Abstract
Investigate the impact of diaphragm sellae competence on surgical outcomes and risk factors for postoperative hypothalamic injury (HI) in patients undergoing endoscopic transsphenoidal surgery (ETS) for infradiaphragmatic craniopharyngiomas (ICs). A retrospective analysis of 54 consecutive patients (2016–2023) with ICs treated by ETS was conducted. All tumors originated from the sellar region inferior to the diaphragm sellae and were classified into two subtypes in terms of diaphragm sellae competence: IC with competent diaphragm sellae (IC-CDS) and IC with incompetent diaphragm sellae (IC-IDS). Clinical features, intraoperative findings, and follow-up data were compared between subtypes. Postoperative HI was assessed using an magnetic resonance imaging-based scoring system. Fifty-four patients (29 males, 25 females) were included in this study, with 12 (22.2%) under 18 years old. Overall, 35 cases were IC-CDS, while 19 were IC-IDS. Compared with IC-CDS, patients with IC-IDS tended to have hormone hypofunction before surgery (p = 0.03). Tumor volume in IC-IDS group (9.0 ± 8.6 cm3) was also higher than that in IC-CDS group (3.3 ± 3.4 cm3, p = 0.011). Thirty-seven patients underwent standard endoscopic transsphenoidal approach (SEA) and 17 underwent an extended endoscopic transsphenoidal approach (EEA). Gross total resection (GTR) was achieved in 50 cases (92.6%). Postoperative CSF leak was observed in four patients (7.4%). Permanent diabetes insipidus (DI) occurred in 13 patients (27.7%), six in IC-CDS and seven in IC-IDS. Postoperative HI occurred in 38.9% of patients. Univariate analysis revealed that large tumor size (p = 0.014), prior hypopituitarism(p = 0.048) and IC-IDS (p < 0.001) were significantly associated with postoperative HI. Multivariate analysis revealed that IC- IDS was the sole predictor of postoperative HI. To our knowledge, this is the largest case series in the literature to describe IC resected by endoscopic surgery in a single institution. Classification based on diaphragm sellae competence highlights distinct clinical features and surgical outcomes between IC-CDS and IC-IDS subtypes. Notably, IC-IDS is an independent risk factor for postoperative HI. Preoperative identification of subtype can guide surgical strategy and potentially minimize complications.
Similar content being viewed by others
Introduction
Craniopharyngiomas are rare benign tumors that are still challenging for neurosurgeons. They have historically been classified into different types in terms of ___location and origin1,2,3. Yasagil et al. proposed a classic classification based on tumor ___location in the 1990s and recommended the microscopical transsphenoidal approach for the intrasellar-infra-diaphragmatic craniopharyngiomas1. Furthermore, according to tumor origin from beneath or above the diaphragm sellae, Wang et al. suggested infradiaphragmatic and supradiaphragmatic craniopharyngiomas4. Recently, the emergence of endoscopic transsphenoidal surgery (ETS) has revolutionized CP management due to its superior visualization and avoidance of brain injury in surgical passages5,6,7. Infradiaphragmatic craniopharyngiomas (ICs), arising below the diaphragm sellae, are particularly suited for ETS1,8,9. Anatomically, diaphragm sellae is a dual sheath and the roof of sella turcica, it covers the upper surface of pituitary gland with a medial opening for pituitary stalk passing. According to the literature, there is a remarkable variation in the size of diaphragm opening, resulting in the tight or open type of diaphragm10,11,12. This distinction has significant surgical and functional implications. ICs can be confined infradiaphragmatically with a competent diaphragm sellae (IC-CDS) or extend superiorly through an incompetent diaphragm sellae, contacting suprasellar structures (IC-IDS) including suprasellar cistern, the third ventrical floor, etc4. There is still no consensus on the nature of the structure adhering superiorly to the tumor, whether it be arachnoid membrain, diaphragma sellae, or pseudocapsule13.
Several studies described IC. Jamshidi et al. demonstrated IC as a complement to the Kassam classification, a classification based on the relationship to infundibulum through endoscopic observation2,10. Fan et al. also described IC in their “QST” classification which was based on tumor origin and preoperative radiological evaluation14. However, these studies either focus solely on ICs with intact diaphragm sellae or categorize tumors based on ___location, neglecting the crucial distinction of diaphragm competence. This distinction significantly impacts surgical strategy and potential complications9,15.
Hypothalamic injury (HI) after surgery for craniopharyngiomas can lead to diabetes insipidus (DI), sodium disturbances, and impaired quality of life16,17. The severity of HI determines residual hypothalamic function and associated sequelae. Data on the degree and risk factors of HI specifically in ICs remain scarce.
This study addresses this gap by analyzing a series of endoscopically resected ICs categorized as IC-CDS or IC-IDS based on diaphragm sellae competence. We compare clinical features, surgical outcomes, and follow-up data between the two groups. Additionally, we assess the severity and risk factors of postoperative HI. Our study proposes a supplementary classification scheme for ICs that can inform surgical decision-making and outcome prediction.
Methods
Patients selection
After obtaining institutional review board approval, a total of 54 patients with IC resected via an endoscopic endonasal approach at Xiangya Hospital, Central South University between May 2016 and January 2023 were identified. Patients with recurrent tumors were excluded from the study. Demographic, clinical, radiological, and surgical results were retrospectively examined and analyzed.
Preoperative evaluation
All patients underwent contrast-enhanced magnetic resonance imaging (MRI) before surgery and 1–2 days postoperatively. CT scans were used to evaluate calcification before surgery and hematoma after surgery. All MRI were reviewed using a picture archiving and communication system. IC were defined as those tumors that originated from the sella with an enlarged pituitary fossa, and the gland was scarcely recognizable.
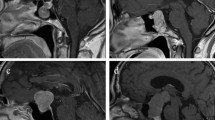
As described above, IC could be categorized into two subtypes based on the aperture size and the competence of diaphragm sellae. There are several noticeable MRI features to identify two subtypes of IC. In IC-CDS, the tumor elevated and stretched the diaphragm sellae upward which formed a smooth bulging along with the dura mater of the skull base, indicating the integrity of diaphragm sellae, and the suprasellar extension of the tumors were rounded shaped and symmetrical with clear borders on the MRI. Even with considerable suprasellar extension, the tumors remain confined below diaphragm sellae. While in IC-IDS, the tumor passed through the opening of diaphragm sellae and directly contacted suprasellar structures with a snowman-like appearance. The suprasellar aspects of these tumors were irregular on MRI and matched the suprasellar cistern morphology. All those characteristics suggest the diaphragm sellae was incomplete (Fig. 1).
Schematic diagrams and T2-weighted MRI showing the opening of diaphragma sellae in IC-CDS and IC-IDS. In IC-CDS, the lesion confined below the competent diaphragma sellae (Orange line) even with a suprasellar extension (A,B). In IC-IDS, the lesion went through the large opening of diaphragma sellae (Orange line) and directly contacted suprasellar structures (C,D).
Tumor volume was calculated by a senior radiologist. Hormone levels, including free thyroxine, fasting morning cortisol, growth hormone, prolactin, testosterone, estrogen, follicle-stimulating hormone, and luteinizing hormone were evaluated before and after surgery for each patient.
Surgical procedure and postoperative management
Tailored surgical approach was adopted based on the recommendation of “The EANS task force”1. Specifically, in the IC-CDS group, tumors were resected using the standard endoscopic transsphenoidal approach (SEA, Fig. 2). In IC-IDS, most tumors were removed via extended endoscopic transsphenoidal approach (EEA, Fig. 3). Both SEA and EEA procedures have been described in previous reports2,4,5,6. Under intraoperative endoscopic observation, we certified all tumors originating from the pituitary stalk below the level of the diaphragm sellae. After resection of the tumor, the skull base reconstruction protocol was done in a slightly different manner from the previous algorithm7,18. In our series, in EEA, we used multiple layer reconstruction, including fat tissue, a fascial graft, an in situ bone flap, and finally the premade pedicled septal flap. In SEA, the fat tissue or fascial graft was not prepared to avoid an additional wound; only artificial dura patch and pedicled septal flaps were used instead. Lumbar drainage was not routinely performed unless CSF leakage occurred postoperatively. Hormone levels were evaluated on the day after surgery and before discharge.
Illustrative case of IC-CDS in a 12-year-old boy with preoperative hypopituitarism. Preoperative coronal (A) and sagittal (B) postcontrast T1-weighted MR images showed a cystic mass intra sellar region with the intact diaphragm sellae. Intraoperative images indicated the tumor was cystic and filled with a yellowish content (C). After removal of the tumor, the compressed pituitary and intact diaphragm sellae were displayed (D). Postoperative MR images showed the mass was totally removed, both pituitary and the stalk were discernable (E,F).
Illustrative case of IC-IDS in a 58-year-old male with headache and preoperative hypopituitarism. Preoperative coronal (A) and sagittal (B) enhanced T1-weighted MRI revealed a solid mass located intrasellar region and extended to suprasellar area. Intraoperative photographs indicated tumor originating from an intrasellar ___location grew through the opening of diaphragm sellae and adhered with third ventricle floor (C). After total removal of the tumor, optic chiasm, third ventricle and residual pituitary stalk were observed (D). Post-operative MRI certified tumor was totally removed and pituitary stalk was preserved (E,F).
Assessment of postoperative HI
Postoperative HI was assessed based on a neuroimaging scoring scale slightly modified from previous reported semi-quantitative scoring system8,9. In our scoring system, we focused on integrity of floor and lateral wall of third ventricle, which were thoroughly assessed using three coronal sections of MRI within 48 h after surgery, including section through the anterior commissure, section midway between the anterior commissure and mammillary bodies, and section through the mammillary bodies. A score of 1 was assigned to the defect of each structure, and score of 0.5 for a unilateral injury of the third ventricle wall. Finally, the score of each structure was added up. Patients with score ≥ 0.5 were considered as postoperative HI.
Follow-up
Patients were followed-up face-to-face at 3 and 6 months after surgery and annually thereafter. MRI and endocrinological evaluations were performed for each patient. The extent of resection was evaluated on MRI 3 months after surgery. Gross total resection referred to 100% volume removed and no calcification left; near-total resection referred to 90–99% of the volume removed.
Statistical analysis
Data were collected using Microsoft Excel 2019 (Microsoft Corp.) and analyzed using SPSS version 26 (IBM Corp.). Data were compared using the t-test, Mann-Whitney test, Chi-square test, or Fisher’s exact test, as appropriate. Univariate and multivariate logistic regression analysis were performed to determine independent predictive factors for postoperative HI. Statistical significance was set at p < 0.05.
Results
Clinical characteristics and surgical outcomes
Fifty-four patients (29 men and 25 women) were included in the study (Table 1). Overall, 77.8% (42) of the patients were adults, and only 22.2% (12) were children. Age and sex distributions were not significantly different between the two groups. With regard to onset symptoms, hormonal deficiency (59.3%) was the most prevalent symptom, followed by headache (55.6%) and vision impairment (46.3%). Hormonal deficiency included panhypopituitarism in 22 cases, hypogonadism in 8 cases, and hypothyroidism in 3 cases. Patients with IC-IDS were more prone to suffer from pituitary hypofunction than IC-CDS (78.9% vs. 48.6%, p = 0.03). Contrarily, headaches more frequently occurred in IC-CDS than IC-IDS (p = 0.041). Other symptoms such as vision impairment and DI showed no differences between the IC-CDS and IC-IDS groups. Preoperative MRI revealed an average tumor volume of 5.3 cm3 (range 0.5–29.6 cm3). Patients with IC-IDS were likely to harbor larger tumors (9.0 ± 8.6 cm3) than IC-CDS (3.3 ± 3.4 cm3, p = 0.011). No cavernous sinus invasion was observed in our series.
A total of 37 and 17 patients underwent SEA and EEA, respectively (Table 2). In patients with IC-CDS, we used SEA in almost all patients except one. That patient harbored a considerable suprasellar extension of the tumor with an unexpanded sella, thus we used EEA to broaden the visualization. In IC-IDS group, we performed EEA in 16 patients and SEA in the other 3 patients. Histopathological examination showed 38 cases (70.4%) were adamantinomatous craniopharyngiomas, 16 cases (29.6%) were papillary type. There was no difference in histopathological type between IC-CDS and IC-IDS subgroups.
The average postoperative follow-up duration was 32 months (range, 6–66 months). Gross-total resection was achieved in 92.6% (50/54) of the patients, while 4 patients including 1 IC-CDS and 3 IC-IDS had near-total resection; among these patients, tumor of the only patient in the IC-CDS group was considered to be totally removed during surgery, with postoperative MRI scan showing no tumor left. However, the tumor reappeared on MRI examination after 3 months. Tumors of the other three patients in the IC-IDS were not totally resected because of tight adhesion of a small piece of tumor to the normal vital suprasellar structures. We did not remove the residue aggressively as it may result in severe complications. These three patients were carefully observed, and the residual tumors showed no enlargement during follow-up.
Preoperative headaches in all patients relieved after surgery. Of the 25 patients with vision impairment, 20 exhibited improvement after surgery, including 11 patients with IC-CDS and 9 with IC-IDS. Preexisting DI relieved in all seven patients during follow-up. No new neurological deficits were observed postoperatively. After the surgery, 24 patients suffered from hormone hypofunction. 14 patients exhibited single hormonal axis insufficiency, and 10 patients with multiple axes insufficiency. The conditions of 10 patients with normal anterior pituitary function onset worsened postoperatively, including six in the IC-CDS group, and four in the IC-IDS group. A total of 18 of the 32 patients with hypopituitarism preoperatively improved after surgery, including 13 patients with IC-CDS and five with IC-IDS. Intraoperative CSF leak occurred in 17 patients (48.6%) in the IC-CDS group and in all patients in the IC-IDS group (p < 0.001). Postoperative CSF leak occurred in four cases, two of which ceased after lumbar drainage, while the other two patients underwent a repair surgery. Meningitis occurred in two patients in the IC-CDS group and four in the IC-IDS group, all of which relieved after treatment with antibiotics. Pituitary stalk was preserved in all patients with IC-CDS, while in the IC-IDS group, the lower part of the stalk was sacrificed in six cases to ensure radical resection. Permanent DI occurred in 13 patients, six in IC-CDS, and seven in IC-IDS.
Postoperative HI and the predictors
Then we assessed the HI based on the defects in the hypothalamic structure on postoperative MRI. Totally 21 patients including 7 IC-CDS and 14 IC-IDS showed different degrees of HI on the postoperative MRI. The details of HI score were listed in Table 3. Third ventricle floor injury was most common in both IC-CDS and IC-IDS subgroups. The proportion of patients with a score ≥ 2 in IC-IDS group (42.9%) was much higher than that in IC-CDS group (9.5%), indicating patients in IC-IDS had more severe postoperative HI. We then evaluated the association between postoperative hypopituitarism and HI, the results showed no significant differences between postoperative hypopituitarism and HI either in the whole cohort or in each subgroup (Fig. 4A). Further, to identify risk factors associated with HI in IC, we performed logistic regression analysis using clinical characteristics data. Univariate analyses indicated tumor size, prior pituitary hypofunction and IC-IDS were associated with HI. After adjustment for confounding factors in multivariate analysis, only IC-IDS was a significant predictor for postoperative HI (OR: 6.934, 95%CI: 1.59-30.241, p = 0.01, Fig. 4B).
Discussion
Several endoscopic classifications of craniopharyngiomas exist, focusing primarily on suprasellar lesions10. While Tang et al. included an “intrasellar stalk subtype” corresponding to IC in thier novel classification based on the stalk and the origin site11, it does not distinguish between competent and incompetent diaphragm sellae. This distinction may significantly impact surgical approach and potential complications. Our study focused on IC and compared clinical characteristics and outcomes categorized as IC-CDS or IC-IDS based on diaphragm sellae integrity.
In our series, 77.8% of the patients were adults, indicating a higher prevalence of suprasellar tumors in children. Patients with IC-IDS exhibited a significantly higher rate of preoperative hormone hypofunction (78.9%) compared to IC-CDS (48.6%, p = 0.03). This likely correlates with the larger tumor size observed in the IC-IDS group (9.9 cm3 vs. 3.9 cm3, p = 0.011), potentially causing greater compression to the pituitary gland and stalk.
Endoscopic transsphenoidal surgery (ETS) achieved gross-total resection (GTR) in most cases due to the intrasellar origin of ICs. This approach offers higher GTR rates compared to surgery for supradiaphragmatic tumors12,14,15,16,17,19,20. Generally, extended endoscopic approaches (EEA) were reserved for tumors located in the extrasellar space or third ventricle21,22,23,24,25. A recent multicenter study reported 84 cases of IC resected through SEA in the Italian population with satisfactory clinical outcomes26. We advocated the tailored approach based on the IC subtype. In IC-CDS, the intact diaphragm sellae separates the tumor from the subarachnoid space. Therefore, SEA is sufficient for majority of the tumors, even with suprasellar extension. The endoscope’s wide view allows for safe detachment of the tumor from the compressed pituitary and diaphragm sellae without requiring tuberculum opening. Conversely, in IC-IDS, the tumor breaches the diaphragm sellae and directly contacts suprasellar structures27. Thus, for tumors with a large portion of the supra-diaphragmatic part, we preferred EEA for safe resection. Because EEA offers a wider operative field and superior visualization, enabling meticulous dissection and minimizing the risk of hypothalamic injury associated with blind maneuvers16.
The rate of postoperative CSF leak was significantly higher in the IC-IDS group (15.8%) compared to IC-CDS (2.9%) due to the inherent disruption of the diaphragm sellae in IC-IDS. Interestingly, the intraoperative CSF leak rate in IC-CDS (48.6%) was higher than reported elsewhere12. This might be because we meticulously dissected along the stalk for complete tumor removal, potentially exposing CSF pathways. In our view, the tumor could still extend along the stalk, despite the diaphragm sellae being intact. Because of the relatively high incidence of intraoperative CSF leak, we harvested a tailored nasoseptal flap with a pedicle in almost every patient who was diagnosed with craniopharyngioma preoperatively. For cases that were hardly distinguishable from Rathkes’ cyst, rescue flaps were used instead28. The incidence of postoperative meningitis in our series (11.1%) was within the range reported for EEA (1 − 21.9%)17,29,30,31. Meningitis in the postoperative period is probably associated with postoperative CSF leaks (bacterial meningitis), or chemical irritation by the tumor content and artificial dura patch used for reconstruction (chemical meningitis).
Pituitary stalk preservation was achieved in all IC-CDS patients and most IC-IDS patients (68.4%). Notably, some cases in IC-IDS extensively involved the stalk, necessitating sacrifice for complete resection. In our study, tumors growing along the stalk were observed in both groups, mimicking transinfundibular craniopharyngiomas. However, IC-IDS arises from the intrasellar region with suprasellar extension through the diaphragm sellae, whereas transinfundibular tumors originate from the suprasellar area. On the other hand, IC-IDS can grow exophytically of the stalk through the aperture of diaphragm sellae, resulting in variable suprasellar extension patterns (anterior or posterior to the infundibulum) distinct from the transinfundibular type.
The incidence of permanent DI in our series was lower than reported previously14,17. Interestingly, seven patients even experienced preoperative DI relief during follow-up, suggesting potential for hypothalamic functional recovery despite stalk sacrifice. This aligns with other studies showing DI improvement after stalk sectioning11,32, highlighting the importance of hypothalamic preservation beyond just stalk integrity.
Due to the intrasellar stalk origin, postoperative HI is less frequent in IC than the supradiaphragmatic craniopharyngiomas. Only a few studies explored the HI in IC, one study revealed worsened hypothalamic impairment occurred in 16.67% of IC patients after endoscopic transsphenoidal surgery33. Another report evaluated the HI based on endoscopic observation and indicated that only 9.1% of IC had HI34. In our group, HI was assessed through postoperative MRI, 21/54 patients had HI, which was higher than previous studies. This difference might be attributed to the use of postoperative MRI for HI assessment, potentially detecting subtle injury not captured by other methods. Prior studies mentioned degree of postoperative hypothlamic damage catergorized using postoperative MR significantly correlated with patient outcome including BMI score and Health Utility Index Mark 235. Another study utilizing the hypothalamo-hypophyseal injury score also linked higher scores to severe hypernatremia and adipsic DI in patients undergoing transcranial surgery for craniopharyngiomas. Additionally, they reported a correlation between tumor size and postoperative hypothalamo–hypophyseal injury8. Consistent with these findings, univariate analysis identified large tumor size, prior hypopituitarism, and IC-IDS as factors associated with HI in our study. However, only IC-IDS emerged as an independent risk factor in the multivariate analysis. Identifying high-risk patients preoperatively not only enables us to provide patients and their families with informed consent regarding potential high hypothalamic injury risks and post-operative management, but also allows us to adopt a more cautious approach during surgery involving the hypothalamus and adjacent regions in specific patients, such as avoiding coagulation and excessive manipulation in the hypothalamus. We aim for a maximal safe resection and leaving suspicious residual tumor in some instances if it is tightly attached to vital structures. This is in accordance with previous studies36,37.
In our study, large tumor size, prior hypopituitarism and IC-IDS were also asscociated with postoperative HI in univarite analysis, However, in multivariate analysis in our study, the IC-IDS is the only risk factor for postoperative HI.
This suggests that the intact diaphragm sellae in IC-CDS likely acts as a protective barrier, shielding the hypothalamus from surgical injury compared to IC-IDS where the tumor directly contacts the suprasellar structures including hypothalamus. Consequently, patients with IC-IDS may be at a higher risk of developing complications like recurrent hypernatremia and adipsic DI.
Conclusions
The current study described two different subtypes of infradiaphragmatic craniopharyngiomas (ICs) indicating distinct clinical characteristics and surgical outcomes. Endoscopic transsphenoidal surgery achieved high gross-total resection rates in both IC subtypes. Importantly, IC-IDS emerged as an independent risk factor for postoperative hypothalamic injury (HI) in our series. The distinction between IC subtypes is crucial for guiding surgical decision-making and potentially minimizing complications and preserving hypothalamic function. Future studies with larger cohorts are warranted to validate this classification system and its impact on long-term functional outcomes.
Data availability
All data generated or analysed during this study are included in this published article.
References
Cossu, G. et al. Surgical management of craniopharyngiomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir. (Wien) 162(5), 1159–1177 (2020).
Hofstetter, C. P. et al. Endoscopic endonasal transsphenoidal surgery for functional pituitary adenomas. Neurosurg. Focus. 30(4), E10 (2011).
Sainte-Rose, C. et al. Craniopharyngioma: The pendulum of surgical management. Childs Nerv. Syst. 21(8–9), 691–695 (2005).
Frank, G. et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 59(1 Suppl 1), ONS75–83 (2006).
Gardner, P. A. et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: A case series. J. Neurosurg. 109(1), 6–16 (2008).
Gong, X. et al. Outcome of endoscopic transsphenoidal surgery for recurrent or residual pituitary adenomas and comparison to non-recurrent or residual cohort by propensity score analysis. Front. Endocrinol. (Lausanne). 13, 837025 (2022).
Patel, K. S. et al. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J. Neurosurg. 119(3), 661–668 (2013).
Du, C. et al. Relationship between postoperative hypothalamic injury and water and sodium disturbance in patients with craniopharyngioma: A retrospective study of 178 cases. Front. Endocrinol. (Lausanne) 13, 958295 (2022).
Roth, C. L. et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obes. (Silver Spring). 23(6), 1226–1233 (2015).
Kassam, A. B. et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: A new classification based on the infundibulum. J. Neurosurg. 108(4), 715–728 (2008).
Tang, B. et al. A novel endoscopic classification for craniopharyngioma based on its origin. Sci. Rep. 8(1), 10215 (2018).
Jamshidi, A. O. et al. A modern series of subdiaphragmatic craniopharyngiomas. J. Neurosurg. 131(2), 526–531 (2018).
Cabuk, B. et al. Anatomic and histologic features of diaphragma sellae that effects the suprasellar extension. J. Clin. Neurosci. 71, 234–244 (2020).
Nishioka, H. et al. Endoscopic endonasal surgery for subdiaphragmatic type craniopharyngiomas. Neurol. Med. Chir. (Tokyo). 58(6), 260–265 (2018).
Mou, J. et al. Endoscopic Endonasal Surgery for Craniopharyngiomas: A Series of 60 Patients (World Neurosurg, 2019).
Tang, B. et al. Clinical features and operative technique of transinfundibular craniopharyngioma. J. Neurosurg. 1–10 (2019).
Koutourousiou, M. et al. Endoscopic endonasal surgery for craniopharyngiomas: Surgical outcome in 64 patients. J. Neurosurg. 119(5), 1194–1207 (2013).
Hadad, G. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope 116(10), 1882–1886 (2006).
Almeida, J. P. et al. Current results of surgical treatment of craniopharyngiomas: The impact of endoscopic endonasal approaches. World Neurosurg. 142, 582–592 (2020).
Almeida, J. P. et al. Surgical anatomy Applied to the resection of craniopharyngiomas: Anatomic compartments and surgical classifications. World Neurosurg. 142, 611–625 (2020).
Cavallo, L. M. et al. Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: Anatomic considerations–part 1. Neurosurgery 62(6 Suppl 3), 1202–1212 (2008).
Cappabianca, P. et al. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surgery. Adv. Tech. Stand. Neurosurg. 33, 151–199 (2008).
de Divitiis, E. et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 61(5 Suppl 2), 219 – 227 (2007).
Cavallo, L. M. et al. The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle. Neurosurg. Rev. 36(1), 27–37 (2013). discussion 38.
Cavallo, L. M. et al. Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: Anatomic considerations–part 1. Neurosurgery 61(3 Suppl), 24–33 (2007).
Solari, D. et al. Endoscopic endonasal approach for infradiaphragmatic craniopharyngiomas: A multicentric Italian study. J. Neurosurg. 1–11 (2022).
Wang, K. C. et al. Growth patterns of craniopharyngioma in children: Role of the diaphragm sellae and its surgical implication. Surg. Neurol. 57(1), 25–33 (2002).
Rawal, R. B. et al. Minimizing morbidity in endoscopic pituitary surgery: Outcomes of the novel nasoseptal rescue flap technique. Otolaryngol. Head Neck Surg. 147(3), 434–437 (2012).
Seo, Y. et al. Outcomes of the endoscopic endonasal approach for tumors in the third ventricle or invading the third ventricle. J. Clin. Neurosci. 90, 302–310 (2021).
Algattas, H. et al. Endoscopic endonasal approach for craniopharyngiomas with intraventricular extension: Case series, long-term outcomes, and review. World Neurosurg. 144, e447–e459 (2020).
Cavallo, L. M. et al. The endoscopic endonasal approach for the management of craniopharyngiomas: A series of 103 patients. J. Neurosurg. 121(1), 100–113 (2014).
Ogawa, Y., Niizuma, K. & Tominaga, T. Recovery from diabetes insipidus and preservation of thyroid function after craniopharyngioma removal and pituitary stalk sectioning. Clin. Neurol. Neurosurg. 162, 36–40 (2017).
Solari, D. et al. Endoscopic endonasal approach for infradiaphragmatic craniopharyngiomas: A multicentric Italian study. J. Neurosurg. 138(2), 522–532 (2023).
Yang, L. et al. Hypothalamic injury patterns after resection of craniopharyngiomas and correlation to tumor origin: A study based on endoscopic observation. Cancer Med. 9(23), 8950–8961 (2020).
Puget, S. et al. Pediatric craniopharyngiomas: Classification and treatment according to the degree of hypothalamic involvement. J. Neurosurg. 106(1 Suppl), 3–12 (2007).
Elowe-Gruau, E. et al. Childhood craniopharyngioma: Hypothalamus-sparing surgery decreases the risk of obesity. J. Clin. Endocrinol. Metab. 98(6), 2376–2382 (2013).
Rovani, S. et al. Long-term weight gain in children with craniopharyngioma. Eur. J. Endocrinol. 190(5), 363–373 (2024).
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81701285), the Natural Science Foundation of Hunan Province (Grant No. 2023JJ30908 and Grant No. 2018JJ3824), and Health Research Project of Hunan Provincial Health Commission (grant number: D202304049299).
Author information
Authors and Affiliations
Contributions
XG: idealization, data collection, writing, and review; ZC, KY, CTL, and SSF: data collection and review; HSZ: data collection, writing, and review; MYZ, ZYL and ZXL: senior surgeon, review, and supervision. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All research was performed in accordance with relevant guidelines/regulations. Studies involving human participants were reviewed and approved by the Xiangya Hospital, Central South University. Written informed consent was obtained from all the individual(s) for publication of any potentially identifiable images included in this article. Informed consent was obtained from all subjects and/or their legal guardian(s).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gong, X., Chen, Z., Yang, K. et al. Endoscopic transsphenoidal surgery for infradiaphragmatic craniopharyngiomas and impact of diaphragm sellae competence on hypothalamic injury. Sci Rep 14, 30127 (2024). https://doi.org/10.1038/s41598-024-81347-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81347-5
Keywords
This article is cited by
-
Hypopituitarism in non-neuroendocrine pituitary tumors: a systematic review
Reviews in Endocrine and Metabolic Disorders (2025)