically, age was associated with increased risk, with hazard ratios reported at 1.7 per 10-year increase and 1.05 per year in similar studies29,37, while HIV infection and history of TB treatment were also linked to higher mortality12. These factors underscore the need for individualized care strategies to improve survival, especially for patients with additional risk factors such as HIV23,62. Addressing these risk factors through targeted interventions can significantly improve the management and prognosis of TB patients, whether or not they have silicosis. In addition, MDR-TB was significantly associated with lower mortality in univariable analysis (HR = 0.4, 95% CI: 0.2–0.9, p = 0.024) but became non-significant in multivariable analysis. This finding may reflect benefits from newer anti-TB drugs and intensive follow-up63. However, the limited MDR-TB cases (10 in the silico-tuberculosis group vs. 51 in the TB-only group) suggest a need for further research with larger cohorts to clarify MDR-TB’s impact on mortality.
Recommendations for healthcare providers: early identification and intensive management
Healthcare providers should routinely inquire about silica dust exposure when assessing TB patients and suspect silico-tuberculosis early61. Standard TB treatment of 6–12 months may be inadequate for these patients due to their compounded risks of mortality, drug resistance, retreatment, and treatment failure22,24,34. Silico-tuberculosis patients would benefit from more intensive, potentially prolonged treatment regimens, alongside comprehensive follow-up, to improve survival outcomes. Awareness of silicosis and its severe complications with TB remains low among patients and providers, despite the high mortality associated with this comorbidity21,7,7,40,64,65,66,67,68,69,70,42. Clinicians in high-risk regions should receive targeted training to enhance their capacity for diagnosing and managing silico-tuberculosis effectively, ensuring timely and specialized care22. Integrating respiratory health monitoring, such as spirometry, and offering supportive therapies like oxygen, bronchodilators, and pulmonary rehabilitation through comprehensive occupational health programs can further strengthen the management of silico-tuberculosis and improve patient outcomes.
Recommendations for policymakers: implement TB-silicosis collaborative screening and surveillance for unorganized sectors
Policymakers should prioritize bidirectional screening initiatives, encompassing silicosis in silica-exposed TB patients and TB in silicosis patients as well as symptomatic silica-exposed workers, to facilitate early diagnosis and prompt intervention61. Integrating biannual active case finding (ACF) and latent TB infection screening specifically for silica-exposed workers in unorganized sector could further alleviate disease burden71,72. By incorporating silicosis management into the differentiated TB care model, which addresses high-risk factors for mortality from the point of diagnosis, healthcare workers can contribute to more favorable outcomes22. Currently, systemic gaps persist, particularly in post TB treatment follow-up, which should be extended beyond the recommended 24 months, with an emphasis on symptom awareness to reduce TB-related mortality. With two-thirds of TB treatment deaths attributed to TB itself, a community-based death review system under the national TB program is vital for uncovering preventable factors64. Finally, establishing cross-ministry collaboration—between Health, Mines, Labour and Employment, as well as research institutions like ICMR-NIOH—will ensure regulatory enforcement and promote safer silica exposure standards, as mandated by the Factories Act (1948) and Mines Act (1952).
Recommendations for industry stakeholders: reducing silica dust exposure and implementing employer-led TB care models
Industry stakeholders must prioritize silica dust exposure reduction through comprehensive workplace controls to prevent silicosis and silico-tuberculosis, as even moderate silica exposure poses significant health risks59. Employers should establish a hierarchy of exposure controls as the first step to safeguard worker health and prevent disease incidence73. Our findings also emphasize the necessity of employer-led TB-free workplace initiatives in high-risk sectors, particularly for occupations with significant silica exposure74,75,76,77. Effective models, such as those implemented in South Africa, demonstrate that TB surveillance and control among silica-exposed populations reduce TB incidence and mortality71,72,78. South Africa has further addressed the high TB burden in mining by implementing an employer-led model that makes TB a notifiable and compensable disease79. Additionally, biannual medical examinations for TB and silicosis should replace the current annual checks in factories and 3–5-year intervals in mines to enable early detection and timely intervention. For the unorganized sector, district health teams need to play a critical role in supporting these efforts in collaboration with government initiatives61.
Strengths and limitations
Our study has provided a comprehensive survival analysis using a variety of methods: non-parametric Kaplan-Meier, semi-parametric Cox proportional hazards, and parametric accelerated failure time (AFT) models. This multi-faceted approach allowed us to report an predicted median survival time for our cohort. Despite the strengths of our study, some limitations should be acknowledged. We lacked data on silica dust levels and the severity of silicosis, which could have provided unique insights. Additionally, the extended enrolment period from 2006 to 2022 is a major limitation, as it spans multiple advancements in TB management within India’s national TB program. During this period, changes such as the introduction of new drugs, updates in treatment regimens, and improvements in TB screening, including active case finding, were implemented at various intervals. These programmatic shifts could introduce variability in patient outcomes over time, potentially impacting the uniformity of the data and influencing survival estimates in ways that may not fully reflect recent practices. Despite these limitations, our findings remain generalizable to patients with silico-tuberculosis across different occupations in India, highlighting the need for targeted interventions and ongoing research in this area.
Conclusions
Our study reveals that patients with silico-tuberculosis experience significantly earlier mortality than those with TB alone, underscoring the need to prioritize silico-tuberculosis within India’s national TB program. Immediate, collaborative actions are needed from healthcare providers, policymakers, and industry. Healthcare providers should routinely inquire TB patients for silica exposure, promptly suspect and manage silico-tuberculosis, and receive training in comprehensive care for these patients, including monitoring and timely intervention for respiratory support. For policymakers, implementing TB-silicosis collaborative activities and biannual active case finding in unorganized sectors will improve early detection and management. Mandating silica dust exposure assessments and integrating silicosis screening into differentiated TB care models are essential steps to strengthen prevention. Industry stakeholders should focus on reducing silica dust exposure through a structured hierarchy of controls, adopting employer-led TB care models, and supporting worker safety by ensuring free access to high-quality protective equipment. Finally, awareness campaigns for silicosis risks among high-exposure occupations and healthcare providers can ensure earlier detection, better management, and reduced mortality. By advancing these targeted measures, our findings provide a framework for improved survival and quality of life for those impacted by silico-tuberculosis.
Data availability
The datasets utilized in this study are held by the Gujarat State TB Cell and are subject to licensing restrictions, making them unavailable for public access. Interested researchers can request access to the data by contacting Mihir Rupani at [email protected] or Soundarya Soundararajan at [email protected]. Access will be granted contingent upon obtaining permission from the Gujarat State TB Cell (Government of Gujarat, India).
References
World Health Organization. Global Tuberculosis Report 2023. (2023). https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1
Central, T. B. & Division (Ministry of Health & Family Welfare). India TB Report 2023. (2023). https://tbcindia.gov.in/showfile.php?lid=3680
Central, T. B. Division (Ministry of Health & Family Welfare). India TB Report 2024. (2024). https://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-Web_08_10-2024-1.pdf
Intergovernmental Forum on Mining Minerals Metals and Sustainable Development (IGF). Global Trends in Artisanal and Small-Scale Mining (ASM): A Review of Key Numbers and Issues. (2018).
Murie, F. Building Safety—An International Perspective. Int. J. Occup. Environ. Health. 13, 5–11 (2007).
TeamLease. India Labour Report 2009. (2009).
Rupani, M. P. Challenges and opportunities for silicosis prevention and control: need for a national health program on silicosis in India. J. Occup. Med. Toxicol. 18, 11 (2023).
Institute for Health Metrics and Evaluation. Global Health Metrics: Silicosis — Level 4 Cause. (2019).
Pardeshi, G. Survival analysis and risk factors for death in tuberculosis patients on directly observed treatment-short course. Indian J. Med. Sci. 63, 180–186 (2009).
Feng, J. Y. et al. Initial presentations Predict Mortality in Pulmonary Tuberculosis patients - a prospective observational study. PLoS One. 6, e23715 (2011).
Machmud, P. B., Gayatri, D. & Ronoatmodjo, S. A. Survival analysis of successful and poor treatment outcome among patients with drug-resistant tuberculosis and the Associated factors: a retrospective cohort study. Acta Med. Indones. 53, 184–193 (2021).
Xie, Y. et al. Survival Analysis of Risk Factors for Mortality in a Cohort of Patients with Tuberculosis. Can Respir J (2020). (2020).
Balaky, S. T. J., Mawlood, A. H. & Shabila, N. P. Survival analysis of patients with tuberculosis in Erbil, Iraqi Kurdistan region. BMC Infect. Dis. 19, (2019).
Liu, K. et al. Survival Analysis and Associated Factors for pulmonary tuberculosis death: evidence from the Information System of Tuberculosis Disease and Mortality Surveillance in China. Risk Manag Healthc. Policy. 15, 1167–1178 (2022).
Carter, B. B. et al. Survival analysis of patients with tuberculosis and risk factors for multidrug-resistant tuberculosis in Monrovia, Liberia. PLoS One 16, (2021).
Liu, Y. et al. Tuberculosis-associated mortality and its risk factors in a district of Shanghai, China: a retrospective cohort study. Int. J. Tuberculosis Lung Disease. 22, 655–660 (2018).
de Viana, P. V. S. et al. Factors associated with death in patients with tuberculosis in Brazil: competing risks analysis. PLoS One 15, (2020).
Abdullahi, O. A., Ngari, M. M., Sanga, D., Katana, G. & Willetts, A. Mortality during treatment for tuberculosis; a review of surveillance data in a rural county in Kenya. PLoS One. 14, e0219191 (2019).
Drobniewski, F. et al. A national study of clinical and laboratory factors affecting the survival of patients with multiple drug resistant tuberculosis in the UK. Thorax 57, 810–816 (2002).
Ehrlich, R., Akugizibwe, P., Siegfried, N. & Rees, D. The association between silica exposure, silicosis and tuberculosis: a systematic review and meta-analysis. BMC Public. Health. 21, 1–18 (2021).
Rupani, M. P. A mixed-methods study on impact of silicosis on tuberculosis treatment outcomes and need for TB-silicosis collaborative activities in India. Sci. Rep. 13, 2785 (2023).
Rupani, M. P. Silicosis as a predictor of tuberculosis mortality and treatment failure and need for incorporation in differentiated TB care models in India. Archives Public. Health. 81, 173 (2023).
Churchyard, G. J. et al. Factors associated with an increased case-fatality rate in HIV-infected and non-infected South African gold miners with pulmonary tuberculosis. Int. J. Tuberculosis Lung Disease. 4, 705–712 (2000).
Rupani, M. P. Silicosis predicts drug resistance and retreatment among tuberculosis patients in India: a secondary data analysis from Khambhat, Gujarat (2006–2022). BMC Pulm Med. 24, 522 (2024).
Panchadhyayee, P. et al. Rapidly Fatal Silicosis among Jewellery Workers Attending a District Medical College of West Bengal, India. Indian J. Chest Dis. Allied Sci. 57, 165–171 (2015).
Song, X. et al. Survival analysis of 15,402 pneumoconiosis cases in Jiangsu Province of China from 1961 to 2019. Ann. Palliat. Med. 11, 2291–2301 (2022).
Zou, H. et al. Epidemiological characteristics and survival analysis on patients with occupational pneumoconiosis in Zhejiang Province from 1987 to 2019. Front. Public. Health 10, (2022).
Wang, W. et al. [Survival analysis of silicosis patients in Wuxi City]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 39, 430–433 (2021).
Hung, C. L., Su, P. L. & Ou, C. Y. Prognostic effect of tuberculosis on patients with occupational lung diseases a 13-year observational study in a nationwide cohort. Med. (United States) 95, (2016).
Cui, J. W., Hu, J. A. & Zhu, L. [Survival analysis of patients with pneumoconiosis from 1963 to 2014 in Yueyang]. Chin. J. Industrial Hygiene Occup. Dis. 34, 360–362 (2016).
Xue, J. & Chen, L. [Survival analysis of patients with pneumoconiosis from 1956 to 2010 in Changsha]. J. Cent. South. Univ. Med. Sci. 37, 84–88 (2012).
Juan-juan, P. et al. Survival analysis of pneumoconiosis complicated with tuberculosis from 1953 to 2005 in Shanghai. J. Environ. Occup. Med. 26, 224–227 (2009).
Han, L. et al. Survival analysis of coal workers’ pneumoconiosis (CWP) patients in a state-owned mine in the east of China from 1963 to 2014. Int. J. Environ. Res. Public. Health 14, (2017).
Ng, T. P., Chan, S. L. & Lee, J. Predictors of mortality in silicosis. Respir Med. 86, 115–119 (1992).
Haibing, Y., Lei, Y., Junyue, Z. & Jingqiong, C. Natural course of silicosis in dust-exposed workers. J. Huazhong Univ. Sci. Technol. [Medical Sciences]. 26, 257–260 (2006).
Duan, Z., Zhou, L., Wang, T., Han, L. & Zhang, J. Survival and Disease Burden Analysis of Occupational Pneumoconiosis from 1956 to 2021 in Jiangsu Province. J. Occup. Environ. Med. 65, 407–412 (2023).
Infante-Rivard, C. et al. Descriptive study of prognostic factors influencing survival of compensated silicotic patients. Am. Rev. Respir. Dis. 144, 1070–1074 (1991).
Bakan, N. D. et al. Silicosis Denim Sandblasters Chest 140, 1300–1304 (2011).
Maboso, B., te Water Naude, J., Rees, D., Goodman, H. & Ehrlich, R. Difficulties in distinguishing silicosis and pulmonary tuberculosis in silica-exposed gold miners: a report of four cases. Am. J. Ind. Med. 66, 339–348 (2023).
Chaturvedi, O. et al. Challenges in the implementation of the Rajasthan Pneumoconiosis Policy. Ann. Work Expo Health. 66, 1162–1172 (2022).
Saiyed, H. N. Silicosis among children in the agate industry. in Chidren’s Health and the Environment: A Global Perspective (ed Pronczuk-Garbino, J.) (World Health Organization, Geneva, (2005).
Sadhu, H. et al. A follow up study of health status of small-scale agate industry workers. Indian J. Industrial Med. 41, 101–105 (1995).
Patel, J. & Robbins, M. The Agate Industry and Silicosis in Khambhat, India. NEW. SOLUTIONS: J. Environ. Occup. Health Policy. 21, 117–139 (2011).
Central, T. B. & Division (Ministry of Health and Family Welfare). Training Modules for Programme Managers and Medical Officers (Modules 1–4). (2020). https://tbcindia.gov.in/WriteReadData/NTEPTrainingModules1to4.pdf
Mallory, K. F., Churchyard, G. J., Kleinschmidt, I., De Cock, K. M. & Corbett, E. L. The impact of HIV infection on recurrence of tuberculosis in South African gold miners. Int. J. Tuberculosis Lung Disease. 4, 455–462 (2000).
Chaves Torres, N. M., Rodríguez, Q., Andrade, J. J. P., Arriaga, P. S., Netto, E. M. & M. B. & Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS One. 14, e0226507 (2019).
Teferi, M. Y. et al. Tuberculosis treatment outcome and predictors in Africa: a systematic review and Meta-analysis. Int. J. Environ. Res. Public. Health. 18, 10678 (2021).
Central, T. B. Division (Ministry of Health & Family Welfare). Guidelines for Programmatic Management of Drug Resistant Tuberculosis in India. (2021). https://tbcindia.gov.in/showfile.php?lid=3590
R Core Team. R: A Language and Environment for Statistical Computing. Preprint at (2022). https://www.r-project.org/
Kassambara, A., Kosinski, M., Biecek, P., Fabian, S. & Survminer Drawing Survival Curves Using ‘Ggplot2’. Preprint at (2021). https://doi.org/10.32614/CRAN.package.survminer
Ripley, B. et al. Modern Applied Statistics with S (MASS) package. Preprint at (2024). https://doi.org/10.32614/CRAN.package.MASS
Sjoberg, D. D. et al. Flexible Time-to-Event Figures. Preprint at (2023). https://cran.r-project.org/package=ggsurvfit (2023).
Therneau, T. M., Lumley, T., Elizabeth, A. & Cynthia, C. A Package for Survival Analysis in R. Preprint at (2023). https://cran.r-project.org/package=survival
Rastogi, S. K. et al. A study of the prevalence of respiratory morbidity among agate workers. Int. Arch. Occup. Environ. Health. 63, 21–26 (1991).
Chaudhury, N., Phatak, A., Paliwal, R. & Raichaudhari, C. Silicosis among agate workers at Shakarpur: an analysis of clinic-based data. Lung India. 27, 221–224 (2010).
MUKHOPADHYAY, K. et al. Exposure to respirable particulates and silica in and around the Stone Crushing Units in Central India. Ind. Health. 49, 221–227 (2011).
Semple, S., Green, D. A., McAlpine, G., Cowie, H. & Seaton, A. Exposure to particulate matter on an Indian stone-crushing site. Occup. Environ. Med. 65, 300–305 (2008).
MUKHERJEE, A. K., SAIYED, H. N. & BHATTACHARYA, S. K. & Assessment of Respirable Dust and its free silica contents in different Indian coalmines. Ind. Health. 43, 277–284 (2005).
Dhatrak, S. & Nandi, S. Assessment of silica dust exposure profile in relation to prevalence of silicosis among Indian sandstone mine workers: need for review of standards. Am. J. Ind. Med. 63, 277–281 (2020).
Turner, H. M. & Martin, W. J. Mortality and survival rates in Silicosis. BMJ 2, 1148–1150 (1949).
Rupani, M. P., Nimavat, P., Patel, Y., Shah, H. D. & Sau, A. Framework for implementing collaborative TB-silicosis activities in India: insights from an expert panel. Archives Public. Health. 82, 91 (2024).
Corbett, E. L. et al. HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. Aids 14, 2759–2768 (2000).
Vambe, D. et al. Bedaquiline and delamanid result in low rates of unfavourable outcomes among TB patients in Eswatini. Int. J. Tuberc. Lung Dis. 24, 1095–1102 (2020).
Shah, H. D. et al. A step up to end tuberculosis: lessons from a community-based death review of patients with tuberculosis from western India. Clin. Epidemiol. Glob Health. 19, 101205 (2023).
Yadav, S. P., Anand, P. K. & Singh, H. Awareness and practices about Silicosis among the Sandstone Quarry Workers in Desert Ecology of Jodhpur, Rajasthan, India. J. Hum. Ecol. 33, 191–196 (2011).
Nandi, S., Burnase, N., Barapatre, A., Gulhane, P. & Dhatrak, S. Assessment of silicosis awareness among stone mine workers of Rajasthan state. Indian J. Occup. Environ. Med. 22, 97 (2018).
Sharma, D. C. Miners fight for breath in Indian state. Lancet Respir Med. 3, 181 (2015).
Aggarwal, B. D. Worker Education Level is a factor in self-compliance with dust-preventive methods among small-scale Agate Industrial Workers. J. Occup. Health. 55, 312–317 (2013).
Shamim, M. & Saraf, A. Examining legal and policy provisions on silicosis in the Context of Sandstone Mining in Karauli, Rajasthan-India. Indian J. Public. Health Res. Dev. 8, 407 (2017).
Falk, L. et al. Reducing agate dust exposure in Khambhat, India: protective practices, barriers, and opportunities. J. Occup. Health. 61, 442–452 (2019).
Shah, A. P., Dave, J. D., Makwana, M. N., Rupani, M. P. & Shah I. A. A mixed-methods study on impact of active case finding on pulmonary tuberculosis treatment outcomes in India. Archives Public. Health. 82, 92 (2024).
Rupani, M. P. et al. Mixed methods study on latent tuberculosis among agate stone workers and advocacy for testing silica dust exposed individuals in India. Sci. Rep. 14, 13830 (2024).
Occupational Safety and Health Administration (OSHA). Identifying Hazard Control Options: The Hierarchy of Controls. (2016). https://www.osha.gov/sites/default/files/Hierarchy_of_Controls_02.01.23_form_508_2.pdf
Ministry of Health and Family Welfare; Government of India. Employer Led Model for Tuberculosis Care and Prevention: Operational Guidelines. (2019).
Sachdeva, K. S. et al. Paradigm shift in efforts to end TB by 2025. Indian J. Tuberculosis. 67, S48–S60 (2020).
Central, T. B. & Division (Ministry of Health & Family Welfare). India TB Report 2020. (2020). https://tbcindia.gov.in/showfile.php?lid=3538
World Health Organization (WHO) Regional Office for South-East Asia. TB Control in the Workplace: Report of an Intercountry Consultation. (2004). https://iris.who.int/bitstream/handle/10665/206461/B3683.pdf?sequence=1&isAllowed=y
Chimoyi, L. A. et al. Estimating the yield of tuberculosis from key populations to inform targeted interventions in South Africa: a scoping review. BMJ Glob Health. 5, e002355 (2020).
Baleta, A. Southern African declaration targets TB in mining sector. Lancet 380, 1217–1218 (2012).
Acknowledgements
We express our gratitude to the National Institute of Occupational Health (NIOH) for providing the time and support necessary for this study. We also acknowledge the Gujarat State TB Cell and the Gujarat State TB Operations Research Committee for their permission to access the data. We also wish to convey our sincere thanks to the TB health workers and staff at the Khambhat TB unit for their indispensable assistance in data collection and provision. Additionally, we acknowledge the financial support for travel expenses to Khambhat, generously covered by intramural funding from the ICMR-National Institute of Occupational Health (NIOH). We also wish to express our sincere thanks to Dr. Rivu Basu, Bankura Sammilani Medical College, West Bengal, for suggesting the use of accelerated failure time models for our dataset, and to Dr. Pankaj Nimavat, an expert in TB in Gujarat, for his insightful feedback on the study’s recommendations.
Author information
Authors and Affiliations
Contributions
Both authors made significant contributions to the study’s conception, design, literature review, data interpretation, manuscript preparation, and overall intellectual content. M.R. was primarily responsible for data acquisition and drafting the manuscript. S.S. took the lead on conducting the survival analysis. Both authors reviewed and critically revised the manuscript, and have approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by both the Scientific Advisory Committee and the Institutional Human Ethics Committee at the National Institute of Occupational Health (NIOH), under the Indian Council of Medical Research (ICMR). Access to national tuberculosis (TB) program data was granted by the Gujarat TB Operations Research Committee. Data confidentiality was upheld using anonymized identifiers, such as Nikshay IDs, ensuring participant privacy. The study adhered to the ethical principles of the Declaration of Helsinki. Individual informed consent was waived by the Institutional Human Ethics Committee of NIOH, based on the retrospective nature of the study and the use of anonymized secondary data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rupani, M.P., Soundararajan, S. Survival analysis shows tuberculosis patients with silicosis experience earlier mortality and need employer-led care models in occupational settings in India. Sci Rep 14, 28891 (2024). https://doi.org/10.1038/s41598-024-80367-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80367-5