Abstract
The ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol, or NHHR, has garnered increased attention because of its connection to metabolic diseases. It is yet unknown, nevertheless, how NHHR and sarcopenia relate to one another in the US population. Examining the relationship between NHHR and the prevalence of sarcopenia in the US population is the main goal of this study. Utilizing information gathered from the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2018, a study was conducted to look at the connection between NHHR and sarcopenia in the US population. The individuals’ baseline features were categorized based on whether or not sarcopenia was present. We used subgroup analysis, restricted cubic spline (RCS), and weighted logistic regression to examine the connection between NHHR and sarcopenia prevalence. Sensitivity analysis was used to confirm that the study’s findings were reliable and consistent. R (version 4.2.3) was used for all analyses, and P < 0.05 was used to indicate statistical significance. There were 10,087 individuals in all, 9,187 of whom were not sarcopenic and 900 of whom were. Sarcopenic patients were generally older, predominantly of Mexican American descent, and had lower educational levels. These patients were generally less active and exhibited higher levels of total cholesterol and BMI, along with lower HDL levels. Following complete adjustment, the weighted logistic regression model indicated that a 7% increased risk of sarcopenia was linked to every unit rise in NHHR (OR = 1.07, 95% CI = 1.02–1.13). An inflection point was identified at 2.90 by RCS analysis, which revealed a nonlinear association between NHHR and the prevalence of sarcopenia. Subgroup analysis showed that, regardless of the demographic group, there was a continuous positive correlation between NHHR and the prevalence of sarcopenia. The results of the interaction test provided evidence in favor of NHHR functioning as a stand-alone risk factor for sarcopenia. Our research suggests that a higher prevalence of sarcopenia may be linked to an increase in NHHR. Consequently, NHHR may function as a separate risk factor for sarcopenia.
Similar content being viewed by others
Introduction
Sarcopenia is a disorder that primarily affects older persons and is characterized by a progressive loss of skeletal muscle mass and strength1. This condition leads to decreased physical function and mobility, increasing the risk of falls, fractures, and disability2,3. Recently, sarcopenia has been noted in younger populations, despite its usual association with older age4. Worldwide, the number of sarcopenia patients is projected to rise from 50 million to 200 million in the next 40 years5. Sarcopenia prevalence ranges from 8 to 36% in adults under 60 and from 10 to 27% in individuals aged 60 and above. Additionally, severe sarcopenia is found in 2–9% of adults with an average age of 68.5 years6. Sarcopenia can lead to a substantial rise in healthcare expenses. Hospitalization costs for patients under 65 years old escalate even more than for those over 65 years old7. Although numerous interventions have been suggested to combat sarcopenia, none have been successful in clinical trials thus far8.
The causes of sarcopenia are multifactorial, including age-related changes in muscle biology, hormonal changes, chronic diseases, and lifestyle factors such as physical inactivity and poor nutrition. Emerging evidence has confirmed a strong and intricate connection between obesity and sarcopenia9,10. Furthermore, obesity is marked by insulin resistance, chronic inflammation, disrupted glucose metabolism, and dyslipidemia11. Lipid abnormalities associated with obesity include decreased levels of high-density lipoprotein cholesterol (HDL-C) and higher levels of triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C). Studies carried out reveal that non-HDL cholesterol is the most common dyslipidemia12,13.
There is growing recognition for the non-HDL-C to HDL-C ratio (NHHR) as a new and comprehensive marker for evaluating the lipid composition in atherosclerosis14. Moreover, research on US populations has revealed a possible link between a greater NHHR and a higher risk of type 2 diabetes15. Research has shown that when it comes to the diagnosis of insulin resistance (IR) and metabolic syndrome, NHHR is more useful than conventional lipid markers16. Together, the studies indicate NHHR could be a valuable tool for predicting diseases related to metabolic dysfunction. Thus, establishing NHHR as a novel marker that may lead to better sarcopenia prediction and treatment is crucial, and further research into the relationship between NHHR and sarcopenia is highly important from a scientific standpoint.
As a result, we carried out a thorough investigation to look at the relationship between NHHR and sarcopenia in the American population. Our aim was to give useful epidemiological insights using data from the National Health and Nutrition Examination Survey (NHANES) gathered between 2011 and 2018.
Methods
Study population
In order to provide thorough insights on the health and nutritional status of the American people, the Centers for Disease Control and Prevention’s multi-stage survey approach is used in the cross-sectional NHANES survey. The survey uses a sophisticated multi-stage probability sampling approach to guarantee the accuracy and representativeness of the sample. Each participant gave informed written agreement when they were enrolled in the NHANES, and the National Center for Health Statistics’ ethics review board approved the study.
This study made use of four cycles’ worth of data from the NHANES between 2011 and 2018. The criteria for inclusion were as follows: (1) individuals who were older than 20 years of age; (2) individuals who had complete data on sarcopenia; and (3) individuals who had complete data on the NHHR.
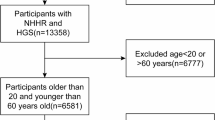
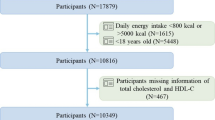
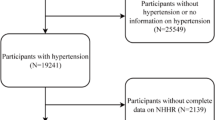
Initially, 39,156 participants were considered across the four waves. Exclusions were made for the following reasons: (1) participants younger than 20 years (n = 17,095); (2) missing sarcopenia data (n = 11,524); and (3) missing NHHR data (n = 450). Ultimately, the analysis comprised 10,087 participants (Fig. 1).
Assessment of sarcopenia
NHANES measured appendicular skeletal mass (ASM), which includes the non-bone and non-fat tissue in the limbs, using dual-energy X-ray absorptiometry (DEXA). The skeletal muscle index (SMI) was determined by the ratio of total ASM (kg) to BMI (kg/m²). Sarcopenia was defined by the Osteoarthritis Biomarkers Network of the National Institutes of Health as a SMI of less than 0.512 for women and 0.789 for males.
Assessment of NHHR
NHHR was employed as an independent variable in this investigation. The non-HDL to HDL cholesterol ratio, or NHHR, is computed based on the lipid profiles of the individuals.
Assessment of covariates
Numerous confounders were taken into account in this study, such as health status, lifestyle factors, and demographics. The demographic factors used in the study were age, sex, race, poverty index ratio (PIR), and level of education. Based on the household income to the poverty line ratio, PIR was divided into three groups: less than 1, 1 to less than 3, and 3 or more. Lifestyle variables comprised smoking behavior and physical activity levels. The findings of a questionnaire intended to assess smoking behavior were used to classify people as smokers if they had smoked more than 100 cigarettes throughout their lives. The Global Physical Activity Questionnaire was utilized to evaluate the degree of physical activity. The metabolic equivalent (MET) value was calculated using the formula: MET (minutes/week) = MET × frequency per week × duration per activity. Physical inactivity was defined as having a MET value below 600 min per week. Health status was determined by physician diagnoses or self-reports and included conditions such as diabetes, hypertension, and chronic kidney disease.
Statistical analysis
From 2011 to 2018, four survey cycles’ worth of data were gathered and entered into the NHANES database. Using descriptive analysis, the baseline characteristics of the final participants were categorized according to the presence or absence of sarcopenia. Continuous variables were presented as mean and standard deviation. Percentages are used to represent categorical variables. Logistic regression analysis was used to examine the relationship between NHHR and sarcopenia after a number of variables were taken into account. To increase the robustness of the findings and investigate potential associations between varying NHHR levels and sarcopenia, NHHR was transformed from a continuous to a categorical variable. To illustrate the dose-response relationship between NHHR and sarcopenia, restricted cubic spline (RCS) analysis was utilized. Additionally, threshold effect analysis was carried out to ascertain the critical point that exists between the two. A subgroup analysis was conducted in order to investigate the possible influence of age, sex, race, education level, and PIR on the association between sarcopenia and NHHR. To check the consistency and robustness of the findings, sensitivity analysis was done in the end, utilizing a statistical significance level of P < 0.05, all analyses were carried out utilizing R software (version 4.2.3).
Results
Baseline characteristics
The baseline characteristics data extracted from the NHANES database are shown in Fig. 1, with a total of 10,087 participants included, comprising 9,187 non-sarcopenic participants and 900 sarcopenic participants. The characteristics of the participants were categorized based on the presence of sarcopenia, as detailed in Table 1. Compared to those without sarcopenia, sarcopenic patients were generally older, predominantly of Mexican American descent, and had lower educational levels. In terms of lifestyle, these patients tended to be less active and had higher levels of total cholesterol and BMI, as well as lower HDL levels. Additionally, sarcopenic patients were more likely to suffer from diabetes, cardiovascular disease, chronic kidney disease, and hypertension. Notably, these patients had higher NHHR levels, indicating a correlation with sarcopenia.
Association between NHHR and Sarcopenia
In this work, logistic regression analysis was used to examine the association between NHHR and sarcopenia prevalence, as shown in Table 2. In model 1, no covariates were adjusted for, and we observed that the frequency of sarcopenia and NHHR showed a strong positive connection (OR = 1.16, 95% CI = 1.10–1.23). After adjusting for age, sex, and race (model 2), a strong positive association between sarcopenia and NHHR remained (OR = 1.11, 95% CI = 1.06–1.17). In Model 3, we further adjusted for additional variables, including educational level, PIR, smoking status, physical activity, hypertension, CAD, CKD, and diabetes. After adjusting for all these factors, the prevalence of sarcopenia increased by 7% (OR = 1.07, 95% CI = 1.02–1.13) for every unit rise in NHHR after other variables were gradually included. Higher NHHR levels were substantially related with an increased prevalence of sarcopenia compared to the lowest quartile (P-trend < 0.001), according to further examination into the relationship between NHHR levels and sarcopenia, when NHHR was converted to a categorical variable. This trend remained for the highest NHHR levels even after adjusting for all variables (OR = 1.77, 95% CI = 1.21–2.59), suggesting a strong positive connection between NHHR and sarcopenia.
Nonlinear relationship and saturation effect analysis between NHHR and Sarcopenia
A nonlinear relationship between NHHR and the prevalence of sarcopenia was found using RCS analysis (P-non-linear < 0.0001), showing an inverted U-shaped positive correlation (Fig. 2). Threshold effect analysis identified a breakpoint at NHHR = 2.90 within the sarcopenia population. Further segmented logistic regression analysis (Table 3) indicated that on the left side of the breakpoint (NHHR < 2.90), each unit increase in NHHR significantly raised the probability of sarcopenia (OR = 1.62, 95% CI = 1.38–1.92). However, the rise in NHHR did not have a statistically significant influence on the prevalence of sarcopenia when NHHR > 2.90 (P = 0.60), with a log-likelihood ratio test result of less than 0.001.
Subgroup analysis
To investigate any possible correlation between NHHR and sarcopenia, subgroup analyses and interaction tests were carried out. We conducted a subgroup analysis of Model 3 by incorporating demographic factors (Fig. 3). The findings showed that, in all populations, there was a positive correlation between NHHR and sarcopenia prevalence. The interaction test results were not statistically significant, further supporting the possibility of NHHR as an independent risk factor for sarcopenia.
Sensitivity analysis
A sensitivity analysis was carried out in order to confirm the stability and coherence of the study findings, as shown in Table 4. After excluding extreme NHHR values (NHHR ± 3SD), 9,972 participants remained. Following full adjustment for covariates, the positive correlation between NHHR and sarcopenia remained stable (OR = 1.13, 95% CI = 1.04–1.23). The results showed a strong positive correlation between high NHHR levels and the prevalence of sarcopenia when NHHR was transformed into a categorical variable.
Discussion
This study investigated the connection between NHHR and sarcopenia in Americans. The correlation held well under several model modifications and statistical methods. A possible nonlinear link is suggested by analysis utilizing RCS regression models, wherein particular NHHR levels are associated with heightened risk indicated by inflection points. These findings provide credence to the theory that NHHR, a measure of lipid metabolic activity, may contribute to the onset of sarcopenia.
According to a European study, sarcopenia prevalence in people 60 years of age and older ranged from 10 to 27% worldwide, whereas severe sarcopenia prevalence varied from 2 to 9%6. According to recent studies, almost 40% of the world’s population suffers from dyslipidemia, with 53.65% of older people being affected17. Sarcopenia is linked to numerous negative health outcomes in older adults, such as increased mortality, disability, and a higher risk of falls supported by highly suggestive evidence18. In a similar way, the prevalence of obesity, defined as an excessive accumulation of fat mass, has been steadily rising. Recent statistics reveal that in the United States, obesity rates among adults over the age of 60 exceed 37.5% for males and 39.4% for females19. Additionally, scientists have found that sarcopenia is more common in people who are overweight or obese20. Sarcopenia obesity (SO) is characterized by the co-occurrence of poor muscle mass and function and excess adiposity. SO is a disorder that is becoming more widely recognized for its clinical and functional characteristics that may have a detrimental impact on significant patient-centered outcomes21. All this evidence suggests that there is a connection between sarcopenia and obesity.
However, the association between sarcopenia and obesity (or dyslipidemia) still lacks clarification. So far, data from a study indicated that females with sarcopenia had LDL-C levels that were 3.1 mg/dL higher compared to those without sarcopenia22. Nonetheless, a study showed that there was no discernible difference in HDL-C and LDL-C levels between individuals with sarcopenia and those without17. The ratio of non-HDL-C to HDL-C, two lipoproteins with different purposes, is represented by the acronym NHHR. A higher NHHR can signify imbalances in lipid metabolism which is typical character of dyslipidemia. Thus, this study primarily uses NHHR as a research indicator to elucidate the relationship between obesity and sarcopenia.
According to the results of this cross-sectional study, which included 10,087 American participants-9,187 of whom were non-sarcopenic and 900 of whom were sarcopenic-sarcopenic patients were typically older, more likely to be of Mexican American descent, and less educated. These patients tended to lead less active lifestyles, with higher BMI, total cholesterol, and HDL levels among their health outcomes. A meta-analysis also suggested that vegetable and fruit intake significantly reduce the risk of sarcopenia23. Additionally, sarcopenic patients were more likely to suffer from diabetes, cardiovascular disease, chronic kidney disease, and hypertension. Researchers have also noted that sarcopenia, an age-related condition, is strongly linked to hypertension due to the vascular damage it causes at both the macrovascular and microvascular levels24,25. Additionally, a review revealed that the most frequent factors linked to sarcopenic obesity are inflammation, hypertension, insulin resistance, dyslipidemia, and inactivity26.
In this study, notably, individuals with elevated NHHR may be more likely to experience sarcopenia, as sarcopenic patients exhibited higher NHHR levels. Subgroup analysis provided more proof that this favorable association is stable. Notably, this link was unaffected by PIR, age, gender, ethnicity, or educational attainment, suggesting that NHHR may function as a separate risk factor for sarcopenia. Furthermore, we discovered that there is a crucial point at an NHHR of 2.90 in the nonlinear association between NHHR and sarcopenia. This suggests that NHHR is positively associated with the prevalence of sarcopenia when the NHHR value is below 2.90. Sensitivity and additional analyses further confirmed the stability of the correlation. Our hypothesis for this observation is that, beyond a certain threshold of NHHR, other factors may come into play that could influence sarcopenia in a non-linear manner. Specifically, at higher levels of NHHR (greater than 2.9), it is possible that the relationship between NHHR and sarcopenia may be confounded by factors such as metabolic dysregulation, systemic inflammation, or other underlying comorbidities that tend to emerge at these higher NHHR levels. Additionally, very high NHHR values could indicate a shift toward a more complex pathophysiological mechanism where the direct association between NHHR and sarcopenia becomes less clear or is attenuated. An experimental study demonstrated that a high-fat diet can reduce muscle mass, strength, and fiber cross-sectional area, while increasing muscle fatty infiltration in naturally aging rats, eventually leading to sarcopenic obesity27. The results of this study indicate that NHHR is a highly valuable tool for early prevention and diagnosis in high-risk populations, as it functions as an independent and early indicator of the likelihood of developing sarcopenia.
Study strengths and limitations
This study has a number of strengths. Above all, by examining a large sample size of 10,087 individuals, this study provides epidemiological evidence of the relationship between NHHR and sarcopenia in the American population. This study also made use of the large NHANES database, which includes a representative sample of people from various parts of the United States. This large dataset offered a solid foundation for investigating the connection between NHHR and sarcopenia risk. The results of this study can be efficiently extended to the total population of the United States by using appropriate sampling weights. Finally, by carefully controlling a variety of confounding variables, this study efficiently minimizes potential biases and improves the study’s robustness and interpretability.
It is crucial to recognize some restrictions. First, due to the cross-sectional nature of the study, a causal relationship between NHHR and sarcopenia cannot be established. Second, depending solely on self-reported information may result in recall bias, especially when it comes to lifestyle choices and sarcopenia history. Furthermore, even after taking into consideration a number of possible confounders, unaccounted for factors may still have an impact on the outcomes. Finally, it is advised to use caution when extrapolating the findings to other groups because the study focused on the U.S. demography. In light of these drawbacks, it will be imperative to conduct additional longitudinal research and develop better techniques for gathering data in order to further our comprehension of the connection between NHHR and the risk of sarcopenia. Additionally, this will help in the advancement of more potent medicinal and preventive approaches.
Conclusion
In conclusion, the significance of NHHR as a predictive biomarker for sarcopenia is highlighted by this study’s result, providing chances for improved risk assessment, focused therapies, individualized management plans, and preventive medicine. By incorporating NHHR tests into clinical practices, healthcare providers can improve patient care by detecting patients who may be at risk for sarcopenia and taking preventative action to reduce this risk, which in turn improves overall health outcomes.
Data availability
The NHANES questionnaires and data involved in this study are all openly available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Abbreviations
- NHDL-C:
-
Non-high-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein
- TC:
-
Total Cholesterol
- NHHR:
-
NHDL to HDL ratio
- NHANES:
-
National Health and Nutrition Examination Survey
- BMI:
-
Body mass index
- IR:
-
Insulin resistance
References
Evans, W. J. & Ferrucci, L. A simplified definition of Sarcopenia: muscle mass/body weight. J. Nutr. Health Aging. 28, 100302 (2024).
Veronese, N. et al. Sarcopenic obesity and health outcomes: An umbrella review of systematic reviews with meta-analysis. J. Cachexia Sarcopenia Muscle (2024).
Vicedomini, A. C. C. et al. Prognostic value of new sarcopenia screening tool in the elderly-SARC-GLOBAL. Nutrients 16. (2024).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 48, 16–31 (2019).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older people. Age Ageing. 39, 412–423 (2010).
Petermann-Rocha, F. et al. Global prevalence of Sarcopenia and severe Sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 13, 86–99 (2022).
Sousa, A. S. et al. Financial impact of Sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 70, 1046–1051 (2016).
Tsai, S. Y. Lost in translation: Challenges of current pharmacotherapy for Sarcopenia. Trends Mol. Med. (2024).
De Lorenzo, A. et al. The association between sarcopenic obesity and DXA-derived visceral adipose tissue (VAT) in adults. Nutrients 16. (2024).
Bahat, G. Sarcopenic obesity: A hot yet under considered evolving concept. Eur. Geriatr. Med. 13, 1023–1024 (2022).
Jang, M. H. & Song, J. Adenosine and adenosine receptors in metabolic imbalance-related neurological issues. Biomed. Pharmacother. 177, 116996 (2024).
Zubirán, R. et al. Performance of the enhanced Sampson-NIH equation for VLDL-C and LDL-C in a population with familial combined hyperlipidemia. Atherosclerosis 386, 117364 (2023).
Raja, V. et al. Non-HDL-cholesterol in dyslipidemia: review of the state-of-the-art literature and outlook. Atherosclerosis 383, 117312 (2023).
Qing, G. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and suicidal ideation in adults: A population-based study in the United States. Lipids Health Dis. 23, 17 (2024).
Tan, M. Y. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with type 2 diabetes mellitus: recent findings from NHANES 2007–2018. Lipids Health Dis. 23, 151 (2024).
Kim, S. W. et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int. J. Cardiol. 168, 2678–2683 (2013).
Bi, B. et al. Dyslipidemia is associated with sarcopenia of the elderly: a meta-analysis. BMC Geriatr. 24, 181 (2024).
Xia, L. et al. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 9, 7964–7978 (2020).
Obesity. Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 894, i–xii (2000).
Jayasinghe, S. & Hills, A. P. Sarcopenia, obesity, and diabetes - the metabolic conundrum trifecta. Diabetes Metab. Syndr. 16, 102656 (2022).
Donini, L. M. et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes. Facts. 15, 321–335 (2022).
Baek, S. J. et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: The 2008–2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Invest. 37, 247–260 (2014).
Hong, S. H. & Bae, Y. J. Association of dietary vegetable and fruit consumption with sarcopenia: A systematic review and meta-analysis. Nutrients 16. (2024).
Mogi, M. Pulse pressure tells the story of Sarcopenia. Hypertens. Res. (2024).
Mirzai, S. et al. Relationship between Sarcopenia and intensive blood pressure control efficacy and safety: A secondary analysis of SPRINT. Hypertension (2024).
Pinel, A. et al. Identification of factors associated with sarcopenic obesity development: Literature review and expert panel voting. Clin. Nutr. 43, 1414–1424 (2024).
Mo, X. et al. High-fat diet induces sarcopenic obesity in natural aging rats through the gut-trimethylamine N-oxide-muscle axis. J. Adv. Res. (2024).
Acknowledgements
The authors thank the NHANES staff, investigators, and participants.
Funding
This work has not received any specific grant from any funding in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HDJ, WXD, and HHM equally contributed to the conceptualization and design of the study. MYM conducted research and drafted the manuscript. YXJ and WYG participated in data collection and analysis. LLJ and WRJ performed statistical analysis. MYM drafted the initial manuscript. LRY reviewed and revised subsequent drafts of the manuscript. All authors critically reviewed and approved the final manuscript, agreeing to take full responsibility for the integrity and accuracy of the work. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The NHANES obtained approval from the National Centre for Health Statistics Research Ethics Review Board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Each participant gave written informed consent agreement when they were enrolled in the NHANES, and the National Center for Health Statistics’ ethics review board approved the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yanming, M., Lingjiang, L., Renji, W. et al. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and sarcopenia based on NHANES. Sci Rep 14, 30166 (2024). https://doi.org/10.1038/s41598-024-81830-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81830-z