Abstract
Studying the implications of multifunctional edible bacteria on the chemical composition and bioactivities of plant extracts could provide one of the best solutions for producing functional juices. This study aimed to develop a high-quality mulberry juice with enhanced antioxidant capacity through fermentation. The juice was fermented using five different mono- and co-cultures of probiotic strains like Lacticaseibacillus casei (LC), Lactobacillus acidophilus (LA), Lacticaseibacillus paracasei (LPC), Lactobacillus helveticus (LH), and Lactiplantibacillus plantarum (LP). Where, four different interval time (24 h, 48 h, 72 h, and 96 h) were used in measuring total phenolic content (TPC), flavonols, along with the antioxidant activity assays and FTIR analysis. The results revealed the formation of new compounds during fermentation, such as organic acids, alcohols, glycerol, and phenols, as evidenced by the FTIR spectra. Besides, LC-LA and LC-LPC co-culture treatments yield the most substantial stability in TPC and total flavonoid content (TFC) after 48 h compared to 24 h and 72 h. After 48 h, the co-cultures of LPC-LH and LPC-LA (1265.5, 1145 GAE/mL, respectively) have increased the TPC than their individual cultures. In conclusion, the novelty of the current study lies in its provision of valuable insights into the industrial applications of fermented mulberry juice. The LC-LPC co-culture was confirmed to enhance the bioactive antioxidant capacity of the mulberry juice, particularly with 72 h of preservation.
Similar content being viewed by others
Introduction
Plants containing a plethora of biologically active polyphenolic moieties play a significant role in human health. Numerous studies have highlighted the nutraceutical effects of plant-derived materials, as they help reduce the risks of cancer, cardiovascular diseases (CVDs), aging, and other chronic conditions due to their phenolic content and antioxidant activity1. Mulberry is a fast-growing woody perennial plant from Moraceaefamily, and its major origin is China and India. The plants are widely distributed globally and have been cultivated in China for more than 5,000 years. Mulberry and Silk worm Committee of China has registered 29 mulberry varieties and 10 have been released by other agency in China2. Mulberries are considered a nutraceutical food in China and are used in traditional Chinese medicine for various purposes, such as improving eyesight, controlling CVDs, maintaining blood pressure, protecting the liver, and reducing fever3. In old herbal medicine of China, this fruit is used to treat different diseases like anemia, diabetes, arthritis and hypertension.
Mulberries are rich in macro- and micronutrients and are well-known for their strong antioxidant properties, attributed to their high polyphenol, pectin, and flavonoid content4. Different nutritive compounds like amino acids minerals, vitamins, fatty acids and compounds like chlorogenic acid, rutin, qurecitin, anthocyanin and polysaccharides are present in mulberry fruit. Notably, mulberries contain significant amounts of iron, which is rare in most berries and essential for hemoglobin production and oxygen transport in the body5. Anthocyanins in mulberries contribute to their color and have neuroprotective, anti-inflammatory, antimicrobial, and antioxidant properties5. Fresh mulberries, rich in biologically active compounds and flavor, deteriorate quickly post-harvest, necessitating immediate processing to preserve their quality. Traditional processing methods like pasteurization and cooking can alter the physico-chemical and nutritional properties of the juice.
Fermentation with probiotic microorganisms is increasingly recognized for its dual role in preserving food and improving its health benefits6,7. Fermented fruit juices, which combine probiotic and nutritional benefits, are increasingly used to deliver live microorganisms8. However, the successful incorporation of probiotics into plant-based products poses several challenges, including the need to select appropriate food matrices and strains of microorganisms that can thrive in plant environments9,10. Researchers are increasingly becoming more interested to understand the mechanism of biotransformation and metabolites functions in food products through Lactic Acid Bacteria (LAB) fermentation11. Different LAB strains show selectivity for various dietary matrices12. Using LAB for fermentation is a beneficial, low-cost, and sustainable method to improve the sensory and nutritional aspects of raw materials, while also extending the shelf-life of fruits and vegetables under sanitary conditions13. Probiotic bacteria offer a viable solution to meet consumer demands, enhance shelf-life, and maintain or improve the sensory and nutritional qualities of raw fruits. LAB co-cultures specifically improve the texture, structure, rheology, and thermal profile of products such as corn dough11,14.
Lactic acid fermentation is one of the ancient means of food storage and is quite inexpensive in rural areas. Lactic acid fermentation enhances fragrance compounds, sensory qualities, nutritional components (primarily folate, riboflavin, cobalamin, and ascorbic acid), low-calorie polyols (mannitol and sorbitol), and decreases anti-nutritive chemicals15. In the indigenous vegetable Momordica charantia(bitter melon), lactic acid (LAB) fermentation enhanced flavonoids, total phenols and antioxidant activity16. Camu-camu (Myrciaria dubia) lactic acid-fermented indigenous fruit and soymilk revealed anti-diabetic and antioxidant effect by suppressing α-glucosidase and α-amylase activity17 Fermentation of African nightshade (Solanum Scabrum) and (Solanum Americanum) with L. plantarumBFE 5092 and Lactobacillus fermentum BFE 6620 starter strains had a substantial influence on bacterial composition by lowering spoilage and harmful microorganism populations owing to pH decrease and lactic acid generation18. Probiotic characteristics may be found in LAB strains, and lactic-fermented foods are advantageous to human gastrointestinal health18. Furthermore, the acidification of foods by organic acids created by LAB stains and bacteriocins during fermentation might lengthen shelf-life and improve product safety19. It is generally understood that fermentation modifies the content of the food product and can drastically modify aromatic molecules and, as a result, sensory qualities20.
Although there are findings on the influence of fermentation on the polyphenolic and antioxidant characteristics of plant-based foods, further research is required (Kwaw et al., 2018). Limited work has been done on the impact of lactic acid fermentation (single and co-culture) on the antioxidant properties of mulberry juice. Given the significance of lactic acid fermentation, particularly co-culture, and its capacity to improve the antioxidant profile and shelf-life of mulberry juice, biotechnological uses of lactic acid bacteria could be beneficial in enhancing its functionality and availability.
The selected cultures (Lactobacillus casei, L. acidophilus, L. paracasei, L. helveticus, and L. plantarum) were chosen for their ability to enhance bioactive compound production, such as phenolics and flavonoids, through enzymatic activity. These strains employ diverse metabolic pathways, efficiently fermenting carbohydrates and breaking down plant cell walls to release valuable bioactive compounds. Additionally, they are well-known for their probiotic potential, promoting gut health and offering functional benefits, making them ideal for fermenting plant extracts and improving the nutritional profile of the final product.
Moreover, co-cultures (LAB-LAB) have not been extensively studied, even though they appear to be superior to single cultures due to the collaborative action of the different metabolic pathways of the strains involved. We conducted a comparison of co-culture with monoculture to determine their effectiveness in enhancing the antioxidant profile of mulberry juice.
Materials and methods
The Mulberry fruits were obtained from a farm in Zhenjiang, Jiangsu Province, China, in May 2023. The fully ripened fruits were manually picked, sorted, and washed with distilled water for further use. The fruits were stored at −18 °C until processing. Crushing and juice extraction were carried out using a juicer (FW100; TAISITE, Co., Tianjin, China). The juice was sieved through a 0.180 mm sieve, and the retained fraction was used for further experiments. Lacticaseibacillus casei (L. casei) ATCC 393 Lactiplantibacillus plantarum (L. plantarum) ATCC 8014, Lacticaseibacillus paracasei (L. paracasei) ATCC 334, Lactobacillus acidophilus (L. acidophilus) ATCC 4356, and Lactobacillus helveticus (L. helveticus) ATCC 15009 were purchased from DuPont China Holding Co. Ltd (Shanghai, China). These strains were propagated in Man Rogosa Sharpe (MRS) broth (Sinopharm Chemical Co., Shanghai, China) at 37 °C and stored at 4 °C. All chemicals used in the study were of analytical grade and were purchased from Sigma-Aldrich (Burlington, Massachusetts, USA).
Fermentation conditions
Isolated L. casei (LC), L. plantarum (LP), L. paracasei (LPC), L. acidophilus (LA), and L. helveticus (LH) were grown in liquid MRS medium and incubated (HZQ-F160; Shanghai, China) to a bacterial concentration of 107–108 cfu/mL. After activation, the cultures were centrifuged at 4000 rpm for 10 min at 25 °C using a Ruijiang RJ-TDL-50 A centrifuge (Ruijiang Analytical Instrument Co., Ltd., Wuxi, China). Following removal of the supernatant, the bacterial cells were washed in a sterile 0.1% NaCl solution. The resulting suspensions were then used as starter cultures for the fermentation process.
Prior to fermentation, the juice was pasteurized at 70 °C for 30 min. A 2% bacterial culture was added to the mulberry juice, which was then incubated at 37 °C for 1–4 days, with the incubator set to 60 rpm, as shown in Table S1. Lactic Acid Bacteria (2%) (L. casei, L. plantarum, L. paracasei, L. helveticus, L. acidophilus) were used individually (2%) and in combination as co-cultures (1% each) for the fermentation of mulberry juice. These were compared with a control sample (without bacteria). Figure 1 presents the study overall flowchart.
Figure 1. presents the study overall flowchart.
Total phenolic contents (TPCs)
The phenolic content of mulberry juice was determined following the procedure of Mehmood, et al.1 with modifications. Briefly, 1 mL of Folin–Ciocalteu (FC) reagent, 3 mL of sodium carbonate (20% w/v), 12 mL of H₂O, and 200 µL of the sample were mixed and placed in a water bath at 70 °C for 10 min. Finally, a SpectraMax i3 spectrophotometer (Molecular Devices, Silicon Valley, CA, United States) was used to measure the absorbance at 765 nm. Gallic acid was used as a standard (500-31.25 mg/mL), and the results were expressed in mg of Gallic acid equivalents per mL of sample.
Total flavonoid contents (TFCs)
The total flavonoid contents (TFCs) of fermented mulberry juice were evaluated as per the method of Chen, et al.21 with slight modifications. Briefly, 25 µL of the sample, 0.1 mL of H₂O, and 10 µL of NaNO₂ (5%) were mixed and allowed to rest for 5 min. Then, 15 µL of AlCl₃ (10%), 50 µL of NaOH, and 50 µL of H₂O were added. The absorbance was measured at 510 nm using a SpectraMax i3 spectrophotometer. The TFCs were calculated using quercetin as the standard (500-31.25 mg/mL), and the results were expressed in mg of rutin equivalents per mL of sample.
Total flavonols contents (TFlavCs)
The TFlavCs of fermented mulberry juice were ascertained using the procedure described by22 with some modifications. Briefly, 2 mL of AlCl₃, 3 mL of CH₃COONa, and 2 mL of the sample were mixed and stored at 20 °C for 150 min. The absorbance was then measured at 440 nm using a SpectraMax i3 spectrophotometer. The TFlavCs were calculated using quercetin as the standard (500-31.25 mg/mL), and the results were expressed in mg of quercetin equivalents per mL of sample.
Total anthocyanin contents (TACs)
The TACs of fermented mulberry juice were determined by the method of23through pH differential method with modifications. Briefly, two buffer solutions KCl (0.025 mol/l) and Sodium acetate (0.4 mol/l) were prepared with pH 1 and pH 4.5, respectively. 200 µl was added in two sets of tubes followed by 1.8mL of KCl buffer in one tube while 1.8 mL of sodium acetate buffer in other tube. The tubes were vortex, and the absorbance of each tube was measured at 700 nm and 520 nm by using spectrophotometer (SpectraMax i3). The results were addressed as mg of cyanidin 3-glucoside equivalent/mL of sample. The calculation can be done through the following formula:
Where K1 = Absorbance at 520 nm, pH1,
K2 = pH1, Absorbance at 700 nm, C3 = pH4.5, Absorbance at 520 nm, C4 = pH4.5, Absorbance at 700 nm, MW = Molecular weight of cyanidin 3glucoside, DF = Dilution factor, Ɛ= molar extinction, L = path length.
Proanthocyanidins contents determination (PACCs)
The proanthocyanidin contents (PACCs) of fermented mulberry juice were determined using the method of24, with modifications. Briefly, 1 mL of the sample, 3 mL of HCl, and 6 mL of vanillin were mixed and stored at 25 °C for 15 min. The absorbance was then measured at 500 nm using a SpectraMax i3 spectrophotometer. The PACCs were calculated using catechin as the standard (500-31.25 mg/mL), and the results were expressed in mg of catechin equivalents per mL of sample.
Reducing power ability (Rp-A)
The reducing power assay (Rp-A) of fermented mulberry juice was determined using the method of25, with small modifications. Briefly, 1 mL of the sample, 50 µL of HCl, 400 µL of potassium ferricyanide (C₆N₆FeK₃), 400 µL of FeCl₃, and 700 µL of H₂O were mixed and incubated in the dark at 37 °C for 30 min. The absorbance was then measured at 720 nm using a SpectraMax i3 spectrophotometer. The results were expressed in mM of ascorbic acid equivalents.
Copper reducing power ability (Cu-Rp)
The cupric reducing power (Cu-RP) of fermented mulberry juice was determined using the method of26 with modifications. Briefly, 250 µL of CuCl₂ (0.01 M), 250 µL of neocuproine (7.5 mM), 250 µL of ammonium acetate (1 M), and various concentrations of the sample were mixed. The solution was allowed to rest at room temperature for 30 min, and the absorbance was measured at 450 nm using a spectrophotometer.
Antioxidant analysis
Determination of DPPH scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the fermented mulberry juice was determined using the method described by Ramirez, et al.27, with modifications. First, DPPH (0.1 mM) was prepared in methanol. Then, 120 µL of the sample was mixed with 4.2 mL of the DPPH solution. The mixture was allowed to rest for 30 min at 25 °C, and the absorbance was measured at 517 nm using a spectrophotometer. The fermented juice without DPPH solution was used as a control. The scavenging activity was calculated using the following formula:
Determination of ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) of the fermented mulberry juice was evaluated using the method described by Benzie and Strain, 199628, with modifications. Briefly, the FRAP solution was prepared by mixing 25 mL of acetate buffer (0.3 M, pH 3.6), 2.5 mL of TPTZ (10 mM in 40 mM HCl), and 2.5 mL of FeCl₃·6 H₂O (20 mM). Then, 0.3 mL of the FRAP solution was mixed with 1 mL of the sample and 0.3 mL of H₂O. The mixture was incubated at 37 °C in a water bath for 30 min, and the absorbance was measured at 595 nm using a spectrophotometer. The results were expressed in mg of trolox equivalents per mL of sample.
Attenuated total reflectance-fourier transforms infrared (ATR-FTIR) spectroscopy
The FTIR spectra of all samples were obtained in the range of 4000 to 700 cm⁻¹, through a Nicolet Is50, Thermo Scientific, USA with a germanium crystal attenuated total reflection accessory. The instrument was configured to perform 16 scans with a resolution of 4 cm[-1.
Molecular docking analysis
Molecular docking was performed using the educational version of MOE software (2015, USA). The molecular interactions were modeled between the bacterial peptidoglycan structure (PDB ID: 2MTZ) as the macromolecule receptor and cyanidin-3-rutinoside (C3R; PubChem CID: 4481459) as the ligand. The structures were retrieved from the Protein Data Bank (https://www.rcsb.org/) and the PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Both structures were optimized by assigning fractional charges and minimizing energy using the Protonate-3D and MMFF94X force fields. Water molecules were removed, and structure refinement, energy minimization, and 3D protonation were carried out via MOE.
This process was repeated for the composite structures, resulting in 4–5 docked conformations that were visualized and analyzed for hydrophobicity, electrostatic potential, hydrogen bonds, and structural fluctuations. These fluctuations were further explored using heatmaps generated by Heatmapper (http://heatmapper.ca/expression/). Molecular dynamic simulations (MDs) were run in 10 ns intervals for a total simulation time of 50 ns, and the root mean square deviation (RMSD) values were calculated to assess structural stability.
The geometry of the peptidoglycan-C3R complex was first optimized using the M062X functional with a 6-31G(d, p) basis set, as implemented in the Gaussian 09 package. Additionally, alcalase was randomly positioned around the peptidoglycan structure, followed by a double-minimization process. Molecular dynamics simulations of this system were performed using GROMACS version 5.1.4 (GNU, Netherlands)29.
Statistical analysis
Unless indicated, all analysis were done in triplicates. The results obtained for each parameter were statistically analyzed using the OriginPro 8.5 software package and presented as mean ± standard deviation. Analysis of variance (ANOVA) and a Completely Randomized Design (CRD) were employed to determine the level of significance at 95% confidence. The principal component analysis (PCA) and Pearson correlation analysis with 0.05, 0.01, and 0.001signifcance levels were achieved via OriginPro 2023 (Origin Lab, Co.,US).
Results
Total phenolic content
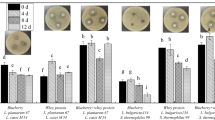
The current investigation revealed a notable rise in total phenolic levels across various fermentation treatments for up to 48 h, followed by a decline, as shown in Fig. 2a. Among the various treatments, LC-LPC (1115.3 ± 27.01 mg GAE/mL) and LP-LPC (1145.3 ± 27.01 mg GAE/mL) fermentation improved total phenolic content up to 48 h, showing a significant difference when compared to unfermented samples. When compared to other treatments, 24-hour fermentation produced higher total phenolics under LC-LPC (873.21 ± 19.35 mg GAE/mL) and LP-LPC (714.23 ± 23.07 mg GAE/mL). Overall, the effect of fermentation was noticeably improved after 24 and 48 h. Co-cultures of LPC-LH (1265.5 ± 19.35 mg GAE/mL), LC-LP (1142.4 ± 27.04 mg GAE/mL), LPC-LA (1145 ± 30.76 mg GAE/mL), LP-LH (1139.9 ± 19.35 mg GAE/mL), and LP-LA (1129.6 ± 23.07 mg GAE/mL) produced more total phenolics after 48 h compared to single cultures. Among all fermentation treatments, the LC-LPC and LC-LH co-treatments were the most effective in increasing total phenolic contents compared to the other groups.
Total flavonoid content
The effect of different fermentation treatments on total flavonoid content was significant, as shown in Fig. 2b. Among all fermentation treatments, the following co-treatments performed the best: LP-LA (3278.8 ± 39.02 mg RE/mL), LC-LPC (1982.5 ± 16.97 mg RE/mL), LP-LPC (1675.1 ± 44.44 mg RE/mL), LA-LH (1545.5 ± 16.97 mg RE/mL), LPC-LA (1538.1 ± 27.96 mg RE/mL), and LC (1489.9 ± 72.29 mg RE/mL). After 48 h of treatment, these mixed fermentation treatments yielded the highest concentration of total flavonoids. In co-cultures, 48 h of fermentation resulted in the highest total flavonoid concentration. All other fermentation treatments produced more total flavonoids than unfermented samples after 48 h.
Total flavonols
The effect of different fermentation treatments on flavonol content was outstanding, as shown in Fig. 2c. Among all fermentation treatments, the co-culture treatments proved to be the most effective. The LC-LPC treatment resulted in flavonol contents of 15.17 ± 3.06 mg QE/mL after 24 h and 14.24 ± 0.22 mg QE/mL after 48 h. The LP-LPC treatment produced 13.71 ± 0.42 mg QE/mL after 24 h and 12.31 ± 0.31 mg QE/mL after 48 h. The LP-LH treatment yielded 10.94 ± 0.10 mg QE/mL after 24 h and 11.98 ± 0.30 mg QE/mL after 48 h. The LPC-LH treatment resulted in flavonol contents of 9.51 ± 0.50 mg QE/mL after 24 h and 10.71 ± 0.50 mg QE/mL after 48 h. Lastly, the LPC-LA treatment produced 7.85 ± 0.18 mg QE/mL after 24 h and 10.00 ± 0.20 mg QE/mL after 48 h. These results indicate that the LC-LPC co-culture treatment was the most effective in producing the highest concentrations of flavonols, followed closely by LP-LPC and LP-LH treatments, which also significantly increased flavonol production after both 24 and 48-hour intervals.
The effect of fermentation time on the chemical compositions of mulberry juice with mono and co-culture of different lactic acid bacteria at 24 h, 48 h, 72 h, and 96 h of fermentation. (a) Total phenolic content (mg gallic equivalent/mL of mulberry juice); (b) Total flavonoid content (mg rutin equivalent/mL of mulberry juice); (c) Total flavonol content (mg quercetin equivalent/mL of mulberry juice); (d) Total anthocyanin content (mg cyanidin 3-glucoside equivalent/mL of mulberry juice). C-Control; LC-Lactobacillus casei; LP-Lactobacillus plantarum; LPC-Lactobacillus paracasei; LA-Lactobacillus acidophilus LH-Lactobacillus helveticus; LC-LP Lactobacillus casei-Lactobacillus plantarum; LC-LPC Lactobacillus casei- Lactobacillus paracasei; LC-LA Lactobacillus casei- Lactobacillus acidophilus ; LC-LH Lactobacillus casei- Lactobacillus helveticus; LP-LPC Lactobacillus plantarum- Lactobacillus paracasei; LP-LA Lactobacillus plantarum-Lactobacillus acidophilus;;LP-LH Lactobacillus plantarum-Lactobacillus helveticus; LPC-LA Lactobacillus paracasei- Lactobacillus acidophilus;LPC-LH Lactobacillus paracasei - Lactobacillus helveticus ;LA-LH Lactobacillus acidophilus - Lactobacillus helveticus.
Anthocyanins content
Fermentation showed improved production of anthocyanins after 24 h of treatment, particularly under LC-LPC (32.888 ± 0.011 mg/mL) and LP-LPC (28.308 ± 0.019 mg/mL) co-culture treatments, as compared to other treatments (Fig. 2d). Additionally, the LPC-LH (25.743 ± 0.01 mg/mL), LC-LP (25.295 ± 0.02 mg/mL), LC (24.948 ± 0.01 mg/mL), and LP (24.623 ± 0.012 mg/mL) treatments also increased anthocyanin production after 24 h. However, extending the fermentation to 48, 72, and 96 h did not show any significant increase in anthocyanin production.
Proanthocyanidins
Proanthocyanidin production significantly increased under various co-culture treatments after 24 and 48 h, compared to the 96-hour fermentation period (Fig. 3). Specifically, the LC-LPC treatment produced 962.17 ± 25.16 mg/mL after 24 h and 1055.5 ± 27.83 mg/mL after 48 h. The LP-LPC treatment yielded 1075.5 ± 26.45 mg/mL after 24 h and 1298.8 ± 32.14 mg/mL after 48 h. The LC-LP treatment resulted in 923.83 ± 16.07 mg/mL after 24 h and 1038.8 ± 20.20 mg/mL after 48 h. The LC-LA treatment produced 692.17 ± 40.41 mg/mL after 24 h and 895.50 ± 31.22 mg/mL after 48 h. The improvement in proanthocyanidin production was more pronounced after 48 h compared to the 24-hour treatment.
(a) the correlation implication of the proanthocyanidins content (mg catechin equivalent/mL of mulberry juice) changes during the fermentation period on the mulberry juice with mono and co-culture of different lactic acid bacteria. (b) The reducing power ability (RP), ferric reducing antioxidant power (RAP), CuCl2-reducing power (Cu), and DPPH radical scavenging activity (mg trolox equivalent/mL of mulberry juice) with mono and co-culture of different lactic acid bacteria at 24 h, 48 h, 72 h, and 96 h of fermentation period. Other abbreviations can be found in Fig. 2 legend.
Antioxidant activity
The impact of LC-LPC (3.4667 ± 0.011) and LP-LPC (3.3800 ± 0.03) co-culture treatments on reducing power ability have been significantly noteworthy, as shown in Fig. 3b. Furthermore, the LP-LA (3.3633 ± 0.035), LPC-LA (3.3367 ± 0.035), LA-LH (3.2633 ± 0.015), LC-LP (3.2467 ± 0.035), and LPC-LH (3.12 ± 0.03) treatments also exhibited remarkable reducing power ability. Both co-culture and mono-culture treatments increased reducing power after 24 h. However, extending the fermentation to 48, 72, and 96 h did not show any significant impact on reducing power.
In this study, the co-culture treatments LC-LPC (32.858 ± 0.498 mM Trolox equivalents), LP-LPC (32.027 ± 0.38 mM Trolox equivalents), LC-LP (31.195 ± 0.627 mM Trolox equivalents), and LPC-LH (30.613 ± 0.498 mM Trolox equivalents) showed a significant increase in ferric reducing antioxidant power (FRAP) after 24 h of fermentation (Fig. 3b). Similarly, other single and co-culture treatments also exhibited an increase in FRAP at the 24-hour mark. However, FRAP levels decreased in samples fermented for 48, 72, and 96 h.
In this study, the co-culture treatments LC-LPC (5612.8 ± 254.58 µM Trolox equivalents and 5096.1 ± 642.54 µM Trolox equivalents) and LP-LPC (5096.1 ± 642.54 µM Trolox equivalents) exhibited a significant increase in CuCl₂ reducing power after 24 h of fermentation (Fig. 3b). Overall, there was a marked improvement in CuCl₂ reducing power compared to the unfermented samples.
The fermentation treatments used in this study had a significant impact on DPPH radical scavenging activity (Fig. 3b). Various treatments showed notable increases in DPPH activity after 24 h of fermentation. Specifically, the LC treatment resulted in 0.6511 ± 0.017, LC-LH in 0.629 ± 0.009, LC-LPC in 0.5532 ± 0.014, LP-LPC in 0.5305 ± 0.011, LPC in 0.5204 ± 0.010, LP in 0.4933 ± 0.080, LA in 0.4925 ± 0.013, LPC-LH in 0.4702 ± 0.009, LP-LA in 0.4694 ± 0.009, LP-LH in 0.4605 ± 0.010, and LPC-LA in 0.4493 ± 0.009. All these treatments significantly increased DPPH activity, demonstrating their potential in enhancing the antioxidant properties of the fermented mulberry juice after 24 h of treatment.
Structural integrity through ATR-FTIR
FTIR spectroscopy is one of the best techniques for understanding molecular interactions, identifying functional groups, confirming different molecules, and observing changes due to fermentation in samples. The FTIR spectra (4000–700 cm-1) of the fermented samples were analyzed (Figs. 4, 5and 6). Where, several differences occurred in the band position and intensity after incorporating the bacteria. The mulberry juice displays distinctive peaks at 3278, 2921, 1339, 1240, and 1027 cm⁻¹. The changes in the LC group were not significant (Fig. 4a) compared to the highly significant shift in 2919 cm[-1 after the treatment with LP (Fig. 4b), LPC (Fig. 4c), LA (Fig. 4d), and LH (Fig. 4e) monocultures.
Figure 4. FTIR spectra of different fermentation time on the chemical compositions of mulberry juice with mono culture of different lactic acid bacteria. (a) LC: Control; (b) LP: Lactobacillus plantarum; (c) LPC: Lactobacillus paracasei; (d) LA: Lactobacillus acidophilus; (d) LH: Lactobacillus helveticus.
In addition, for the co-cultures, as presented in Figs. 5 and 6, the broad peak at 3278 cm⁻¹ indicates strong stretching vibrations of OH functional groups, which is characteristic of water, as fresh juice contains a significant amount of water in the treated juice with LC-LP (Fig. 5a), LP-LPC (Fig. 5b), LC-LH (Fig. 5c), and LP-LPC (Fig. 5e) compared with the changes in the LC-LH (Fig. 5d). The peak at 2921 cm⁻¹ displays the presence of C-H and OH bending/stretching, which comprises aromatic alcohols and alkanes, while 1339 cm⁻¹ indicates moderate OH ability of phenols and alcohols30. The strong peaks at 1241 cm⁻¹ and 1027 cm⁻¹ showed the presence of C-O and C-N stretching, indicating the presence of amines and ethers31.
While, the presence of weak shoulders at 1404 –1230 cm⁻¹ in LP-LA (Fig. 6a), LP-LH (Fig. 6b), LPC-LA (Fig. 6c), LPC-LH (Fig. 6d), and LA-LPC (Fig. 6e) could be attributed to strong C-H and OH bending, which is related to the anomeric configuration of secondary protein structure and is more prominent in co-culture fermented mulberry juice. The presence of organic acid confirmed at 1711 cm⁻¹ represents the C = O bond of carboxylic acid and is regarded as a novel peak due to bacterial inoculation, potentially indicating the stretching vibrations of alkene groups, carboxylic acid, and carbonyl groups.
FTIR spectra of different fermentation time on the chemical compositions of mulberry juice with mono culture of different lactic acid bacteria. (a) LC-LP; (b) LP-LPC; (c) LC-LA; (d) LC-LH; (d) LP-LPC. LC: Control; LP: Lactobacillus plantarum; LPC: Lactobacillus paracasei; LA: Lactobacillus acidophilus; LH: Lactobacillus helveticus.
Consequently, the absorbance at 1408 cm⁻¹ was attributed to formed linkages between the microbial proteins and the juice’s phenolics. The strong shoulder at 3270 cm⁻¹ indicates O-H stretching due to alcohols and carboxylic acids and indicates the reduction of water and its conversion into other compounds. The change of the peak from 3278 to 3270 cm⁻¹ after bacterial fermentation represents the interaction to generate intermolecular hydrogen bonding. The peaks at 2920 cm⁻¹ represent gallic acid, while the change of wavelength from 1339 to 1408 cm⁻¹ indicates an increase in alcohols and phenols, referencing the presence of OH functional groups. The peak at 1150 cm⁻¹ represents saccharides in the sample, and the peak at 1594 cm⁻¹ indicates the amine group, specifically N-H bending. There were some peaks shown at the beginning, mostly at 866 and 817 cm⁻¹, because of bacterial action during fermentation, showing the vibration of CH₂. Absorbances mainly related to C-O bond stretching are related to the fingerprint region.
Figure 6. FTIR spectra of different fermentation time on the chemical compositions of mulberry juice with mono culture of different lactic acid bacteria. (a) LP-LA; (b) LP-LH; (c) LPC-LA; (d) LPC-LH; (d) LA-LPC. LC: Control; LP: Lactobacillus plantarum; LPC: Lactobacillus paracasei; LA: Lactobacillus acidophilus; LH: Lactobacillus helveticus.
Molecular docking stimulation
Figure 7 presents a detailed visualization of the peptidoglycan-C3R interaction including a variety of analyses to clarify the molecular dynamics and binding affinities associated with this interaction and highlighting their binding sites. Peptidoglycans interact with C3R via different forces, indicating a strong binding potential that enables the formation of peptidoglycan-C3R conjugates. The analysis of binding affinity is essential, as it offers valuable insights into the strength and stability of the protein-protein interaction. The binding score of −1.02 and an energy affinity of −1.83 kcal/mol suggest a stable interaction, indicating a moderate binding strength that may impact the functional properties of the conjugates. There are notable differences in the binding interactions when comparing the heat map of peptidoglycan alone to the peptidoglycan-C3R conjugates. The modified patterns in the heat map indicate that C3R was well interacted with peptidoglycan, where this interaction might be responsible for maintaining the stability of C3R and other polyphenols (agreeing well with our experimental results). The analysis conducted by the MDs provides additional support, as it demonstrates the RMSD over time. The RMSD plot provides insights into the structural stability and conformational fluctuations of the peptidoglycan-C3R conjugates throughout the MD simulations. The consistent RMSD values indicate that the conjugates retain their structural integrity over time. Elaborate diagrams highlight precise interactions within peptidoglycan-C3R conjugates. H-bonding is essential for maintaining the stability of protein structures.
FTIR spectra of different fermentation time on the chemical compositions of mulberry juice with mono culture of different lactic acid bacteria. (a) LP-LA; (b) LP-LH; (c) LPC-LA; (d) LPC-LH; (d) LA-LPC. LC: Control; LP: Lactobacillus plantarum ; LPC: Lactobacillus paracasei ; LA: Lactobacillus acidophilus ; LH: Lactobacillus helveticus.
Peptidoglycan-C3R interaction scheme of the bacterial cell wall and anthocyanin structures with rep-resenting their binding affinity, overall conjugates, hydrophobicity alterations, electrostatic potentials, H-bonding, heat-map analysis, and MDs-analysis. The end conjugates showed a binding score and energy affinity of -1.02 and -1.83 kcal/mol, separately.
The diagram illustrates the amino acids that play a crucial role in these interactions, demonstrating the impact of protein binding on their overall conformation and stability. For example, Lys140 and Arg144 of peptidoglycan directly interacted with the anthocyanin structure. Hydrophobic interactions play a crucial role in maintaining the structural integrity of proteins. The visualization demonstrates the alterations in hydrophobic regions, indicating the impact of the conjugation with C3R on the hydrophobic core of peptidoglycan. Electrostatic interactions play a crucial role in determining the strength and selectivity of protein-protein interactions, where other 8 amino acids of peptidoglycan interacted with C3R. The visualization of electrostatic potentials demonstrates the changes in charge distribution that occur during binding, impacting the strength of binding and functional properties of the conjugates32.
Multivariate analysis and correlations
The principal component analysis (PCA) among all studied groups is presented in Fig. 8, and the differences among groups were evaluated according to the contribution rate of PC factors33. In that regard, the loading variances demonstrated a significant discrimination (p < 0.05) among the 16 groups understudy based on the first two components that represented 80.2% of the total variance (Fig. 8). Besides, according to the loadings, the TPCs and TFL showed the highest significant (p < 0.05) patterns on the different group variances compared to the control. This outcome can be attributed to the significant effect of the co-cultures in changing the phytochemical distribution in the mulberry media.
Figure 8. Principal component analysis (PCA). 1-Control; 2-Lactobacillus casei; 3-Lactobacillus plantarum; 4-Lactobacillus paracasei; 5-Lactobacillus acidophilus; 6-Lactobacillus helveticus; 7- Lactobacillus casei-Lactobacillus plantarum; 8-Lactobacillus casei- Lactobacillus paracasei; 9- Lactobacillus casei- Lactobacillus acidophilus ; 10- Lactobacillus casei- Lactobacillus helveticus; 11- Lactobacillus plantarum- Lactobacillus paracasei; 12- Lactobacillus plantarum-Lactobacillus acidophilus;13- Lactobacillus plantarum-Lactobacillus helveticus; 14-Lactobacillus paracasei- Lactobacillus acidophilus;15- Lactobacillus paracasei - Lactobacillus helveticus ;16- Lactobacillus acidophilus - Lactobacillus helveticus. (b) Pearson correlation heatmap. *, **, and *** are the significant levels of 0.05, 0.01, and 0.001, orderly.
The Pearson’s correlation coefficients matrix of the fundamental physicochemical, bioactive components, and antioxidant activities of mulberry juice fermented for 24 h revealed that TPC was highly significantly positively correlated with TFC**, TAC**, RP-A**, CU-A**, FRAP**, and DPPH** (p < 0.01) (Fig. 8). Additionally, it was negatively correlated with PACs. TFC was highly significantly positively correlated with TAC**, RP-A**, FRAP**, and DPPH** (p < 0.01) and negatively correlated with CU-A. Both TPC and TFC play key roles in antioxidant activity. TFLAV is highly significantly positively correlated with PACs**, TAC*, RP-A** (p < 0.01) and negatively correlated with CU-A and DPPH.
PACs are highly positively correlated with TAC** (p < 0.01) and negatively correlated with CU-A and DPPH. TAC is highly significantly positively correlated with RP-A** and FRAP** (p < 0.01) and negatively correlated with CU-A. RP-A is highly significantly positively correlated with FRAP** and DPPH** (p < 0.01). FRAP is highly significantly positively correlated with DPPH** (p < 0.01).
Principal component analysis (PCA). 1-Control; 2-Lactobacillus casei; 3-Lactobacillus plantarum; 4-Lactobacillus paracasei; 5-Lactobacillus acidophilus; 6-Lactobacillus helveticus; 7- Lactobacillus casei-Lactobacillus plantarum; 8-Lactobacillus casei- Lactobacillus paracasei; 9- Lactobacillus casei- Lactobacillus acidophilus ; 10- Lactobacillus casei- Lactobacillus helveticus; 11- Lactobacillus plantarum- Lactobacillus paracasei; 12- Lactobacillus plantarum-Lactobacillus acidophilus;13- Lactobacillus plantarum-Lactobacillus helveticus; 14-Lactobacillus paracasei- Lactobacillus acidophilus;15- Lactobacillus paracasei - Lactobacillus helveticus ;16- Lactobacillus acidophilus - Lactobacillus helveticus. (b) Pearson correlation heatmap. *, **, and *** are the significant levels of 0.05, 0.01, and 0.001, orderly.
Discussion
Numerous scientific investigations have focused on developing and producing new types of fermented foods with probiotic and functional qualities34. Fermentation is a complex process involving multiple stages of breakdown, structural changes, and the formation of new compounds, all of which are influenced by factors such as microbial strains, substrate composition, and fermentation conditions. In the current study, co-culture treatments demonstrated notable improvements in bioactive compound production and antioxidant activity, particularly in treatments like LC-LPC and LP-LPC.
The superior performance of certain co-cultures, such as LC-LPC and LP-LPC, can be attributed to several factors, including the distinct metabolic pathways utilized by different bacterial strains. Co-cultures, which involve the interaction of multiple bacterial species, tend to promote more diverse metabolic activity compared to single-strain fermentations. For example, Lactobacillus plantarumis known for its ability to release phenolic compounds from plant cell walls through enzymatic actions like phenolic acid decarboxylation and glycosidase activity, making these compounds more bioavailable35,36.
In mixed cultures, such as those involving L. plantarum, the bacteria’s ability to produce bioactive peptides and increase polyphenol release is enhanced through the synergistic action of multiple enzymes. For instance, enzymes like glucosidases and esterases can break down complex polyphenols, transforming them into simpler forms that exhibit higher antioxidant activities. This process explains the greater production of phenolics and improved antioxidant capacity seen in co-cultures compared to single-strain fermentation37.
In mixed cultures, such as those involving L. plantarum, the bacteria’s ability to produce bioactive peptides and increase polyphenol release is enhanced through the synergistic action of multiple enzymes. For instance, enzymes like glucosidases and esterases can break down complex polyphenols, transforming them into simpler forms that exhibit higher antioxidant activities. This process explains the greater production of phenolics and improved antioxidant capacity seen in co-cultures compared to single-strain fermentation37.
During fermentation, several key mechanisms come into play, particularly in the liberation and transformation of bioactive compounds. In co-cultures, bacterial enzymes are crucial in hydrolyzing macromolecules such as glycosides, leading to the release of free phenolics from their bound forms. This enhances their bioavailability and antioxidant potential, as free phenolics are more reactive in neutralizing free radicals. Additionally, the production of lactic acid by lactic acid bacteria (LAB) can lower the pH, facilitating the breakdown of plant cell walls and further releasing bioactive compounds. Research indicates that fermentation significantly affects the chemical constituents and antioxidant activity of plant-based food materials. It modifies chemical components through a series of reactions that enhance bioactive compounds and functional properties. Increased production of phenolic compounds, as seen in the LC-LPC and LP-LPC treatments, can also be linked to the bacterial ability to depolymerize complex phenolics into smaller, more bioavailable forms. This enzymatic conversion enhances antioxidant activity, as smaller molecules are more efficient at donating electrons to neutralize free radicals. For instance, Kwaw et al., 2018 reported that lactic acid fermentation significantly impacts the beverage’s total phenolic, anthocyanin, and flavonoid content. Previous research has suggested that fermentation using a complex of strains is superior to single-strain38. Mixed cultures have been shown to promote the growth of Lactobacillus plantarum, which is directly linked to the production of nutrients such as vitamins. For example, mixed cultures of L. plantarum AF1, L. plantarum LP3, and L. plantarum were used to ferment bergamot juice, resulting in the highest radical scavenging and DPPH activity39. Fermentation increased the reducing power of soluble phenolics and antioxidant activity, demonstrating the beneficial effects of fermentation.
In the current study, co-cultures significantly improved the phytocompounds and antioxidant activity of the juice. Dueñas, et al.40 proposed that fermentation by L. plantarum was an effective procedure for increasing the concentration of phenolic components in fermented cowpea (Vigna sinensis) flour. LAB can transform polyphenolic chemicals into smaller molecules with greater bioavailability and release conjugated phenolic compounds from cell walls41. Fermentation hydrolyzes macromolecules into their building blocks, liberating attached phenolics due to microbial influence42. Another study on soy seeds suggested that microbial fermentation increases phenolic content by 120–135%, possibly due to detoxification, degradation, or lignin remobilization. Bacterial fermentation of soy whey by L. plantarummobilizes phenolics and flavonoids, resulting in higher total phenolic contents43.
Another study in line with the current research found that LABs affect the phenolic profile of the juice (Kwaw et al., 2018). Increased antioxidant activity of fermented juice under LAB co-culture has been observed. Different antioxidant components may operate through various mechanisms against oxidative agents, requiring multiple antioxidant capacity tests to comprehensively assess the antioxidant capability of fermented mulberry juice. Assays such as FRAP and DPPH showed high activity under LC-LPC and LP-LPC co-cultures. The rise in free form phenolic compounds and the formation of other byproducts during LAB fermentation may be responsible for the increase in antioxidant capacity44. Zhou et al., 2020 indicated that phenolic and flavonoid content was responsible for enhancing DPPH and FRAP scavenging capabilities. L. plantarumaltered the phenolic profile45.
The current study demonstrates that co-cultures of LC-LPC and LP-LPC act as free radical inhibitors or scavengers, potentially functioning as major antioxidants. It was found that a larger synthesis of phenolic chemicals correlates with stronger antioxidant activity. Natural antioxidants’ activity in terminating free radical processes has been documented46. According to Yao et al., 2010, fermented legume products containing Bacillus sp. showed greater FRAP activity during fermentation47. Vadivel et al., (2011) reported that antioxidant capacity might be connected to phenolic concentration, suggesting that juice extracts with higher phenolic concentration may exhibit higher FRAP and DPPH activity48.
The current study demonstrates that co-cultures of LC-LPC and LP-LPC act as free radical inhibitors or scavengers, potentially functioning as major antioxidants. It was found that a larger synthesis of phenolic chemicals correlates with stronger antioxidant activity. Natural antioxidants’ activity in terminating free radical processes has been documented46. According to Yao et al., 2010, fermented legume products containing Bacillus sp. showed greater FRAP activity during fermentation47. Vadivel et al., (2011) reported that antioxidant capacity might be connected to phenolic concentration, suggesting that juice extracts with higher phenolic concentration may exhibit higher FRAP and DPPH activity48.
Treatments with LC-LPC and LP-LPC co-cultures boosted reducing power. The reducing capacity of polyphenolic compounds was closely connected to their antioxidant activity42,49. Lee et al., 2008 found that the hydrogen-donating capacity of the reductones present was strongly linked to reducing power. The fermentation process may have produced reductants that interacted with free radicals to stabilize and terminate radical chain reactions, resulting in high reducing power in fermented mulberry juice with high phenolic contents. Additionally, the initial organism’s intracellular antioxidants, peptides, and hydrogen-donating capacity might contribute to its improved reducing ability46.
Treatments with LC-LPC and LP-LPC co-cultures boosted reducing power. The reducing capacity of polyphenolic compounds was closely connected to their antioxidant activity42,49. Lee et al., 2008 found that the hydrogen-donating capacity of the reductones present was strongly linked to reducing power. The fermentation process may have produced reductants that interacted with free radicals to stabilize and terminate radical chain reactions, resulting in high reducing power in fermented mulberry juice with high phenolic contents. Additionally, the initial organism’s intracellular antioxidants, peptides, and hydrogen-donating capacity might contribute to its improved reducing ability46.
In the present study, a clear decline in all phytochemicals and antioxidant activity was observed after 72 h of treatment. This decrease can be attributed to LAB bacteria depolymerizing macromolecular phenolics or converting specific phenolic compounds11. Scientists discovered a significant reduction in phytochemicals after 72 h of fermentation, consistent with other50. Microbial metabolism and enzymatic activities can change phenolic compounds during fermentation, with specific enzymes potentially degrading or modifying phenolics, leading to their reduction. Some studies suggest that phenolic degradation may occur after an optimal fermentation period, causing a decrease in their content after a peak51. After an initial increase, certain phenolic compounds may undergo additional reactions or transformations, reducing their concentration over time.
Fermentation with probiotics produces aromatic amine groups, indicating the presence of bacterial proteins in the final fermented product, which have antibacterial properties in different fermented samples52. Studies by Ray, et al.53 and Panda, et al.54 found various phenol groups, aliphatic amines, alcohols, and carboxylic acids at 1000, 1250, and 3270 cm⁻¹ peaks in fruit and purple sweet potato (Ipomoea batatas L.) extracts. During fermentation, bond rearrangements occur, such as C-H stretching vibration changes from 2928 cm⁻¹ to 2925 cm⁻¹, indicating hydrogen bond formation. Ma, et al.55 revealed that hydrogen bonding at the glycosidic bond between the oxygen atom and the presence of the OH group at the 886 cm⁻¹ absorption peak.
In the present study, a clear decline in all phytochemicals and antioxidant activity was observed after 72 h of treatment. This decrease can be attributed to LAB bacteria depolymerizing macromolecular phenolics or converting specific phenolic compounds11. Scientists discovered a significant reduction in phytochemicals after 72 h of fermentation, consistent with other50. Microbial metabolism and enzymatic activities can change phenolic compounds during fermentation, with specific enzymes potentially degrading or modifying phenolics, leading to their reduction. Some studies suggest that phenolic degradation may occur after an optimal fermentation period, causing a decrease in their content after a peak51. After an initial increase, certain phenolic compounds may undergo additional reactions or transformations, reducing their concentration over time.
Fermentation with probiotics produces aromatic amine groups, indicating the presence of bacterial proteins in the final fermented product, which have antibacterial properties in different fermented samples52. Studies by Ray, et al.53 and Panda, et al.54 found various phenol groups, aliphatic amines, alcohols, and carboxylic acids at 1000, 1250, and 3270 cm⁻¹ peaks in fruit and purple sweet potato (Ipomoea batatas L.) extracts. During fermentation, bond rearrangements occur, such as C-H stretching vibration changes from 2928 cm⁻¹ to 2925 cm⁻¹, indicating hydrogen bond formation. Ma, et al.55 revealed that hydrogen bonding at the glycosidic bond between the oxygen atom and the presence of the OH group at the 886 cm⁻¹ absorption peak.
Many positive chemical changes occur during fermentation, improving intermolecular crosslinking and sometimes altering molecular structure to create new covalent bonds, protein interactions, and bioactive compounds56. Fermentation changes hydrogen bonding and increases intermolecular covalent cross-linking, ultimately enhancing juice quality. FTIR results are supported by Panda, et al.54, who fermented sapote fruit to produce wine, identifying peaks at 1201, 1459, 2847, 2925, and 3025 cm⁻¹ linked to secondary amides, aromatic esters, O-H stretching compounds, and C-H stretching. These differences mainly occur between 700 and 2000 cm⁻¹, directly linked to fermentation and the formation of new compounds like phenols, organic acids, glycerol, and alcohol. Peaks at 1352 and 1644 cm⁻¹ indicated amide II and amide I, linked to interactions between amino and hydroxyl groups that modified the secondary structure of pullulan film. The peak at 1148 cm⁻¹ represented a typical saccharide structure56.
Conclusions
The present study demonstrated that the fermentation of mulberry fruit juice using co-cultures led to a substantial increase in metabolites, resulting in enhanced DPPH free radical scavenging activity and ferric reducing antioxidant power (FRAP). Among the tested combinations, the co-cultures of Lactobacillus casei-L. paracasei (LC-LPC) and L. plantarum-L. paracasei (LP-LPC) showed the most significant improvements. Metabolite production peaked after 48 h for the LC-LPC treatment and after 24 h for the LP-LPC treatment, with a subsequent decline in synthesis beyond these time points.
The fermentation process brought about notable structural changes in the juice, as confirmed by FTIR analysis. These changes included the strengthening of hydrogen bonds and the formation of intermolecular covalent cross-links. These structural improvements were attributed to the creation of new compounds, such as alcohols, organic acids, phenols, and glycerol, which contributed to the enhanced properties of the juice. The findings provide valuable insights into the process of designing fermentation systems to transform mulberry fruit into functional food products. This study offers both theoretical and practical guidance for businesses seeking to improve fermentation processes for producing high-quality, bioactive-rich products. Future research should focus on optimizing fermentation conditions to maximize bioactive compound production and exploring other probiotic strains to enhance the functional properties of fermented products. Understanding the molecular mechanisms behind phenolic biotransformation during fermentation is also crucial. Additionally, studying the long-term stability of bioactive compounds in fermented juices and conducting clinical trials to validate their health benefits are essential next steps. Expanding the variety of fermented products and assessing the environmental impact of production processes will further enhance the commercial and health potential of fermented mulberry juice. By addressing these areas, the health benefits and market potential of mulberry fruit-based functional foods can be fully realized.
Data availability
Data will be available upon request with the corresponding author Mostafa Gouda.
References
Mehmood, A. et al. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L). Ultrason. Sonochem. 51, 12–19 (2019).
Rohela, G. K. et al. Indirect regeneration and genetic fidelity analysis of acclimated plantlets through SCoT and ISSR markers in Morus alba L. Cv. Chinese white. Biotechnol. Rep. 25, e00417 (2020).
Kwaw, E. et al. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 250, 148–154 (2018).
Wei-Guo, Z. et al. Construction of fingerprinting and genetic diversity of mulberry cultivars in China by ISSR markers. Acta Genetica Sinica. 33, 851–860 (2006).
Eyduran, S. P. et al. Organic acids, sugars, vitamin C, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 88 (2015).
Ranjith, F. H. et al. Influence of natural antifungal coatings produced by Lacto-fermented antifungal substances on respiration, quality, antioxidant attributes, and shelf life of mango (Mangifera indica L). Postharvest Biol. Technol. 189, 111904 (2022).
Rodríguez, L. G. R. et al. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 140, 109854 (2021).
Perricone, M., Bevilacqua, A., Altieri, C., Sinigaglia, M. & Corbo M. R. Challenges for the production of probiotic fruit juices. Beverages 1, 95–103 (2015).
Aspri, M., Papademas, P. & Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 6, 30 (2020).
Vinderola, G., Burns, P. & Reinheimer, J. In Vegetarian and plant-based Diets in Health and Disease Prevention809–835 (Elsevier, 2017).
Kaprasob, R., Kerdchoechuen, O., Laohakunjit, N., Sarkar, D. & Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 59, 141–149 (2017).
Wang, Z. et al. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 373, 131455 (2022).
Yaqoob, S. et al. Influence of multiple freezing/thawing cycles on a structural, rheological, and textural profile of fermented and unfermented corn dough. Food Sci. Nutr. 7, 3471–3479 (2019).
Yaqoob, S. et al. The effect of lactic acid bacteria and co-culture on structural, rheological, and textural profile of corn dough. Food Sci. Nutr. 10, 264–271 (2022).
Fessard, A. & Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 301, 61–72 (2019).
Gao, H. et al. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Bioscience. 29, 62–72 (2019).
Degrain, A., Manhivi, V., Remize, F., Garcia, C. & Sivakumar, D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African nightshade. Microorganisms 8, 1324 (2020).
Wafula, E. N. et al. Fermentation of African indigenous leafy vegetables to lower post-harvest losses, maintain quality and increase product safety. Afr. J. Hortic. Sci. 9, 1–13 (2016).
Srionnual, S., Yanagida, F., Lin, L. H., Hsiao, K. N. & Chen, Y. -s. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Applied and Environmental Microbiology 73, 2247–2250 (2007).
Wei, M. et al. Comparison of physicochemical indexes, amino acids, phenolic compounds and volatile compounds in bog bilberry juice fermented by Lactobacillus plantarum under different pH conditions. J. Food Sci. Technol. 55, 2240–2250 (2018).
Chen, G. L., Chen, S. G., Xiao, Y. & Fu, N. L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crops Prod. 111, 430–445 (2018).
Kumaran, A. & Karunakaran, R. J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 40, 344–352 (2007).
Tchabo, W. et al. Effects of ultrasound, high pressure, and manosonication processes on phenolic profile and antioxidant properties of a sulfur dioxide-free mulberry (Morus nigra) wine. Food Bioprocess Technol. 10, 1210–1223 (2017).
Sun, J. S. et al. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns 24, 225–231 (1998).
Natić, M. M. et al. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 171, 128–136 (2015).
Apak, R., Güçlü, K., Özyürek, M. & Karademir, S. E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52, 7970–7981 (2004).
Ramirez, J. E., Zambrano, R., Sepúlveda, B., Kennelly, E. J. & Simirgiotis, M. J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 176, 106–114 (2015).
If, B. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Khalifa, I., Nilsuwan, K., Prodpran, T. & Benjakul, S. Covalently phenolated-β-lactoglobulin-pullulan as a green halochromic biosensor efficiency monitored Barramundi fish’s spoilage. Int. J. Biol. Macromol. 243 https://doi.org/10.1016/j.ijbiomac.2023.125189 (2023).
Gouda, M., Chen, K., Li, X., Liu, Y. & He, Y. Detection of microalgae single-cell antioxidant and electrochemical potentials by gold microelectrode and Raman micro-spectroscopy combined with chemometrics. Sens. Actuators B. 329, 129229. https://doi.org/10.1016/j.snb.2020.129229 (2021).
Gouda, M., Zu, L., Ma, S., Sheng, L. & Ma, M. Influence of bio-active terpenes on the characteristics and functional properties of egg yolk. Food Hydrocoll. 80, 222–230. https://doi.org/10.1016/j.foodhyd.2018.02.009 (2018).
Ansari, M. A. & Asiri, S. M. M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 171, 44–58. https://doi.org/10.1016/j.ijbiomac.2020.12.162 (2021).
Gouda, M. et al. Bioprobe-RNA-seq-microraman system for deep tracking of the live single-cell metabolic pathway chemometrics. Biosens. Bioelectron. 261 https://doi.org/10.1016/j.bios.2024.116504 (2024).
Gallo, M. et al. Lactic fermentation of cereal flour: feasibility tests on rice, oat and wheat. Appl. Food Biotechnol. 6, 165–172 (2019).
Khan, S. A. et al. Co-culture submerged fermentation by lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. 326, 126985 (2020).
Leonard, W., Zhang, P., Ying, D., Adhikari, B. & Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 49, 107763 (2021).
Loghman, S. et al. Single and co-cultures of proteolytic lactic acid bacteria in the manufacture of fermented milk with high ACE inhibitory and antioxidant activities. Fermentation 8, 448 (2022).
Ouwehand, A. C., Invernici, M. M., Furlaneto, F. A. & Messora, M. R. Effectiveness of multi-strain versus single-strain probiotics: current status and recommendations for the future. J. Clin. Gastroenterol. 52, S35–S40 (2018).
Yang, W. et al. Changes in nutritional composition, volatile organic compounds and antioxidant activity of peach pulp fermented by lactobacillus. Food Bioscience. 49, 101894 (2022).
Dueñas, M., Fernández, D., Hernandez, T., Estrella, I. & Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food. Agric. 85, 297–304 (2005).
Mantzourani, I. et al. Potential of the probiotic Lactobacillus plantarum ATCC 14917 strain to produce functional fermented pomegranate juice. Foods 8, 4 (2018).
Chandrasekara, A., Naczk, M. & Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 133, 1–9 (2012).
McCUE, P., Horii, A. & SHETTY, K. Solid-state bioconversion of phenolic antioxidants from defatted soybean powders by Rhizopus Oligosporus: role of carbohydrate‐cleaving enzymes. J. Food Biochem. 27, 501–514 (2003).
Wu, C. et al. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. Lwt 122, 109064 (2020).
Zhou, Y. et al. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food. Agric. 100, 3283–3290 (2020).
Lee, I. H., Hung, Y. H. & Chou, C. C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 121, 150–156 (2008).
Yao, Q., JIN, X. & Heui, D. P. Comparison of antioxidant activities in black soybean preparations fermented with various microorganisms. Agricultural Sci. China. 9, 1065–1071 (2010).
Vadivel, V., Stuetz, W., Scherbaum, V. & Biesalski, H. Total free phenolic content and health relevant functionality of Indian wild legume grains: Effect of indigenous processing methods. J. Food Compos. Anal. 24, 935–943 (2011).
Marazza, J. A., Nazareno, M. A., de Giori, G. S. & Garro, M. S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods. 4, 594–601 (2012).
Xu, H. et al. Change of phytochemicals and bioactive substances in Lactobacillus fermented Citrus juice during the fermentation process. Lwt 180, 114715 (2023).
Melini, F. & Melini, V. Impact of fermentation on phenolic compounds and antioxidant capacity of quinoa. Fermentation 7, 20 (2021).
Vaithilingam, M. et al. Fermentation of beet juice using lactic acid bacteria and its cytotoxic activity against human liver cancer cell lines HepG2. Curr. Bioact. Compd. 12, 258–263 (2016).
Ray, R. C., Panda, S. K., Swain, M. R. & Sivakumar, P. S. Proximate composition and sensory evaluation of anthocyanin-rich purple sweet potato (Ipomoea batatas L.) wine. Int. J. Food Sci. Technol. 47, 452–458 (2012).
Panda, S. K., Sahu, U. C., Behera, S. K. & Ray, R. C. Fermentation of sapota (Achras sapota Linn.) Fruits to functional wine. Nutrafoods 13, 179–186 (2014).
Ma, X., Wang, K., Chou, K. C., Li, Q. & Lu, X. Conditional generative Adversarial Network for Spectral Recovery to accelerate single-cell Raman Spectroscopic Analysis. Anal. Chem. 94, 577–582. https://doi.org/10.1021/acs.analchem.1c04263 (2022).
Kang, L. et al. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason. Sonochem. 92, 106242 (2023).
Acknowledgements
The authors extend their appreciation to Jiangsu University central labs for facilitating several ex-periments of the current study. Besides, this research was funded by National Natural Science Foun-dation of China (Grant No. 42301210. Also, it was funded by the “Belt and Road” joint project fund between Zhejiang University, China, and National Research Centre, Egypt (Project No: SQ2023YFE0103360). The authors appreciate the support from Researchers Supporting Project num-ber (RSPD2024R641), King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded by National Natural Science Foundation of China (Grant No. 42301210). Also, it was funded by the “Belt and Road” joint project fund between Zhejiang University, China, and National Research Centre, Egypt (Project No: SQ2023YFE0103360). The authors appreciate the support from Researchers Supporting Project number (RSPD2024R641), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
CRediT authorship contribution statementConceptualization, S.Y., Y.M.; methodology, S.Y., M.M.G., I.K., K.A.A., Y.M.; validation, M.M.G., B.M., A.I., T.A., I.K., Y.M.; formal analysis, M.M.G., B.M., A.I., M.S.M., K.A.A., I.K., Y.M.; inves-tigation, S.Y., B.M., A.I., M.S.M., K.A.A., A.R., T.A.B., T.A.; data curation, S.Y., M.M.G., I.K., Y.M.; writing—original draft preparation, S.Y., M.M.G., A.R., T.A., I.K., Y.M.; writing—review and editing, S.Y., M.M.G., T.A.B., T.A., I.K., Y.M.; visualization, M.M.G., I.K.; supervision, M.M.G., I.K., Y.M.; funding acquisition, M.M.G., I.K., Y.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yaqoob, S., Gouda, M.M., Mubeen, B. et al. Synergistic enhancement in the mulberry juice’s antioxidant activity by using lactic acid bacteria co-culture fermentation. Sci Rep 15, 19453 (2025). https://doi.org/10.1038/s41598-024-81023-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81023-8