Abstract
Invasion and metastasis are the main reasons for the poor prognosis of patients with cervical cancer(CC). SYK is closely related to tumor development. However, the functions of its two isoforms, SYK (L) or SYK (S), are not fully understood to date. In this study, we investigated their biologic functions and possible prognostic values in CC. qRT-PCR was performed to detect the expression of SYK and two variant isoforms in cervical cancer tissues and cells. The association of SYK(L) and SYK(S) with Clinical pathological parameters were evaluated. The migration and invasion was detected by scratch assay and transwell. Western blot was conducted to measure the changes of epithelial mesenchymal transition (EMT)-related markers and PI3K/AKT signaling pathway proteins in cervical cancer cells. LY294002 (inhibitor of PI3K/AKT pathway) and IGF-1 (activator of PI3K/AKT pathway) were applied to evaluate the contribution of PI3K/AKT signaling pathway in cervical cancer cells. The expression of SYK(S) in cervical cancer tissues was significantly higher than that of SYK(L). SYK(L) and SYK(S) were correlated with muscular infiltration, SYK(L) high expression had a better prognosis, whereas SYK(S) high expression predicted a worse disease outcome. Cox multivariate regression analysis demonstrated that SYK(L) expression was an independent prognostic factor. SYK(L) significantly inhibited the proliferation, migration and invasion, while SYK(S) showed the opposite effects. LY294002 blocked SYK (L) knockdown-induced enhancement of migration and invasion as well as the expression EMT-related markers, whereas IGF-1 rescued the decreased migration, invasion and EMT induced by SYK (S) knockdown. The results suggest that SYK(L) and SYK(S) are involved in the progression of cervical cancer through PI3K/AKT signaling pathway, and may serve as potential targets for clinical treatment of advanced cervical cancer.
Similar content being viewed by others
Introduction
Cervical cancer (CC) is one of the world’s top 10 malignant tumors in women, with the fourth highest incidence and sixth highest mortality rate, and remains the second leading cause of cancer deaths in women aged 20–39 years, even though it is one of the most preventable cancers1. Metastasis and recurrence is the main cause of death in the patients with cervical cancer2.Therefore, there is an urgent need to understand the molecular mechanisms of cervical cancer recurrence and metastasis, and to probe the favorable biomarkers and new therapeutic targets.
Spleen tyrosine kinase (SYK) is a non-receptor-type protein tyrosine kinase with a molecular weight of 72 KD, and has its own structural domains at the amino-terminal and carboxyl-terminal ends, respectively, including two SH2 structural domains at the amino-terminal end and one kinase structural ___domain at the carboxyl-terminal end3. SYK has a dual reputation. Coopman et al.4 first reported that SYK expression was reduced in breast cancer, playing the role of tumor suppressor gene, and subsequently reduced SYK expression was observed in hepatocellular carcinoma5, lung cancer6 and melanoma7. Although most studies have shown a positive correlation between SYK loss and neoplastic progression, other studies have indicated that SYK is a necessary condition for tumor cell survival. SYK is upregulated in papillary thyroid cancer, it exhibits a positive correlation with tumor size, lymph node metastasis, and unfavorable disease-free survival8. High SYK expression may facilitate ovarian tumor spread to higher stage9. SYK promotes pancreatic ductal adenocarcinoma growth and metastasis10. The knockdown of SYK efficiently decreases the motility and migration in lung cancer cells11. The pro-carcinogenic effect of SYK has also been observed in studies of retinoblastoma, where treatment with SYK knockdown or use of SYK inhibitor cause reinforced apoptosis of cancer cells12. Some studies suggested that the reason for the dual reputation of SYK is due to the presence of its two variant isoforms, the full-length SYK [SYK(L)] and the short isoform SYK [SYK(S)], SYK(S) lacks a stretch of 23 amino acids in linker B, referred to as the linker insert or DEL. While preserving the major structural domains (two SH2 domains and a kinase ___domain), SYK(S) does not share the biologic responses elicited by SYK(L)13. The role of the linker insert in SYK has been proposed with two functions. In one, the linker insert enhances the ability of kinases to interact with immune receptor tyrosine activated motifs carrying receptors14. In another, it contains a signal sequence that allows kinases transport to the nucleus15.
Accumulating evidences suggest an active but opposing role of SYK (L) and SYK (S) isoforms on the growth properties of cancer cells, possibly due to different biologic functions for the two isoforms16. Overexpression of SYK(L) inhibits cell proliferation and invasion in breast cancer, but SYK(S) did not affect cell invasion15. Expression of two types of variant isoforms of SYK is also different in hepatocellular carcinoma, with decreased expression of SYK(L) and increased expression of SYK(S) in hepatocellular carcinoma compared to that in the normal liver tissue. Overexpression of SYK(S) induce epithelial mesenchymal transition and promote lung metastasis of hepatocellular carcinoma cells17.
The different biological functions of SYK(L) and SYK(S) may be attributed to their different intracellular localization, SYK(L) exists in the nucleus and cytoplasm, while SYK(S) exists only in the cytoplasm15,18. The interdomain B of SYK(L) contains important nuclear localization signals, and its tumor suppressor function is closely related to its nuclear localization, however, due to the alternatively splicing of SYK (S) within interdomain B, the 23 amino acid sequences required for nuclear localization are lacking19. The 5-year survival rate was found to be significantly lower among patients with negative SYK expression than in those with nuclear SYK expression20. However, the biological significance of SYK(S) in carcinogenesis is unclear. Decreased SYK expression was observed in cervical cancer21. In our previous research, we found that SYK was strongly expressed in well-differentiated keratinized area in the center of cancer nests, and weakly expressed in poor differentiated area in the peripheral of cancer nests in cervical cancer tissues (unpublished data). However, the expression and biological functions of SYK variant isoforms in cervical cancer are not fully understood. The aim of this study is to investigate the effects of SYK variant isoforms in cervical cancer.
Materials and methods
Clinical sample collection
The fresh tissue samples used in this study were from biobank of Affiliated Cancer Hospital of Xinjiang Medical University (Urumqi, China) between 2017 and 2022, containing 30 cases of normal cervical tissues and 40 cases of cervical cancer tissues. Paraffin tissues was provided by Department of Pathology of Affiliated Cancer Hospital of Xinjiang Medical University. All patients did not receive radiotherapy or chemotherapy before surgery. The clinical stage of the patients was determined according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO). All subjects signed an informed consent form, and this study was approved by the Ethics Committee of Affiliated Cancer Hospital of Xinjiang Medical University (approval number 2023BC030). All methods were performed in accordance with relevant guidelines and regulations.
Cell culture and transfection
Human cervical cancer cell lines SiHa, HeLa, CaSki, C33A and human cervical epithelial immortalized cell line H8 were obtained from the Pathology Laboratory of Xinjiang Medical University. CaSki cell line was purchased from the Cell Bank of the Chinese Academy of Sciences. DMEM (Gibco, USA) was used to cultivate HeLa, SiHa, C33A and H8 cell lines, and RPMI-1640 medium (Gibco, USA) was used to cultivate CaSki cell line. All cell lines were cultured at 37 ℃ in a humidified incubator containing 5% CO2. sh-SYK(L), sh-SYK(S) and negative control (sh-NC) were designed and synthesized by Rebel Bio Ltd. (RiboBio, China). SYK(L) target sequences: 5′-CCTGGCCACAGAAAGTCCT-3′; SYK(S) target sequences: 5′-GGTTCCCATCCTGCGTCCT-3′. When cell confluence rate reached to 60–70%, cells were transfected with lipofectamine8000 (Beyotime, China).
Quantitative real‑time polymerase reaction (qRT‑PCR)
Total RNA from tissues and cells was extracted using TriQuick Reagent (Solarbio, China) and cDNA was synthesised using cDNA Synthesis SuperMix (TransGen Biotech, China) according to the manufacturer’s instruction. cDNA was reversed transcription using PerfectStart® Green, the cDNA was amplified using PerfectStart® Green qPCR SuperMix (TransGen Biotech, China). The primer sequences were as follows: SYK(F), 5′-CATACTCCCTATCAGCCAGCCC-3′; SYK(R), 5′-TGACAATGCAGCTGTAGCCTCT-3′; NM_003177.7(SYK L) F, 5′-CTTGGTCAGCGGGTGGAATA-3′; NM_003177.7(SYK L) R, 5′-GGCAGGGGAGGACTTTCTGT-3′; NM_001135052.4(SYK S) F, 5′-GTTCCCATCCTGCGTCCTC-3′; NM_001135052.4(SYK S) R, 5′-CGTAGGGGCTCTCGTACACCTC-3′. GAPDH was used as housekeeping gene, the fold changes of SYK(L) or SYK(S) are given by 2–ΔΔCT. The patients were divided into high and low expression groups according to the median of the relative expression of SYK(L) or SYK(S) in cervical cancer tissues. The expression of SYK(L) or SYK(S) with greater than the median was considered as higher, and the expression of SYK(L) or SYK(S) with less than or equal to the median was considered as lower.
Nucleocytoplasmic fractionation
The extraction and isolation of nuclear and cytoplasmic protein were carried out by using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, China). The cytoplasmic and nuclear fractions were analyzed by immunoblotting. PARP was used as the internal reference for the nuclear fraction, and α-tubulin was used as that for the cytoplasmic fraction.
Immunofluorescence (IF) staining
Tissue paraffin sections were closed for 30 min at room temperature after routine steps of dewaxing and washing, antigen repair and hydrogen peroxide (H2O2) blocking, and SYK primary antibody was added overnight at 4 ℃. Then the fluorescently labelled secondary antibody was incubated at 37 ℃ for 1 h. Afterwards, the nuclei were re-stained with 4–6-diamidino-2-phenylindole (DAPI) for 15 min and photographed under fluorescence microscope.
Cell counting kit‑8 (CCK‑8) assay
Cells were inoculated into 96-well plates at a density of 2 × 103 cells/well, and after incubation for 0, 24, 48 and 72 h, 10 μL CCK-8 solution (Elabscience, China) was added to each well, and incubation was continued in the incubator for 2 h, and then a spectrophotometer was used to evaluate the absorbance at 450 nm.
5‑Ethynyl‑20‑deoxyuridine (EdU) assay
After cell transfection, cells were digested and counted and inoculated in 24-well plates, about 3 × 104 cells per well, incubated at 37 ℃, 5% CO2 incubator for 24 h and then labelled with EdU using Cell Proliferation Detection Kit (Beyotime, China), each well was incubated for 2 h by adding 100 μL of EdU-containing medium, and then treated with fixation (4% paraformaldehyde) and permeabilization (0.3% Triton X-100), 100 μL Click reaction solution was added, and finally the nuclei were stained using Hoechst. Cell counting was performed under fluorescence microscope (Leica, Germany).
Cell migration (Scratch wound) assay
The migration of cells after transfection was detected by the wound scratch method. Cells were cultured into confluent monolayers, placed in 6-well plates and synchronized in 1% FBS for 24 h. Cell strips 300–500 μm wide were scratched in the wells with a standard 10 μL pipette tip, and then the floating cells were washed away with PBS. Culture medium with 10% FBS was added to the wells and incubated for 0 and 24 h, respectively. Finally, the wound area was calculated using Image J software (NIH, Bethesda, MD, USA).
Transwell assay
Transwell experiments were performed in 24-well plates, matrix gel mix (BD Biosciences, USA) was used to detect invasion, and resuspension cells with serum-free medium using transwell chambers (8 μm pore size, Corning, USA), the upper chamber inoculated with 5 × 104 cells, and the lower chamber added with 600 μL of medium containing 10% FBS. After incubation for 20–24 h (to assess migratory ability) or 30–36 h (to assess invasive ability), the cells were fixed with 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet for 20 min, washed with PBS, and the upper chamber of the transwell was wiped with a cotton swab. Cell counting was next performed under a microscope (Leica, Germany).
Western blot
One percent PMSF added to RIPA lysis buffer (Beyotime, China) was used to extract total proteins, Protein concentrations were determined using a BCA protein quantification kit (Beyotime, China). Separated by 10% SDS-PAGE gel electrophoresis and transferred to polyvinylidene fluoride (PVDF, Sigma-Aldrich LLC.; 3,010,040,001) membranes, which were then closed with 5% skimmed milk or bovine serum protein (for phosphorylated proteins) for 2 h at room temperature and incubated with the primary antibody overnight at 4 °C. The applied primary antibodies were as follows: anti-SYK (#sc1240, Santa Cruz, USA), anti-PI3Kinase p110α (#4255, Cell Signaling Technology, USA), anti-Phospho-PI3Kinase p85α (#ab182651, Abcam, UK), anti-Phospho-Akt (Ser473) (#9271, Cell Signaling Technology, USA), anti-Akt (#4691, Cell Signaling Technology, USA), anti-E-cadherin (#ab40772, Abcam, UK), anti-N-cadherin (#ab76011, Abcam, UK), anti-MMP2 (sc-13595, Santa Cruz, USA), anti-α-Tubulin (11224–1-AP , Proteintech, China), anti-PARP (#9532, Cell Signaling Technology, USA), anti-β-actin (#66009–1-Ig, Proteintech, China). Afterwards, horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated for 1 h at room temperature. Next, the membrane was rinsed 5 times with TBST and then visualized by the Western Bright ECL detection system (Bio-Rad, USA). ImageJ software was used to quantify the protein levels.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 and SPSS 26.0 software. Measurements were expressed as mean ± SD. Comparisons between two groups were statistically analyzed using Student’s t-test, comparisons between three groups were analyzed using One-way ANOVA. The correlations between SYK(L) and SYK(S) expression and clinical pathology characteristics were analyzed using Chi-square test and rank sum test. For survival analysis, Kaplan–Meier curve was generated with the log-rank test. The prognostic significance of both SYK variant isoforms was also evaluated by Cox regression analysis. P values < 0.05 were considered to be statistically significant.
Results
The expression of SYK(L) and SYK(S) in cervical cancer tissues
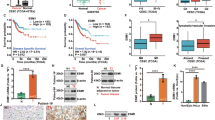
We first detected the expression of SYK in normal cervical tissues and CC, and the results showed that SYK expression was reduced in CC (Fig. 1A,B). Next, the expression of SYK(L) and SYK(S) in normal cervical tissues and CC was detected by qRT-PCR. The results showed that SYK(L) expression was significantly down-regulated in CC, while the decreasing trend of SYK(S) was not obvious (Fig. 1C,D). The relative expression abundance of SYK(L) in CC (0.41 ± 0.09) was 2.7 fold lower than that in normal cervical tissues (1.12 ± 0.23), whereas SYK(S) expression abundance in CC (0.82 ± 0.13) was 1.2 fold lower than that in normal cervical tissues (0.99 ± 0.26). The expression of SYK (L) is higher than that of SYK (S) in normal cervical tissues, while the markedly opposite trend is observed in CC (Fig. 1E,F).
The expression of SYK, SYK(L) and SYK(S) (A) Relative protein expression of SYK in normal cervical tissues (n = 4) and CC (n = 4). (B) Relative mRNA expression of SYK in normal cervical tissues and CC. (C, D) Relative mRNA expression of SYK(L) and SYK(S) in normal cervical tissues and CC. (E) Relative mRNA expression of SYK(L) and SYK(S) in normal cervical tissues. (F) Relative mRNA expression of SYK(L) and SYK(S) in CC. (B–F) Normal cervical tissues n = 30; CC n = 40. Data are shown as mean ± SD. * P < 0.05, ** P < 0.01, ****P < 0.0001. CC Cervical cancer.
Correlation of SYK(L) and SYK(S) with clinical pathology variables and prognostic value
To verify the functions of SYK(L) and SYK(S), we correlated SYK(L) or SYK(S) expression in cervical cancer specimens with clinical pathological parameters. Our results showed that SYK(L) high expression was associated with better tumor differentiation and superficial muscular infiltration, thereby predicting a favorable clinical outcome. By contrast, SYK(S) high expression was strongly correlated with poor tumor differentiation, lymph node metastasis, deep muscular infiltration and advanced FIGO stage (Table S1). Univariate analysis of 3-year survival revealed that lymph node metastasis, muscular infiltration, SYK(L) and SYK(S) expression were associated with overall survival (OS) (Table 1). Kaplan‑Meier analysis disclosed that the SYK(L) high expression had a better prognosis, whereas the SYK(S) high expression predicted a worse disease outcome (Fig. 2A,B). Cox multivariate analysis revealed that SYK(L) expression was an independent predictor of OS (Table 2).
Subcellular distribution of SYK
qRT‑PCR was used to detect the expression of SYK(L) and SYK(S) in four cervical cancer cell lines SiHa, HeLa, CaSki, C33A and H8. The results showed that SYK(L) expression was highest in SiHa cell line and SYK(S) expression was highest in CaSki cell line among the four cervical cancer cell lines and H8 (Fig. 3A, Fig. S1). Therefore, we chose SiHa and CaSki as target cells for SYK(L) and SYK(S) plasmid transfection, the results showed that SYK(L) or SYK(S) knockdown decreased the expression of SYK variant isoforms in SiHa and CaSki cells, respectively, compared with the NC and control, sh-SYK(L)-2 showing the highest knockdown efficiency, leading us to select it for further tests (Fig. 3B,C). Nucleocytoplasmic fractionation experiments displayed that SYK expression was reduced in the nucleus of SiHa cell line or in the cytoplasm of CaSki cell line after knockdown SYK (L) or SYK (S). (Fig. 4A,B). These results partially suggested that SYK (L) is mainly localized in the nucleus, while SYK (S) is mainly localized in the cytoplasm in the subjected cervical cancer cell lines. Immunofluorescence results showed that SYK was mainly localized in the cytoplasm of cervical cancer and normal cervical tissues, and partially in the nucleus of cervical intraepithelial neoplasia (CIN). (Fig. 4C). The results indicated that the cellular localization of SYK may be related to its biological function.
The expression of SYK(L) and SYK(S) in cervical cancer cells and H8 (A) The relative expression of SYK(L) and SYK(S) in cervical cancer cells and H8 was detected by qRT-PCR, GAPDH was used as a loading control. (B, C) qRT-PCR analysis of transfection efficiency of SiHa and CaSki cells. (mean ± SD; n = 3; * P < 0.05, ** P < 0.01).
Differential subcellular distribution of SYK in cervical cancer cells. (A, B) SiHa [SYK(L) positive], CaSki [SYK(S) positive] and knockdown SYK(L) or SYK(S) were subjected to subcellular fractionation. The cytoplasmic (C) and nuclear (N) fractions were then processed together with whole cell lysate (W) for immunoblotting. PARP and α-Tubulin were used as nuclear and cytoplasmic markers, respectively. (C) Localization of SYK in different cervical tissues by immunofluorescence. SYK expressed in SiHa and CaSki cell lines were immunostained with anti-SYK antibody that does not recognize both SYK(L) and SYK(S) (green), cell nuclei were stained with DAPI (blue). Scale bar, 200 μm. (mean ± SD; n = 3; * P < 0.05).
Opposing effect of SYK(L) and SYK(S) on proliferation, migration and invasion
To assess the proliferative effect of SYK(L) and SYK(S) on cervical cancer cells, we performed CCK8 and EdU assays. CCK8 assay showed that SYK(L) knockdown was significantly enhanced cell viability, whereas SYK(S) knockdown decreased cell viability compared with NC and Control (Fig. 5A,B). To further validate the cell proliferation, EdU assay was performed. The results displayed that the number of proliferating cell was significantly increased after SYK(L) knockdown, while which was diminished after SYK(S) knockdown (Fig. 5C,D). These data indicate that SYK(L) inhibits the proliferation of cervical cancer cells, while SYK(S) may promote the proliferation. The migration and invasion effect of SYK(L) and SYK(S) was further evaluated by wound healing and transwell. The results demonstrated that SYK(L) knockdown increased the migration and invasion in SiHa cell line, whereas SYK(S) knockdown decreased those in CaSki cell line (Fig. 5E,H).
SYK(L) and SYK(S) exhibited opposing activities in cervical cancer cells (A–D) CCK-8 and EdU were used to detect the effect of SYK(L) and SYK(S) knockdown on cell proliferation ability. Scale bar, 200 μm. (E, F) Scratch was made in 6-well plates, and 24 h later, light microscope was used to examine the migration ability of each group of cells. Scale bar, 200 μm. (G, H) Transwell detected the migration and invasion ability of each group of cells. Scale bar, 100 μm. (mean ± SD; n = 3; * P < 0.05, ** P < 0.01).
SYK(L) and SYK(S) regulates epithelial-mesenchymal transition (EMT) in cervical cancer cells
EMT reflect the epithelial and mesenchymal status of cells, and is closely related to the invasion and metastasis. SYK(L) or SYK(S) exhibited the opposite effects on proliferation, migration and invasion in cervical cancer cells, therefore, we tested the EMT-related proteins expression by western blot. The result suggested that E-cadherin expression was decreased, N-cadherin and MMP-2 expression was elevated after SYK(L) knockdown, whereas E-cadherin expression was elevated, N-cadherin and MMP-2 expression was decreased after SYK(S) knockdown, compared with those in NC and control (Fig. 6A,B).
SYK (L) and SYK (S) regulated EMT and participated PI3K/AKT signaling pathway (A, B) Western blot and quantification of E-cadherin, N-cadherin and MMP-2 in SiHa and CaSki cells. (C) Western blot and quantitative analysis of PI3K, p-AKT and AKT proteins in normal cervical tissues and cervical cancer tissues. (D-E) Western blot and quantification of PI3K, p-PI3K p85α, p-AKT and AKT proteins in SiHa and CaSki cells. β-actin was used as a loading control. (mean ± SD; n = 3; * P < 0.05, ** P < 0.01). EMT epithelial mesenchymal transition, CC Cervical cancer.
SYK(L) and SYK(S) regulates EMT through PI3K/AKT signaling pathway in cervical cancer cells
Previous studies have shown that PI3K/AKT signaling pathway is closely associated with the development of cervical cancer22. Therefore, we firstly detected the expression of PI3K/AKT signaling pathway proteins. The results indicated that PI3K p110, p-AKT and AKT expression were significantly higher in cervical cancer tissues than that in the normal cervical tissues (Fig. 6C). Next, we explored whether SYK variant isoforms were involved in cervical cancer progression through PI3K/AKT signaling pathway. The results showed that SYK(L) knockdown increased the levels of phosphorylated PI3K p85α and AKT in SiHa cell line. In contrast, SYK(S) knockdown downregulated the levels of phosphorylated PI3K p85α and AKT in CaSki cell line (Fig. 6D,E).
Next, we sought to determine the signaling mechanisms involved in SYK-mediated EMT, increasing evidence suggests that activated PI3K/AKT pathway bolster EMT in cervical cancer23,24,25. We treated with PI3K inhibitor LY294002 in SiHa cell line and found that it partially abolished the migration (Fig. 7A), reversed the expression of E-cadherin, N-cadherin, MMP-2, and p-AKT (Fig. 7B). Then, PI3K activator growth factor-1 (IGF-1) was used to treat in CaSki cell line, and found that it enhanced the migration (Fig. 7C). Similarly, the expression of E-cadherin, N-cadherin, MMP-2, and p-AKT was restored (Fig. 7D). Based on these findings, we speculated that SYK(L) and SYK(S) regulate EMT through PI3K/AKT signaling pathway in cervical cancer.
SYK(L) and SYK(S) regulated EMT through PI3K/AKT signaling pathway (A) Wound healing assays were used to analyze migration ability in SiHa cell line after SYK(L) knockdown treated with or without LY294002 (20 μM). Scale bar, 200 μm. (B) Relative expression of E-cadherin, N-cadherin, MMP-2, p-AKT and AKT in SiHa cell line after SYK(L) knockdown treated with or without LY294002. (C) Migration ability of CaSki cell line after SYK (S) knockdown treated with or without IGF-1 (100 ng/mL). Scale bar, 200 μm. (D) Relative expression of E-cadherin, N-cadherin, MMP-2, p-AKT and AKT in CaSki cell line after SYK(S) knockdown treated with or without IGF-1. β-actin was used as a loading control. (mean ± SD; n = 3;* P < 0.05, ** P < 0.01, *** P < 0.001).
Discussion
Invasion and metastasis remain the main factors affecting the long-term survival of cervical cancer patients26. Therefore, there is an urgent need to search for molecular markers for the prognosis of cervical cancer patients. SYK is now considered closely related to the development of cancer, and studies have shown that it plays a role in tumor suppression or promotion, respectively27. The reason for this dual reputation may be related to its two variant isoforms. So far, the roles of SYK variant isoforms, namely SYK(L) and SYK(S), in cancer are still unclear and even controversial8,28,29,30. Consistent with the results obtained in hepatocellular carcinoma17, we found in the present study that SYK (L) and SYK (S) also exhibited the differential expression in cervical cancer. The expression of SYK (L) mRNA in cervical cancer tissues decreased by 2.7 fold compared with normal cervical tissues, while SYK (S) mRNA only lowered by 1.2 fold, the expression of SYK(S) was significantly higher than that of SYK(L) in cervical cancer tissues. It prompts that SYK(L) plays an inhibitory role, yet SYK (S) plays a role of tumor activation in cervical cancer. Aberrant hypermethylation represents an epigenetic mechanism that inactivates tumor suppressor genes. Ma et al.31 found that the loss of expression of SYK gene in lung cancer is due to high methylation of the promoter. The decrease in SYK (L) expression resulting from promoter methylation was an adverse prognostic factor among patients with human hepatocellular carcinoma32. Indeed, down-regulation of SYK(L) has a positive correlation with metastasis in multiple cancer types, including colorectal cancer33 and lung cancer34, while increased SYK(S) expression was markedly associated with the risk of liver metastasis in colon cancer35. The high expression of SYK (S) in tumor tissue was believed to be associated with overexpressed genes related to translation and mitochondria, and down regulated genes implicated in the progression of mitosis16.
In order to further explore the functions of SYK variant isoforms, Subsequently, we analyzed the relationship between the expression of SYK(L) and SYK(S) and the clinical pathological variables in the patients with cervical cancer. The data hinted that SYK(L) expression was higher in the group with middle differentiation and superficial muscular infiltration and revealed a clear upward trend in the group with tumor size of ≤ 4 cm, and meanwhile evaluation of 3-year survival rate also demonstrated that the patients with high SYK(L) expression had a better prognosis, which supported SYK(L) as a tumor suppressor. While SYK(S) expression was higher in the patients with the lower clinical stage, and related to lymph node metastasis, deeper muscular and vascular invasion, and moreover, analysis about 3 year survival also displayed that the patients with high SYK(S) expression had a poorer prognosis. which favored SYK(S) as a tumor promotor.
The functional differences between SYK(L) and SYK(S) may be mainly related to the position in cells. SYK(L) is located both in the cytoplasm and the nucleus, whereas SYK(S) only exists in the cytoplasm15,36. Due to the lack of specific antibodies against the two SYK isoforms, it seems feasible to evaluate the expression of SYK in cytoplasmic and nuclear by nucleocytoplasmic fractionation and immunoblotting with a pan-SYK antibody. Ovarian cancer patients with high nuclear Syk expression have better overall survival9. In this study, the result of immunofluorescence showed that SYK expression was localized in the cytoplasm of cervical cancer and normal cervical epithelial cells, and partially in the nucleus of CIN. It suggests that the expression of SYK gradually disappears in the nucleus and increases in the cytoplasm as precancerous lesions progressing into invasive cervical cancer. SYK can be found both in the cytoplasm and in the nucleus due to an unconventional shuttling sequence. Localization is relevant, as nuclear expression correlates with better prognosis37.The results of nucleocytoplasmic fractionation showed that, in agreement with studies of breast cancer, knocking down the isoform in SiHa with high expression of SYK (L) caused a decrease in the expression of SYK in the nucleus, while interfering the isoform in CaSki with high expression of SYK (S) significantly reduced the expression of SYK in the cytoplasm15. SYK(S) was distributed mainly in cytoplasm, whereas SYK(L) was mainly localized in the nucleus. However, whether SYK cytoplasmic and nuclear distribution offers the prognostic value in cervical cancer needs to be further investigated.
To further understand the roles of SYK (L) and SYK (S) in the progression of cervical cancer, we knocked down SiHa with high expression of SYK (L) and performed EdU, wound healing, and transwell. The results exhibited that SYK(L) knocking down promoted the proliferation, migration, and invasion. However, knocking down CaSki with high expression of SYK (S) showed the opposite biological functions. Coincided with the aforementioned ones in cervical cancer tissue, the results strongly suggest that SYK (L) can play a potential role as a tumor suppressor gene, while SYK (S) as an oncogene. Wang et al.15 reported that SYK(L) exerted a tumor-suppressive role and SYK(S) promoted tumor development in breast cancer. The overexpression of SYK (S) promoted the proliferation and invasion in hepatocellular carcinoma17. EMT activation may enhance cancer cell motility and metastasis38. In this study, SYK(L) knockdown down-regulated E‐cadherin expression, and increased the expression of N‐cadherin and MMP-2 in SiHa cell line, whereas SYK(S) showed the opposite effects in CaSki cell line. EMT is induced by various signaling pathways39, including PI3K/AKT signaling pathway40. Under normal conditions, the activation of PI3K/AKT pathway is tightly controlled and is dependent on extracellular growth signals as well as the supply of amino acids and glucose41, whereas over-activation of the signaling cascade promotes the proliferation and invasion in a wide range of cancer cells, including prostate cancer42, pancreatic43 and lung cancer44. PI3K/AKT pathway activation is associated with a number of factors, including RTK family45, toll-like receptors (TLRs)46 and PTK family47. It is found that SYK belongs to the PTK family and is an upstream effector of PI3K/AKT signaling pathway48. when SYK was phosphorylated due to increased H2O2 entering into the cell, the PI3K/AKT signaling pathway was activated, promoting invasion and metastasis in HeLa49. In this study, the protein expression of PI3Kp110, p-AKT and AKT in cervical cancer tissues were significantly higher than those in the normal group, suggesting that there is an aberrant activation of PI3K/AKT pathway in cervical cancer development. Knocking down SYK (L) significantly enhanced the expression of PI3K/AKT pathway-related proteins and the migration and invasion, treatment with PI3K inhibitor LY294002 partially restored the biological effects in SiHa cell line. While SYK (S) presented the opposite effect, treatment with PI3K activator IGF-1 partially restored the biological effects in CaSki cell line. Accumulating data suggests that the linker insert provides a crucial role in the biological activity of SYK, this importance has been ascribed to the presence of tyrosine phosphorylation sites14. Checkpoint kinase 1 phosphorylated SYK(L) at Ser295 in hepatocellular carcinoma5. It has been reported that Ser291 is the main site of SYK phosphorylation, and protein kinase C affects SYK activity by phosphorylating SYK Ser291 in colorectal cancer cells50. Teresa et al.51 found that although SYK (S) lacks the linker insert like ZAP70, unlike ZAP70, SYK (S) was involved in CD19-PI3K signaling. The function of SYK (S) was not caused by the absence of crucial tyrosine phosphorylation sites, but may be related to its reduced ability to bind phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) in vitro and in vivo14. This requires us to conduct more in-depth research in the future. The above suggests that the variant isoforms of SYK may regulate EMT through PI3K/AKT signaling pathway. Some studies have shown that SYK activates PI3K/AKT pathway to drive the growth and survival of EBV (Epstein-Barr Virus) + B-cell lymphomas52. This is consistent with the results of this study.
Taken together, using cervical cancer cell lines and human cervical cancer tissue samples, we provided the compelling evidence that SYK(L) or SYK(S) were strongly correlated with clinical stage, infiltration and lymph node metastasis, which regulate EMT to involve in the progression of cervical cancer through PI3K/AKT signaling pathway. The variant isoforms of SYK could be a potentially effective target for the treatment of cervical cancer. In addition, in vivo were not included in our study for the time being, which warrants further subsequent studies.
Data availability
The data that support the findings of this study are available on request from the corresponding authors.
Abbreviations
- SYK:
-
Spleen tyrosine kinase
- EMT:
-
Epithelial mesenchymal transition
- CC:
-
Cervical cancer
- H2O2 :
-
Hydrogen peroxide
- DAPI:
-
4–6-Diamidino-2-phenylindole
- CIN:
-
Cervical intraepithelial neoplasia
- OS:
-
Overall survival
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. https://doi.org/10.3322/caac.21763 (2023).
Li, H., Wu, X. & Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 27 (4), e43. https://doi.org/10.3802/jgo.2016.27.e43 (2016).
Shao, Y., Zhang, S., Zhang, Y. & Liu, Z. Recent advance of spleen tyrosine kinase in diseases and drugs. Int. Immunopharmacol. 90, 107168. https://doi.org/10.1016/j.intimp.2020.107168 (2021).
Coopman, P. J. et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 406 (6797), 742–747. https://doi.org/10.1038/35021086 (2000).
Hong, J. et al. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J. Clin. Invest. 122 (6), 2165–2175. https://doi.org/10.1172/jci61380 (2012).
Chuanliang, P. et al. Syk expression in non-small-cell lung cancer and its relation with angiogenesis. J. Cancer Res. Ther. 12 (2), 663–666. https://doi.org/10.4103/0973-1482.154082 (2016).
Tang, H. et al. Silencing of microRNA-27a facilitates autophagy and apoptosis of melanoma cells through the activation of the SYK-dependent mTOR signaling pathway. J. Cell Biochem. 120 (8), 13262–13274. https://doi.org/10.1002/jcb.28600 (2019).
Tan, G. et al. Spleen tyrosine kinase facilitates the progression of papillary thyroid cancer regulated by the hsa_circ_0006417/miR-377–3p axis. Environ. Toxicol. 39 (1), 421–434. https://doi.org/10.1002/tox.23982 (2024).
Zhang, S., Deen, S., Storr, S. J., Yao, A. & Martin, S. G. Expression of Syk and MAP4 proteins in ovarian cancer. J. Cancer Res. Clin. Oncol. 145 (4), 909–919. https://doi.org/10.1007/s00432-019-02856-9 (2019).
Rohila, D. et al. Syk inhibition reprograms tumor-associated macrophages and overcomes gemcitabine-induced immunosuppression in pancreatic ductal adenocarcinoma. Cancer Res. 83 (16), 2675–2689. https://doi.org/10.1158/0008-5472.Can-22-3645 (2023).
Boudria, R. et al. Regulatory interplay between Vav1, Syk and β-catenin occurs in lung cancer cells. Cell Signal 86, 110079. https://doi.org/10.1016/j.cellsig.2021.110079 (2021).
Zhang, J. et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481 (7381), 329–334. https://doi.org/10.1038/nature10733 (2012).
Rowley, R. B., Bolen, J. B. & Fargnoli, J. Molecular cloning of rodent p72Syk. Evidence of alternative mRNA splicing. J. Biol. Chem. 270 (21), 12659–12664. https://doi.org/10.1074/jbc.270.21.12659 (1995).
Latour, S., Zhang, J., Siraganian, R. P. & Veillette, A. A unique insert in the linker ___domain of Syk is necessary for its function in immunoreceptor signalling. Embo J. 17 (9), 2584–2595. https://doi.org/10.1093/emboj/17.9.2584 (1998).
Wang, L. et al. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 63 (15), 4724–4730 (2003).
Denis, V. et al. Targeting the splicing isoforms of spleen tyrosine kinase affects the viability of colorectal cancer cells. PLoS ONE 17 (9), e0274390. https://doi.org/10.1371/journal.pone.0274390 (2022).
Hong, J. et al. Expression of variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. Cancer Res. 74 (6), 1845–1856. https://doi.org/10.1158/0008-5472.Can-13-2104 (2014).
Ulanova, M. et al. Syk tyrosine kinase participates in beta1-integrin signaling and inflammatory responses in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 288 (3), L497-507. https://doi.org/10.1152/ajplung.00246.2004 (2005).
Zhou, F., Hu, J., Ma, H., Harrison, M. L. & Geahlen, R. L. Nucleocytoplasmic trafficking of the Syk protein tyrosine kinase. Mol. Cell Biol. 26 (9), 3478–3491. https://doi.org/10.1128/mcb.26.9.3478-3491.2006 (2006).
Nakashima, H. et al. Clinical significance of nuclear expression of spleen tyrosine kinase (Syk) in gastric cancer. Cancer Lett. 236 (1), 89–94. https://doi.org/10.1016/j.canlet.2005.05.022 (2006).
Zhao, S., Sun, G., Tony, P. W., Ma, D. & Zhao, C. Expression and methylation status of the Syk gene in cervical carcinoma. Arch. Gynecol. Obstet. 283 (5), 1113–1119. https://doi.org/10.1007/s00404-010-1546-6 (2011).
Jiang, E. et al. Dehydrocostus lactone inhibits proliferation, antiapoptosis, and invasion of cervical cancer cells through PI3K/Akt signaling pathway. Int. J. Gynecol. Cancer 25 (7), 1179–1186. https://doi.org/10.1097/igc.0000000000000474 (2015).
Yang, X. & Zhu, W. ERBB3 mediates the PI3K/AKT/mTOR pathway to alter the epithelial-mesenchymal transition in cervical cancer and predict immunity filtration outcome. Exp. Ther. Med. 25 (4), 146. https://doi.org/10.3892/etm.2023.11845 (2023).
Chen, L. et al. TIM-1 promotes proliferation and metastasis, and inhibits apoptosis, in cervical cancer through the PI3K/AKT/p53 pathway. BMC Cancer 22 (1), 370. https://doi.org/10.1186/s12885-022-09386-7 (2022).
Zhang, X., Liu, S. & Zhu, Y. A-kinase-interacting protein 1 promotes EMT and metastasis via PI3K/Akt/IKKβ pathway in cervical cancer. Cell Biochem. Funct. 38 (6), 782–791. https://doi.org/10.1002/cbf.3547 (2020).
Asthana, S., Busa, V. & Labani, S. Oral contraceptives use and risk of cervical cancer-A systematic review & meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 247, 163–175. https://doi.org/10.1016/j.ejogrb.2020.02.014 (2020).
Krisenko, M. O. & Geahlen, R. L. Calling in SYK: SYK’s dual role as a tumor promoter and tumor suppressor in cancer. Biochim. Biophys. Acta 1853 (1), 254–263. https://doi.org/10.1016/j.bbamcr.2014.10.022 (2015).
Black, M. et al. Spleen tyrosine kinase expression is correlated with human papillomavirus in head and neck cancer. Oral Oncol. 101, 104529. https://doi.org/10.1016/j.oraloncology.2019.104529 (2020).
Wang, T., Xu, Y., Liu, X., Zeng, Y. & Liu, L. miR-96–5p is the tumor suppressor in osteosarcoma via targeting SYK. Biochem. Biophys. Res. Commun. 572, 49–56. https://doi.org/10.1016/j.bbrc.2021.07.069 (2021).
Aguirre-Ducler, A. et al. Tumor cell SYK expression modulates the tumor immune microenvironment composition in human cancer via TNF-α dependent signaling. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-005113 (2022).
Ma, L. et al. The relationship between methylation of the Syk gene in the promoter region and the genesis of lung cancer. Clin. Lab. 56 (9–10), 407–416 (2010).
Yuan, Y. et al. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin. Cancer Res. 12 (22), 6687–6695. https://doi.org/10.1158/1078-0432.Ccr-06-0921 (2006).
Ni, B. et al. Alternative splicing of spleen tyrosine kinase differentially regulates colorectal cancer progression. Oncol. Lett. 12 (3), 1737–1744. https://doi.org/10.3892/ol.2016.4858 (2016).
Gao, D. et al. Spleen tyrosine kinase SYK(L) interacts with YY1 and coordinately suppresses SNAI2 transcription in lung cancer cells. Febs J. 285 (22), 4229–4245. https://doi.org/10.1111/febs.14665 (2018).
Coebergh van den Braak, R. R. J. et al. High mRNA expression of splice variant SYK short correlates with hepatic disease progression in chemonaive lymph node negative colon cancer patients. PLoS ONE 12 (9), e0185607. https://doi.org/10.1371/journal.pone.0185607 (2017).
Prinos, P. et al. Alternative splicing of SYK regulates mitosis and cell survival. Nat. Struct. Mol. Biol. 18 (6), 673–679. https://doi.org/10.1038/nsmb.2040 (2011).
Moncayo, G. et al. SYK inhibition blocks proliferation and migration of glioma cells and modifies the tumor microenvironment. Neuro Oncol. 20 (5), 621–631. https://doi.org/10.1093/neuonc/noy008 (2018).
Aiello, N. M. & Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 216 (5), 1016–1026. https://doi.org/10.1084/jem.20181827 (2019).
Deshmukh, A. P. et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.2102050118 (2021).
Karimi Roshan, M. et al. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie 165, 229–234. https://doi.org/10.1016/j.biochi.2019.08.003 (2019).
Danielsen, S. A. et al. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim. Biophys. Acta 1855 (1), 104–121. https://doi.org/10.1016/j.bbcan.2014.09.008 (2015).
Hashemi, M. et al. Targeting PI3K/Akt signaling in prostate cancer therapy. J. Cell Commun. Signal 17 (3), 423–443. https://doi.org/10.1007/s12079-022-00702-1 (2023).
Mortazavi, M., Moosavi, F., Martini, M., Giovannetti, E. & Firuzi, O. Prospects of targeting PI3K/AKT/mTOR pathway in pancreatic cancer. Crit. Rev. Oncol. Hematol. 176, 103749. https://doi.org/10.1016/j.critrevonc.2022.103749 (2022).
Almalki, W. H. Beyond the genome: lncRNAs as regulators of the PI3K/AKT pathway in lung cancer. Pathol. Res. Pract. 251, 154852. https://doi.org/10.1016/j.prp.2023.154852 (2023).
Sabbah, M. et al. RTK inhibitors in melanoma: From bench to bedside. Cancers (Basel) https://doi.org/10.3390/cancers13071685 (2021).
Huang, L. et al. Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway. Open Med. (Wars) 17 (1), 626–637. https://doi.org/10.1515/med-2022-0453 (2022).
Zhang, Q. L. et al. The protein tyrosine kinase inhibitor genistein suppresses hypoxia-induced atrial natriuretic peptide secretion mediated by the PI3K/Akt-HIF-1α pathway in isolated beating rat atria. Can. J. Physiol. Pharmacol. 99 (11), 1184–1190. https://doi.org/10.1139/cjpp-2020-0503 (2021).
Agrawal, R., Carpino, N. & Tsygankov, A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J. Cell Biochem. 104 (3), 953–964. https://doi.org/10.1002/jcb.21678 (2008).
Wang, Q. et al. AQP3 promotes the invasion and metastasis in cervical cancer by regulating NOX4-derived H(2)O(2) activation of Syk/PI3K/Akt signaling axis. J. Cancer 15 (4), 1124–1137. https://doi.org/10.7150/jca.91360 (2024).
Dangelmaier, C. et al. Phosphorylation of (Ser 291) in the linker insert of Syk negatively regulates ITAM signaling in platelets. Platelets 35 (1), 2369766. https://doi.org/10.1080/09537104.2024.2369766 (2024).
Sadras, T. et al. Developmental partitioning of SYK and ZAP70 prevents autoimmunity and cancer. Mol. Cell 81 (10), 2094–111.e9. https://doi.org/10.1016/j.molcel.2021.03.043 (2021).
Incrocci, R. et al. Epstein-Barr virus LMP2A utilizes Syk and PI3K to activate NF-κB in B-cell lymphomas to increase MIP-1α production. J. Med. Virol. 91 (5), 845–855. https://doi.org/10.1002/jmv.25381 (2019).
Acknowledgements
The authors thank all staffs at the biobank of Affiliated Cancer Hospital of Xinjiang Medical University for their effort in collecting and managing the bio-sample.
Funding
This work was supported by National Natural Science Foundation of China (81660427, to Yonghua Shi) and Natural Science Foundation Project of Xinjiang Autonomous Region, China (2021D01A47, to Yonghua Shi).
Author information
Authors and Affiliations
Contributions
Y H S developed the study concept and design. B J L, Q X W, X W, H J W, X J N performed the experiments and collected the data. B J L, Q X W co-wrote the paper. B J L and Y H S contributed to the pathological analysis. Y H S supervised the research, the writing and revision of the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All clinical experiments in this study were approved by the Ethics Committee of Xinjiang Medical University Affiliated Cancer Hospital. All included patients have signed informed consent forms.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, B., Wang, Q., Wang, X. et al. Expression of variant isoforms of the tyrosine kinase SYK differentially regulates cervical cancer progression through PI3K/AKT pathway. Sci Rep 14, 29080 (2024). https://doi.org/10.1038/s41598-024-80579-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80579-9