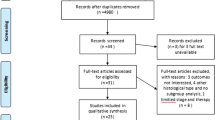
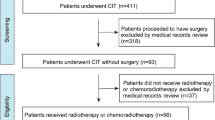
Abstract
To compare the survival outcomes of patients with stage T2N0M0 esophageal cancer treated with surgery alone versus those treated with neoadjuvant chemoradiotherapy followed by surgery. Patients with stage T2N0M0 esophageal cancer, who either underwent surgery alone or received neoadjuvant chemoradiotherapy followed by surgery, were extracted from the Surveillance, Epidemiology, and End Results database covering the period from 2000 to 2020. Cancer-specific survival (CSS) and overall survival (OS) between the two treatment groups were compared. A total of 583 patients were included: 267 (45.8%) received surgery alone, while 316 (54.2%) underwent neoadjuvant chemoradiotherapy followed by surgery. Prior to propensity score matching, no significant differences were observed between the surgery alone and neoadjuvant chemoradiotherapy groups in terms of 5-year CSS (60.86% vs. 59.02%; hazard ratio [HR] = 1.01, 95% confidence interval [CI]: 0.79–1.29; P = 0.916) and OS (50.64% vs. 49.81%; HR = 0.91, 95% CI: 0.75–1.12; P = 0.375). After propensity score matching, the 5-year CSS (66.43% vs. 56.67%; HR = 1.21, 95% CI: 0.89–1.64; P = 0.225) and OS (56.49% vs. 47.37%; HR = 1.09, 95% CI: 0.85–1.40; P = 0.481) remained statistically similar between the two groups. Subgroup analyses of patients with squamous cell carcinoma and adenocarcinoma revealed no significant differences in survival outcomes between the treatment modalities for either histological subtype. Neoadjuvant chemoradiotherapy followed by surgery does not confer a survival advantage over surgery alone in patients with stage T2N0M0 esophageal cancer, irrespective of histological subtype.
Similar content being viewed by others
Introduction
Esophageal cancer ranks as the sixth leading cause of cancer-related deaths globally, representing a significant burden on public health1. Recent advancements in screening techniques, including endoscopic surveillance and imaging modalities, have markedly improved early detection rates for esophageal cancer, thereby enhancing opportunities for timely therapeutic intervention2. Despite these advancements, the management of locally advanced esophageal cancer remains challenging, with neoadjuvant chemoradiotherapy followed by surgical resection currently recognized as the standard of care3,4.
However, while this multimodal approach has been validated in landmark trials such as the CROSS and NEOCRTEC5010 studies, these pivotal trials included only a small subset of patients with stage T2N0M0 disease3,4. As a result, the applicability of these findings to this specific subgroup remains unclear. This study aims to address this clinical uncertainty by comparing survival outcomes between patients undergoing surgery alone and those receiving neoadjuvant chemoradiotherapy followed by surgery, specifically in the context of stage T2N0M0 esophageal cancer. By doing so, this research seeks to provide more definitive evidence to guide treatment strategies and optimize patient outcomes in this distinct cohort.
Materials and methods
Study population and data source
This retrospective cohort study utilized data from the Surveillance, Epidemiology, and End Results (SEER) database, covering the period from 2000 to 2020. The SEER program, maintained by the National Cancer Institute, collects and publishes cancer incidence and survival data from population-based cancer registries, representing approximately 34.6% of the U.S. population. Patients included in the analysis met the following inclusion criteria: (1) histologically confirmed adenocarcinoma (SEER codes: 8140–8389, based on the International Classification of Diseases for Oncology, 3rd Edition) or squamous cell carcinoma (SEER codes: 8050–8089); (2) clinical stage T2N0M0 esophageal cancer, as defined by the American Joint Committee on Cancer (AJCC) staging manual; and (3) those who underwent surgical resection as part of their treatment.
Patients were excluded from the study if they met any of the following criteria: (1) those who received radiotherapy or chemotherapy as a standalone treatment; (2) patients who underwent definitive concurrent chemoradiotherapy without subsequent surgery; (3) those who did not receive any form of cancer-directed therapy; (4) individuals who received adjuvant therapies (either chemotherapy, radiotherapy, or both) after surgery; (5) patients treated with neoadjuvant radiotherapy alone or neoadjuvant chemotherapy alone. This approach ensured the selection of patients who either received surgery alone or neoadjuvant chemoradiotherapy followed by surgery, to directly compare these treatment modalities.
Key demographic and clinical characteristics, including age, gender, race, primary tumor site, histological subtype, tumor grade, and treatment modality, were extracted for comprehensive analysis. The cohort was then stratified into two distinct groups: patients who underwent surgery alone and those who received neoadjuvant chemoradiotherapy followed by surgery. Age was stratified based on the median value, while other categorical variables were analyzed to determine baseline comparability between the two groups.
Ethics approval was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital. Informed consent was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Statistical analyses were conducted using SPSS Statistics (Version 26.0, IBM Co., Armonk, NY, USA) and R software (version 4.4.0). The distribution of categorical variables, including age, gender, race, primary tumor site, histological type, and tumor grade, between the two treatment groups was compared using either the χ² test or Fisher’s exact test, as appropriate. Factors associated with the choice of neoadjuvant chemoradiotherapy followed by surgery were evaluated using logistic regression analysis.
Overall survival (OS) served as the primary endpoint of our study, defined as the time until death from any cause, as recorded in the SEER database. Cancer-specific survival (CSS) was designated as the secondary endpoint, defined as the duration until death attributed specifically to esophageal cancer within the same database.
OS and CSS outcomes were estimated using the Kaplan-Meier method. Log-rank tests were performed to assess differences in survival between the surgery alone group and the neoadjuvant chemoradiotherapy followed by surgery group. Cox proportional hazards regression models were employed for multivariable analysis to identify independent prognostic factors influencing CSS and OS. Adjustments were made for relevant clinical covariates, including age, gender, race, tumor site, histological subtype, and tumor grade.
To mitigate selection bias between the treatment groups, propensity score matching (PSM) was performed. The propensity scores were calculated with surgery alone serving as the reference group. One-to-one matching without replacement was executed using the nearest-neighbor approach on the logit of the propensity score, with confounders such as age, gender, race, tumor site, histological type, and tumor grade included in the matching algorithm. A caliper width of 0.01 was applied to optimize the matching precision.
A two-tailed P-value of less than 0.05 was considered statistically significant in all analyses, ensuring that the findings met the conventional standards of statistical rigor.
Results
Patient characteristics
A total of 583 patients diagnosed with esophageal cancer were included in the analysis, derived from the SEER database. Of these, 267 patients (45.8%) underwent surgery alone, while 316 patients (54.2%) received neoadjuvant chemoradiotherapy followed by surgery. Table 1 presents a detailed summary of the clinical characteristics of these patients, both prior to and following PSM.
Before PSM, there was an observed imbalance between the two groups in factors such as age, race, and tumor grade. However, after PSM, all baseline characteristics, including age, gender, race, primary tumor site, histological type, and tumor grade, were well-balanced between the surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups.
Factors associated with treatment patterns
Logistic regression analysis identified several factors influencing the choice of neoadjuvant chemoradiotherapy followed by surgery over surgery alone.(Fig. 1) Patients aged 66 years or older were significantly less likely to receive neoadjuvant chemoradiotherapy followed by surgery (odds ratio [OR] = 0.40, 95% confidence interval [CI]: 0.29–0.57; P < 0.001). Additionally, patients with tumors located in the middle third of the esophagus were less likely to undergo this combined treatment approach (OR = 0.57, 95% CI: 0.33–0.98; P = 0.042).
Conversely, black patients were more likely to receive neoadjuvant chemoradiotherapy followed by surgery (OR = 3.57, 95% CI: 1.43–10.28; P = 0.010), as were patients with grade 3/4 tumors (OR = 1.47, 95% CI: 1.00-2.15; P = 0.048) and those with unknown tumor grade (OR = 2.99, 95% CI: 1.55–6.06; P = 0.001).
Survival before PSM
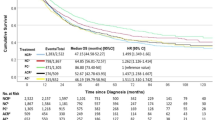
Prior to PSM, the 5-year CSS rates between the surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups were similar, with rates of 60.86% and 59.02%, respectively (hazard ratio [HR] = 1.01, 95% CI: 0.79–1.29; P = 0.916, Fig. 2A). In multivariable Cox proportional hazards regression analysis, neoadjuvant chemoradiotherapy followed by surgery was not identified as an independent prognostic factor for CSS (HR = 1.13, 95% CI: 0.87–1.45; P = 0.363, Fig. 2B).
Survival outcomes of patients with stage T2N0M0 in the unmatched cohort. (A) Cancer-specific survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (B) Multivariable proportional hazards regression analysis of cancer-specific survival. (C) Overall survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (D) Multivariable proportional hazards regression analysis of overall survival.
Similarly, the 5-year OS rates were comparable between the surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups, with rates of 50.64% and 49.81%, respectively (HR = 0.91, 95% CI: 0.75–1.12; P = 0.375, Fig. 2C). Multivariable analysis confirmed that neoadjuvant chemoradiotherapy followed by surgery did not independently influence OS (HR = 1.03, 95% CI: 0.84–1.27; P = 0.776, Fig. 2D).
Survival after PSM
After propensity score matching, the analysis continued to show no significant differences in the 5-year CSS rates between the two treatment groups, with rates of 66.43% for surgery alone and 56.67% for neoadjuvant chemoradiotherapy followed by surgery (HR = 1.21, 95% CI: 0.89–1.64; P = 0.225, Fig. 3A). Multivariable Cox regression analysis further confirmed that neoadjuvant chemoradiotherapy followed by surgery was not an independent prognostic factor for CSS (HR = 1.25, 95% CI: 0.92–1.71; P = 0.152, Fig. 3B).
Survival outcomes of patients with stage T2N0M0 in the matched cohort. (A) Cancer-specific survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (B) Multivariable proportional hazards regression analysis of cancer-specific survival. (C) Overall survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (D) Multivariable proportional hazards regression analysis of overall survival.
Similarly, the 5-year OS rates remained statistically similar between the surgery alone group (56.49%) and the neoadjuvant chemoradiotherapy followed by surgery group (47.37%) (HR = 1.09, 95% CI: 0.85–1.40; P = 0.481, Fig. 3C). Multivariable analysis confirmed that neoadjuvant chemoradiotherapy followed by surgery was not an independent predictor of OS (HR = 1.12, 95% CI: 0.87–1.43; P = 0.372, Fig. 3D).
Subgroup analysis of patients with squamous cell carcinoma
In patients with squamous cell carcinoma, the 5-year CSS rates were similar between the surgery alone group (55.40%) and the neoadjuvant chemoradiotherapy followed by surgery group (46.00%) (HR = 1.08, 95% CI: 0.70–1.68; P = 0.717, Fig. 4A). Multivariable analysis confirmed that neoadjuvant chemoradiotherapy followed by surgery was not an independent prognostic factor for CSS in this subgroup (HR = 1.03, 95% CI: 0.63–1.68; P = 0.909, Fig. 4B).
Survival outcomes of patients with esophageal squamous cell carcinoma. (A) Comparison of cancer-specific survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (B) Multivariable proportional hazards regression analysis of cancer-specific survival. (C) Comparison of overall survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (D) Multivariable proportional hazards regression analysis of overall survival.
The 5-year OS rates were likewise comparable between the two groups, with 46.81% for surgery alone and 36.56% for neoadjuvant chemoradiotherapy followed by surgery (HR = 0.94, 95% CI: 0.65–1.36; P = 0.750, Fig. 4C). Multivariable analysis indicated that neoadjuvant chemoradiotherapy followed by surgery was not an independent prognostic factor for OS in patients with squamous cell carcinoma (HR = 0.92, 95% CI: 0.61–1.39; P = 0.698, Fig. 4D).
Subgroup analysis of patients with adenocarcinoma
Among patients with adenocarcinoma, the 5-year CSS rates were similar between the surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups (62.93% vs. 63.64%; HR = 0.99, 95% CI: 0.74–1.33; P = 0.934, Fig. 5A). Multivariable analysis confirmed that neoadjuvant chemoradiotherapy followed by surgery was not an independent prognostic factor for CSS in patients with adenocarcinoma (HR = 1.09, 95% CI: 0.80–1.48; P = 0.586, Fig. 5B).
Survival outcomes of patients with adenocarcinoma. (A) Comparison of cancer-specific survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (B) Multivariable proportional hazards regression analysis of cancer-specific survival. (C) Comparison of overall survival between surgery alone and neoadjuvant chemoradiotherapy followed by surgery groups. (D) Multivariable proportional hazards regression analysis of overall survival.
The 5-year OS rates were similarly comparable between the two treatment groups, with 52.09% for surgery alone and 54.72% for neoadjuvant chemoradiotherapy followed by surgery (HR = 0.90, 95% CI: 0.71–1.15; P = 0.406, Fig. 5C). Multivariable analysis confirmed that neoadjuvant chemoradiotherapy followed by surgery did not independently influence OS in patients with adenocarcinoma (HR = 0.99, 95% CI: 0.77–1.27; P = 0.944, Fig. 5D).
Discussion
Although neoadjuvant chemoradiotherapy followed by surgery is widely regarded as the standard treatment for locally advanced esophageal cancer3,4, our study indicates that this combined treatment approach does not provide a survival advantage in terms of CSS or OS compared to surgery alone in patients with stage T2N0M0 esophageal squamous cell carcinoma and adenocarcinoma. These findings suggest that initial surgical intervention may be preferable for this specific subgroup of esophageal cancer patients, with neoadjuvant chemoradiotherapy not recommended as the standard approach.
Several studies have previously reported that preoperative therapies for T2N0M0 esophageal cancer did not significantly impact survival outcomes5,6,7,8,9. However, these studies included preoperative treatments such as neoadjuvant chemotherapy, radiotherapy, or chemoradiotherapy, and did not perform subgroup analyses to assess the effects of each treatment modality. Therefore, their results should be interpreted with caution. On the other hand, neoadjuvant chemoradiotherapy is often favored as the preoperative treatment of choice3,4, as it has been shown to achieve superior tumor downstaging compared to neoadjuvant chemotherapy. In our study, we evaluated the impact of neoadjuvant chemoradiotherapy on survival outcomes. The results indicated that the downstaging achieved with neoadjuvant chemoradiotherapy did not translate into a CSS or OS benefit for patients with stage T2N0M0 esophageal cancer. A possible explanation for this finding is that stage T2 disease is typically resectable from the outset, making the potential additional benefits of neoadjuvant therapy less pronounced.
The optimal treatment strategy for T2N0M0 esophageal cancer is heavily dependent on the accuracy of staging. Currently, the T stage is primarily determined through endoscopic ultrasound10. However, the limitations of the SEER database prevent us from assessing the specific diagnostic methods used for preoperative staging in the patients analyzed, which could have influenced the decision to administer neoadjuvant therapies. Previous studies have highlighted that the accuracy of staging between clinical T2N0M0 (cT2N0M0) and pathological T2N0M0 (pT2N0M0) is low, with reported accuracy rates as low as 21.4%.11 Moreover, it has been found that between 42.9% and 63.4% of patients are understaged as T2N0M011,12,13,14.
The understaging of T2N0M0 patients has significant implications for survival outcomes. In one study, the 5-year OS rate for patients undergoing surgery alone was 34.2%, compared to 55.7% for those receiving neoadjuvant chemoradiotherapy followed by surgery (P = 0.0088).11 Several key factors have been identified as associated with understaging, including tumor size greater than 3 cm, high-grade histology, and the presence of lymphovascular invasion15. For patients with stage T2N0M0 esophageal cancer who exhibit any of these risk factors, upfront surgery followed by adjuvant therapies has been demonstrated to yield survival outcomes comparable to those who undergo neoadjuvant chemoradiotherapy (48.3 vs. 45.9 months, respectively)15. Consequently, the recommended treatment strategy for cT2N0M0 esophageal cancer involves initial surgical resection. After surgery, adjuvant radiotherapy and/or chemotherapy should be considered for patients found to be understaged or who possess high-risk prognostic factors16. For those correctly staged as pT2N0M0 without high-risk prognostic factors, post-surgical observation is generally recommended.
It is important to note that stage T2N0M0 esophageal cancer represents a heterogeneous disease, and treatment decisions should be tailored to individual patient risk factors. The recommended treatment approach for this stage has evolved over time. Initially, the 2010 National Comprehensive Cancer Network (NCCN) guidelines recommended neoadjuvant therapy as the standard approach. By 2015, however, the guidelines had been revised to allow for more clinical discretion, offering clinicians the choice between neoadjuvant therapy and surgery alone. The most recent guidelines advocate for surgery in “low-risk” T2N0M0 lesions, defined as tumors less than 2 cm in size, well-differentiated, and without evidence of lymphovascular invasion.
Our study supports these guidelines, as logistic regression analysis demonstrated that patients with higher-grade tumors (grade 3/4) were more likely to receive neoadjuvant chemoradiotherapy followed by surgery compared to those with lower-grade tumors (grade 1/2). Interestingly, older patients (age ≥ 66 years) were less likely to receive neoadjuvant chemoradiotherapy followed by surgery, though this finding does not necessarily indicate that age ≥ 66 years is a low-risk factor. Rather, it suggests that older patients may be at greater risk for adverse events or postoperative complications, including increased mortality17,18. Therefore, further research is needed to identify the factors most strongly associated with prognosis in this population. A prognostic model based on these factors would be valuable for guiding clinical treatment decisions.
A notable limitation of our study must be acknowledged. Due to the constraints of the SEER database, we were unable to obtain data on the regimen, cycles, and doses of chemotherapy. Similarly, information on the technique, radiation target volume, and radiation dose of radiotherapy was unavailable. The specific combination of chemotherapy and radiotherapy is a pivotal prognostic factor that influences survival outcomes19,20,21. Consequently, our results should be interpreted with caution. Further randomized controlled trials, with detailed subgroup analyses, are required to validate our findings.
In conclusion, our study suggests that for patients with stage T2N0M0 esophageal cancer, neoadjuvant chemoradiotherapy followed by surgery does not offer a significant survival benefit compared to surgery alone. These findings support the consideration of initial surgery as the preferred treatment strategy for this patient population.
Data availability
The data are available from the corresponding author upon request.
Abbreviations
- SEER:
-
the surveillance epidemiology, and end results
- OS:
-
overall survival
- CSS:
-
cancer-specific survival
- PSM:
-
propensity score matching
- OR:
-
odds ratio
- HR:
-
hazard ratio
- CI:
-
confidence interval
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Zhu, H. et al. Esophageal cancer in China: practice and research in the new era. Int. J. Cancer. 152, 1741–1751. https://doi.org/10.1002/ijc.34301 (2023).
Yang, H. et al. Neoadjuvant Chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the Esophagus (NEOCRTEC5010): a phase III Multicenter, Randomized, open-label clinical trial. J. Clin. Oncol. 36, 2796–2803. https://doi.org/10.1200/JCO.2018.79.1483 (2018).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl. J. Med. 366, 2074–2084. https://doi.org/10.1056/NEJMoa1112088 (2012).
Speicher, P. J. et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J. Thorac. Oncol. 9, 1195–1201. https://doi.org/10.1097/JTO.0000000000000228 (2014).
Lv, H. W., Xing, W. Q., Shen, S. N. & Cheng, J. W. Induction therapy for clinical stage T2N0M0 esophageal cancer: a systematic review and meta-analysis. Med. (Baltim). 97, e12651. https://doi.org/10.1097/MD.0000000000012651 (2018).
Chen, W. H. et al. Long-term outcomes following neoadjuvant chemoradiotherapy in patients with clinical T2N0 esophageal squamous cell carcinoma. Dis. Esophagus. 25, 250–255. https://doi.org/10.1111/j.1442-2050.2011.01243.x (2012).
Mota, F. C. et al. Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. Int. J. Surg. 54, 176–181. https://doi.org/10.1016/j.ijsu.2018.04.053 (2018).
Markar, S. R. et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur. J. Cancer. 56, 59–68. https://doi.org/10.1016/j.ejca.2015.11.024 (2016).
Deboever, N., Jones, C. M., Yamashita, K., Ajani, J. A. & Hofstetter, W. L. Advances in diagnosis and management of cancer of the esophagus. BMJ 385, e074962. https://doi.org/10.1136/bmj-2023-074962 (2024).
Capovilla, G. et al. Optimal treatment of cT2N0 esophageal carcinoma: is upfront surgery really the way? Ann. Surg. Oncol. 28, 8387–8397. https://doi.org/10.1245/s10434-021-10194-9 (2021).
Samson, P. et al. Clinical T2N0 esophageal Cancer: identifying pretreatment characteristics Associated with Pathologic Upstaging and the potential role for induction therapy. Ann. Thorac. Surg. 101, 2102–2111. https://doi.org/10.1016/j.athoracsur.2016.01.033 (2016).
Li, M. et al. Clinicopathologic factors associated with pathologic upstaging in patients clinically diagnosed stage T2N0M0 squamous cell esophageal carcinoma. J. Cancer Res. Ther. 16, 1106–1111. https://doi.org/10.4103/jcrt.JCRT_1171_19 (2020).
Hardacker, T. J. et al. Treatment of clinical T2N0M0 esophageal cancer. Ann. Surg. Oncol. 21, 3739–3743. https://doi.org/10.1245/s10434-014-3929-6 (2014).
Semenkovich, T. R. et al. Comparative effectiveness of upfront esophagectomy versus induction chemoradiation in clinical stage T2N0 esophageal cancer: a decision analysis. J. Thorac. Cardiovasc. Surg. 155 (e2221), 2221–2230. https://doi.org/10.1016/j.jtcvs.2018.01.006 (2018).
Situ, D. et al. Do tumor ___location and grade affect survival in pT2N0M0 esophageal squamous cell carcinoma? J. Thorac. Cardiovasc. Surg. 146, 45–51. https://doi.org/10.1016/j.jtcvs.2013.01.034 (2013).
Yang, H. et al. Long-term efficacy of Neoadjuvant Chemoradiotherapy Plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg. 156, 721–729. https://doi.org/10.1001/jamasurg.2021.2373 (2021).
Eyck, B. M. et al. Ten-year outcome of Neoadjuvant Chemoradiotherapy Plus surgery for esophageal Cancer: the Randomized Controlled CROSS Trial. J. Clin. Oncol. 39, 1995–2004. https://doi.org/10.1200/JCO.20.03614 (2021).
Kato, K. et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int. J. Radiat. Oncol. Biol. Phys. 81, 684–690. https://doi.org/10.1016/j.ijrobp.2010.06.033 (2011).
Kato, K. et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 gy with elective nodal irradiation for stage II-III esophageal carcinoma. Jpn J. Clin. Oncol. 43, 608–615. https://doi.org/10.1093/jjco/hyt048 (2013).
Onozawa, M. et al. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol. 92, 266–269. https://doi.org/10.1016/j.radonc.2008.09.025 (2009).
Acknowledgements
The authors thank the Surveillance, Epidemiology, and End Results program for providing the data used in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: Yan Lin. Methodology: Huan-Wei Liang and Yang Liu. Formal Analysis: Shou-Feng Wang and Huan-Wei Liang. Investigation: Yan Lin. Resources: Shou-Feng Wang and Huan-Wei Liang. Validation: Wei Huang. Writing-Original Draft Preparation: Yan Lin. Writing-Review & Editing: Xin-Bin Pan.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital.
Informed consent
Informed consent was waived by the ethics committee/Institutional Review Board of Guangxi Medical University Cancer Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, Y., Wang, SF., Liang, HW. et al. Surgery alone versus neoadjuvant chemoradiotherapy followed by surgery in patients with stage T2N0M0 esophageal cancer. Sci Rep 14, 28898 (2024). https://doi.org/10.1038/s41598-024-80653-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80653-2