Abstract
The industrial sector faces a significant challenge in finding the highly effective and efficient treatments for harmful dye-based color effluents. In this study, pure and cobalt doped barium hexaferrite of chemical formula, Ba1-xCoxFe12O19 (x = 0—0.06) are made via sol–gel auto-combustion (SC) methodology. These nano hexaferrite based catalysts are employed for the photodegradation of Congo Red (CR) pollutant. X-rays diffraction investigation confirms the creation of pristine M-type with a hexagonal structure for the prepared hexaferrites. Field emission scanning electron microscopy analysis shows the existence of the hexagonal-shaped grains with well-defined grain boundaries. The reduction in the band gap of prepared hexaferrites are observed with the cobalt doping which is helpful in enhancing the photocatalytic performance. The X-ray photoelectron spectroscopy examination verifies the oxidation states of all elements found in the fabricated specimens. From the photocatalytic measurements, it is observed that the CR dye attains the removal percentage of 87.90%, 90.73%, 91.86% and 94.88% for the BaFe12O19 (x = 0.00), Ba0.08Co0.02Fe12O19 (x = 0.02), Ba0.06Co0.04Fe12O19 (x = 0.04), and Ba0.04Co0.06Fe12O19 (x = 0.06) hexaferrites under the natural sunlight of two hours. In addition, the reusability potential of prepared hexaferrites is also studied over the six consecutive experimental cycles. The excellent photodegradation performance of the Co- doped barium M-type hexaferrites for the removal of CR dye makes them highly useful for the wastewater remediation.
Similar content being viewed by others
Introduction
In general, the treatment of different types of contaminates like heavy-metals, drugs, and dye have been recognized as one of the primary goals in managing the environmental pollution all over the world1,2. Dyes are widely used in sectors like textiles, rubber, paper, leather, and cosmetics. Textile dyes are among the largest groups of organic compounds that increasingly threaten the environment. The textile industry produces the waste streams that are highly contaminated with inorganic salts and non-fixed colors, particularly named as azo dyes3. It is generally known that the several azo dyes and their breakdown products, such as aromatic amines, are extremely carcinogenic. The existence of aromatic rings in azo dyes make them hardy to naturally removal. One significant azo dye is Congo red (CR) dye. Its pigmented compounds have large molecular weights and intricate chemical structures. CR dye, containing benzidine known for its carcinogenic properties, is highly toxic and water-soluble, resisting degradation caused by its complex aromatic structure. Consequently, the remediation of polluted water with CR dyes is imperative4.
Textile dyes are commonly eliminated from the wastewater through the three primary methods: chemical treatments like, chlorination and ozonation, biological processes like biodegradation, and physical techniques including the adsorption. Additional methods include reverse osmosis, flocculation, and adsorption onto activated carbon5. However, none of all these methods can completely degrade the dyes due to their inherent limitations. The initial step in the degradation of azo dyes typically involves the discoloration resulting from the cleavage of chromophore bonds, followed by the production of the non-color toxic aromatic amine compounds6. These intermediates pose a long-term threat to the ecosystem, highlighting the necessity for complete dye degradation. While the aforementioned techniques transfer contamination from one phase to another without causing any damage, which therefore, create a different form of pollution, necessitating for further treatment7. Extensive research has focused on advanced oxidation processes, particularly the use of photocatalysis for dye degradation. The degradation process in photo-catalysis takes place at the photocatalyst’s surface, and visible light is suitable for an efficient and cost-effective degradation process. Photocatalysis is a sustainable process, which can degrade organic compounds and demonstrates the excellent durability and stability, enabling them to endure harsh conditions without considerable degradation, which extends their lifespan and enhances process’s sustainability. Photocatalysis involves the acceleration of chemical transformations by a photocatalyst, with efficiency dependent on various factors like light absorption capacity, porosity, bandgap, and charge transfer traits8.
Among the photocatalysts, the ferrite-based nanomaterials are utilized for the photocatalytic degradation due to their exceptional magnetic and optical characteristics. Divalent iron oxide materials, with the general formula MFe12O19 (M = Sr, Ba), exhibit a hexagonal structure exhibiting notable traits such as cost-effectiveness, significant magnetization, and the superior chemical stability against the erosion. Barium hexaferrite (BaFe12O19) possesses a hexagonal structure contributing to the high single-axis magnetic anisotropy characterized by the elevated coercivity values9. M-type hexaferrites are particularly promising as the photocatalytic materials, capable of absorbing visible light with band gaps ranging from 1.1 to 2.3 eV. The magnetic nature of M-type hexaferrite facilitates easy recovery in the presence of strong magnetic field. Nanocrystalline materials are engineered to maximize the active sites on their surfaces, enhancing the reactivity of nano-catalysts for improved organic dye degradation under the solar light exposure. The introduction of the elements such as Yttrium, Manganese10, in M-type hexaferrite enhance the magnetic and catalytic properties. The coercivity, multiferroic behavior, and customization of several other properties can be improved via doping. Bibi et al., Cr and Co added barium hexaferrite nanoparticles were synthesized by microemulsion method. The resulting Ba1-xCoxFe12-yCryO19 (x = y = 0–0.6) nanoparticles showed crystallite size of 18 nm. Evaluation of photocatalytic activities involved the testing of the removal of crystal violet (CV). The doping process significantly enhanced the photocatalytic performance as compared to pristine BaFe12O19 nanoparticles. Some literature shows that BaFe12O19degrade CV 52% in 80 min11. Remarkably, in this study, under visible light, up to 64.23% of the crystal violet dye was degraded within 60 min by co-doped samples. This highlights the promising potential of Ba1-xCoxFe12-yCryO19nanoparticles as an economical and efficient photocatalyst, utilizing solar light for the dye removal12. Luna et al., fabricated the BaFe12O19 hexaferrite via a co-precipitation method for the elimination of methylene blue (MB). This catalyst exhibited notable catalytic activity under visible irradiation. The catalyst’s ability to harness photons from the visible light spectrum, because of small band gap (estimated at ∼1.77 eV), facilitated its activation. The BaFe12O19effectively decolorize and mineralize MB by achieving removal percentages of 70.8% and 63.7%, within 360 min13.
There are many different synthesis approaches to fabricate the hexaferrite based nanomaterials including the sol–gel auto-combustion (SC), solid-state, hydrothermal, microemulsion, green approach, co-precipitation and so on. Out of these, the most prominent approach is sol–gel auto-combustion route because of the unique traits such as effectively manages the growth of the crystals, ensures chemical uniformity, results in high purity and crystallinity of the end product, achieves a fine particle size with a narrow distribution, requires minimal external energy input, and economically advantageous. This study involved the synthesis of, Ba1-xCoxFe12O19 (x = 0.00–0.06) using the SGA technique. The impact of doping with divalent cations (Co) on the, magnetic, structural, optical, and photocatalytic traits of nanoparticles is thoroughly inspected. The hexaferrites synthesized in the present investigation are utilized for the photodegradation of CR dye, via a photocatalytic process under sunlight, with the goal of treating wastewater.
Experimental procedure and characterization
Raw precursors used
The substrates of AR grade (> 98%), such as barium (Ba(NO3)2, Merck), ferric (Fe(NO3)3, SimSon), cobalt nitrate (Co(NO3)2, SimSon), and citric acid (C6H8O7, SimSon), was used to produce the Ba1-xCoxFe12O19 (x = 0.00–0.06) ferrite nanoparticles. Furthermore, a solution of ethylene glycol (from Rankem) and ammonia (from SimSon) helped in the gelation and pH control. The CR dye is bought from Central Drug House (CDH), New Delhi, India.
Procedure for fabrication of Ba1-xCoxFe12O19 (x = 0.00, 0.02,0.04 and 0.06) hexaferrites
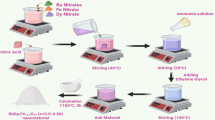
The SGA approach successfully produced the cobalt doped barium hexaferrite nanoparticles. Firstly, the stoichiometry quantity of Ba, Co, and Fe nitrates are dissolved in 80 mL of distilled water. C6H8O7 is also dissolved in the same quantity of distilled water separately. Each solution is stirred on separate hot plate stirrers by keeping the temperature below 50 °C for 30 min. The solutions are continuously stirred until a clear mixture is achieved. Then, the nitrate solution is combined with an aqueous citric acid solution, followed by continuous stirring on a hot plate stirrer. The mixture’s pH is raised to a parameter of 7 using an ammonia (NH3) solution. This stirring continues for an hour, following which a stoichiometric quantity of ethylene glycol is added to the mixture, acting as a gelling agent and causing gel formation. As the temperature gradually augmented up to 100 °C, the mixture is transformed into a brown wet gel. The gel is then burned at 100 °C and hence, during this process, the dried gel swelled up and released an enormous number of gases. After the complete combustion, a brown-colored ash like powder is obtained. This powder is subjected to the further stepwise thermal treatment in a furnace for 5 h at 1150 °C.
Figure 1 illustrations the scheme for fabrication of Ba1-xCoxFe12O19 nanoferrites. Here, BaFe12O19 (x = 0.00) is named as BCF1, Ba0.98Co0.02Fe12O19 (x = 0.02) is named as BCF2, Ba0.96Co0.04Fe12O19 (x = 0.04) is named as BCF3, and Ba0.94Co0.06Fe12O19 (x = 0.06) is named as BCF4, respectively.
Characterization
We used an X-ray diffractometer (XRD) of Bruker D8 advance to investigate the phase formation and other structural characteristics such as crystallite size and lattice constant. The morphology of hexaferrite nanoparticles is analysed by the field emission scanning electron microscopy (FESEM JSM IT 800-Jeol). Utilizing FTIR (PerkinElmer), UV–Visible-NIR spectrophotometer (LAMBDA 750) and RAMAN (RAMAN (RAM HR evolution by Horiba), the optical nature of the samples is noted. For the confirmation of the oxidation states of every element, X-Ray Photoelectron Spectroscopy (NEXSA Surface analysis via Thermo Scientific) available at the IIT Mandi is utilized. The BET examination of the specimens is carried out via BET surface analyser (available at Panjab University, India. The magnetic nature was noted via vibrating sample magnetometry (VSM) (Microsense, USA, VSM) at the University of Kashmir.
Procedure for photocatalytic degradation study
The photocatalytic degradation capability of the Ba1-xCoxFe12O19hexaferrite nanoparticles is investigated using the CR dye as the target pollutant. The experimentations are conducted under the controlled conditions to assess the efficiency of these nanoparticles for degrading the dye14. Specifically, the experiments are carried out across a range of pH values from 1 to 9 to determine the optimal pH for the maximum degradation. The different dosages of hexaferrites, ranging from 0.02 to 0.08 g, are also tested in 0.1 L of a 10 mg/L CR solution. Natural sunlight is used as the irradiation source for 2 h to simulate the environmental conditions. During the photocatalytic experiments, samples of the reaction mixture are collected at the 20-min intervals. For each interval, 4 mL of the supernatant is extracted for the analysis. This step is crucial for monitoring the progress of the degradation process over time. The absorbance of the CR dye in these hexaferrite samples is measured using a UV–VIS spectrophotometer both before and after irradiation with sunlight. These measurements are used to evaluate photocatalytic performance of the nano hexaferrites by comparing the starting and terminal concentrations of dye. The efficiency of the dye removal is quantified using the following expression15:
Where, \({\text{C}}_{\text{t}}\) is the concentration of the dye and \({\text{C}}_{\text{o}}\)is the initial concentration of the dye, at a specific time interval during the irradiation16. This formula allowed for the calculation of the percentage of dye degraded and providing a conclusion of the photocatalytic nature of the Ba1-xCoxFe12O19 hexaferrite. The results demonstrated the effectiveness of the nanoparticles in the elimination of CR exposed to the natural sunlight.
Results and discussions
XRD study
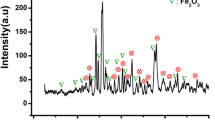
XRD analysis demonstrated the identification of the phase development and the structure of the prepared nanohexaferrites. The patterns for Ba1-xCoxFe12O19 (where x = 0.00–0.06) hexaferrites are shown in Fig. 2(a). Peaks are identified at (110), (008), (112), (107), (114), (201), (203), (205), (206), (217), (2011), and (220) diffraction planes, indicating a pure hexagonal structure with the P63/mmc space group. The observed data totally matches with JCPDS no.: 84–075717. No additional peaks are observed, indicating the purity of the obtained phase. With the help of XRD data, the different structural parameters are computed including, the lattice parameter ‘a’ and ‘c’, crystallite size (D), unit cell volume (Vcell), strain, and c/a ratio demonstrated in the Table 1. The ‘a’ and ‘c’ and (Vcell), are computed via the formulas as listed below18,19:
The distance, denoted as 'd', the lattice constants 'a' and 'c', and Miller indices (hkl). The result indicates a noticeable variation in 'a' and 'c' in response to the cobalt doping levels, as detailed in Table 1. However, with the rising cobalt concentration up to x = 0.04, the values of “a” increases from 5.866 to 5.875 Å. Instead, by increasing the substitution upto x = 0.06, then it decreases from 5.875 to 5.862 Å. For “c” with increasing the cobalt content, the values are increasing upto x = 0.02 and then, decreases. This significant variation occurs because of the smaller Co2+ cations replace the larger Ba2+ cations. This substitution is primarily responsible for the anomalous behavior observed in a & c. Because of the irregularities in the (a & c), there exist an irregular trend in the (Vcell) of the samples, as shown in Table 1. The (Vcell) raising from 690.38 to 693.12 Å3 with the cobalt concentration upto x = 0.04 and then, it decreases to 688.91 Å3 at x = 0.06. In addition, (D) of the pristine and cobalt-doped BaFe12O19nanoferrites is estimated from the (114) peak of patterns by Debye–Scherrer formula as shown below20:
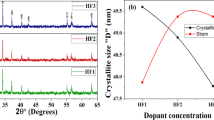
where k is the shape factor, θ is diffraction angle, \(\upbeta\) is the FWHM. The ‘D’ is determined between 43.91–55.92 nm. The analyses demonstrated a consistent decline in its value corresponding to the augmented concentration of the Co dopant. The material exhibited such behavior due to the influence of doping content on introducing strain that hinders the process of crystal growth, consequently diminishing the size of crystallites. This phenomenon is clearly demonstrated by microstrain values derived from the XRD data, indicating that an augmentation in the doping concentration directly correlates to an increase in system strain. The relationship between strain and crystallite size, in response to the rise in dopant levels, is graphically represented in Fig. 2 (b)21. Also, the c/a ratio for the prepared nanohexaferrite is less than 3.98, which proves that there is a successful production of the hexagonal phase. The density of the unit cell is equivalent to the (dx) and the strain of the sample could be determined by the formula listed below:
In this equation, N is the Avogadro number, n = 2 represents the number of molecules per unit cell, M is the molecular weight, V is the volume, and β is the FWHM22,23. Table 1 illustrated that when the Co-substituted rise, the X-ray density of the fabricated material declines from 5.35 to 5.31 g/cm3 before increasing at x = 0.06.
To ensure the purity of all hexaferrite samples, the Rietveld refinement technique is employed. The outcomes of the Rietveld refinement, as depicted in Fig. 3, unveiled that the empirical data (Y observed) corresponds closely with the hypothetical data (Y calculated), yielding a goodness of fit metric of under 2 for every specimen. This suggests the effective establishment of a singular phase in both the pristine and cobalt-doped specimens.
Surface and elementary study
FESEM analysis is conducted to observe the impact of cobalt on the surface analysis of the fabricated nanohexaferrite. Figure 4(a, d, g) presents the FESEM images of BCF1, BCF2 and BCF4 hexaferrite nanoparticles with x = 0.00, 0.02, and 0.06. The images show the hexagonal-shaped particles, characteristic of the magnetoplumbite structure, with well-defined grain boundaries and a tendency to agglomerate across all samples. Image J software is employ to computed the grain sizes of the specimens. The micrographs reveal the significant variations in grain sizes, which are further illustrated by the grain size distribution histograms in Fig. 4(b, e, h). The study shows that the grains in the BCF1 sample have size of 264.51 nm. Similarly, the BCF2 exhibits the grains with size of 253.20 nm, whereas the BCF3 sample demonstrates an average grain size of 198.84 nm. The grains that are formed demonstrate the distinct boundaries between them, and presence of agglomeration among the grains indicates a strong magnetic nature. This phenomenon of agglomeration is believed to be the primary factor contributing to the greater size as opposed to the ‘D’ that has been reported through XRD. Figure 4(c, f, i) displays the EDX results of the fabricated specimens, confirming the elemental purity of the hexaferrites. The spectra depicted the peaks only Ba, Co, O, and Fe with no extra elemental, indicating the creation of pure Ba1-xCoxFe12O19 hexaferrite.
UV–VIS spectroscopy study
The study utilized a UV–VIS spectrophotometer to investigate the absorption characteristics of Ba1-xCoxFe12O19 hexaferrites across 200 to 800 nm. The absorbance spectra of fabricated samples are illustrated in Fig. 5 and it is explained that the addition of Co into the BaFe12O19significantly improved the photocatalytic capabilities. This feature is critical in photocatalytic applications because it determines the material’s ability to collect light and form electron–hole pairs, which are crucial for photocatalytic removal24.
In pure BaFe12O19, electron–hole recombination can occur and which diminishes the material’s photocatalytic efficiency. However, doping of BaFe12O19with cobalt ions introduces the defects and electron traps that reduce recombination rate. This is because the doped materials can capture photogenerated electrons and thereby enhancing the separation of charge carriers and improving photocatalytic performance. This mechanism effectively lowers the band gap energy and making it easier for the material to utilising visible light and formation of the necessary charge charrier for the photocatalytic process25. Tauc plots are employed to compute the Egof the hexaferrites. The Tauc plot method is based on the equation as defined below15.
Here \(\alpha\) is the absorption coefficient, \(\upnu\) is the photon’s frequency, Egis the band gap energy, h is Planck’s constant, K is a proportionality constant, electronic transition (n = 1 for direct, n = 4 for indirect transitions)24. For pure BaFe12O19, the Eg is found to be 2.19 eV. Doping with cobalt ions reduced this band gap, which is a desirable effect for the photocatalytic applications. Specifically, Ba0.04Co0.06Fe12O19 with the highest concentration of dopants exhibited the most significant reduction in Eg to 1.69 eV. Other two samples, Ba0.08Co0.02Fe12O19 and Ba0.06Co0.04Fe12O19, showed Eg of 1.99 and 1.76 eV, respectively. The Tauc plot is illustrated in Fig. 6. The drop in Egdue to doping correlates with an enhancement in photocatalytic activity. A smaller band gap means that the nanoparticles absorb lower-energy photons (i.e., visible light), which is abundant in the environment26.
In summary, the UV–VIS spectrophotometric study demonstrated that the doping of BaFe12O19 with the cobalt ions significantly reduced the Eg and thereby enhancing the photocatalytic performance of the hexaferrites. The reduction in electron–hole recombination, because of trapping of electrons by the dopants and contributed to this improvement and making these materials more effective in photocatalytic degradation of the environmental pollutants from wastewater.
FTIR study
FTIR is a spectroscopic technique utilized for the recognition of inorganic, polymeric, and organic compounds. The emergence of a hexagonal configuration in the nanocrystalline state is substantiated by the mid-infrared spectra. Ferrites can be perceived as perpetually connected crystals, wherein the atoms are linked to all adjacent atoms through comparable forces (ionic, covalent, or van der waals). Figure 7(a) presents the FTIR spectra for both undoped and Co-doped barium hexaferrites, measured over the range of 400–3000 cm−1. IR absorption bands within the 1000–100 cm−1 range are typically due to vibrations of inorganic ions in the crystal lattice. The spectra display in the 400–800 cm−1 range, corresponding to the Fe–O vibrations at both octahedral and tetrahedral sites. The initial peak at 410–415 cm⁻1 represents the elongation vibrations of Fe–O bonds at the tetrahedral sites, while the subsequent peak at 537-568 cm⁻1 is indicative of Fe–O stretching at the octahedral sites. These bands are denoted as υ1 (cm⁻1), υ2 (cm⁻1), and υ3 (cm−1), respectively. Additionally, due to the presence of the large Ba2+ in the hexagonal layer, a band at 895 cm⁻1 was found, corresponding to the vibration of the Ba2+–O2-27. The tiny absorption peaks in the range of 1057-1058 cm−1are associated with Fe–O–Fe and Co–O–Co bonds28. The bands are labelled as υ4 (cm−1) and υ5 (cm−1) in Table 2.
Raman study
Raman analysis, a method of optical spectroscopy, is employed for the investigation of crystal structure and ion distribution within materials. According to the group theory, which adhere to the D6h symmetry, ascertains the presence of 42 Raman active modes, 30 IR active modes, with the remaining modes being inactive (4A2g + 3A1u + 13B1g + 4B1u + 4B1g + 12B2u + 3B2g + 15E2u). Here, A represents 1-dimensional, B represent 2-dimensional, and E represent 3-dimensional of optical modes, while 'g' and 'u' denotes the symmetry and anti-symmetry in relation to the center of inversion. The Raman spectra of BCF1, BCF2, BCF3, and BCF4 nanoparticles are described in Fig. 7(b) whereas, the Table 3 shows the presence of each vibration mode at their respective wavenumber. The main peaks identified around 170-176, 198-203, , 317-325, 403, 453-459, 509-517, 599-607, and 669-676 cm−1 align with the hexagonal structure. Specifically, the vibrational frequencies at 170-176, 198-203, and 403 cm−1 correspond to E1g modes, while those at 317-325, 453-459, 509-517, 599-607, and 669-676 cm−1 are associated with A1g modes31. The peaks at 198-203 and 170-176 cm−1 are attributed to E1gband of the spinel block29,30. The vibrational bands around 453-459 and 509-517 cm⁻1 are associated with the Fe–O bonds in octahedral sites. Additional peaks observed at 317-325, 453-459, and 599-607 cm⁻1are attributed to A1g band of Fe–O in the octahedral positions4,6. The peak observed at 403 cm−1 is associated with the E1gband occurring at octahedral positions (12 k)32.
XPS study
XPS is a surface-analysis method extensively utilized for examining the elemental composition and chemical state of samples. The XPS spectral data of the BCF1, BCF2, and BCF4 are depicted in Fig. 8, Fig. 9, and Fig. 10. The spectra confirmed that the pure sample contain four peaks of C1s, O1s, Fe2p, and Ba3d, with the additional presence of Co2p and Co3s in BCF2 and BCF4 samples. The peaks observed around 284 eV in each sample shows the presence of carbon (C1s) which is because of the utilization of carbon for instruments calibration (Fig. 8e, 9f, 10f). The analysis of the C1s spectra reveals the existence of two discrete peaks at 284.4 and 285.1 eV, which resemble to the C–O and O–C–O, respectively. Figure (8b, 9b, 10b) shows the presence of Ba in each sample, where two peaks are observed for the binding energies of 778.86-779 and 794.15 eV, representing the Ba3d5/2 and Ba3d3/2 states, respectively, with an additional satellite peak at 783.92-784 eV arising from the electron removal from the atom’s core level shell. The finely resolved Fe2p spectra is observed in the Fig. (8c, 9c, 10c) which exhibits the two distinct peaks at 709.81-709.99 eV and 723.04-723.50 eV. This corresponds to Fe2p3/2Fe2+ and Fe2p1/2Fe3+ ions, respectively, with the satellite peaks at 718.19- 718.69 eV and 731.91-732.52 eV. For every fabricated specimen, a notable portion of Fe2+ peak is present. This occurrence is related to the existence of oxygen vacancies within lattice. The oxygen vacancies have the capability to reduce the Fe3+ cations into Fe2+ cations in order to balance the total charge distribution of the specimen. The O1s spectrum displays in Fig. (8d, 9d, 10d) shows the peak at 529.23-529.27 eV, with deconvolution revealing an additional peak at 530 eV which is an indicative of the lattice oxygen. The observed energy difference between the oxygen and Ba3d5/2 peaks is higher than 249 eV which is the characteristic for the BaM as shown in Table 433. The characteristic peaks for Co2p3/2 are observed at 779 eV and for Co2p1/2 at 794 eV Fig. (9e, 10e).
BET study
BET study is executed to find the specific surface area of the samples. The analysis is performed via the nitrogen adsorption–desorption isotherm34. Figure 11 depicts the obtained isotherms for both BCF1 and BCF3 samples. A hysteresis loop formation is observed for all the samples with the relative pressure (P/Po) approaching to 1. The isotherm shape resembles to the IV isotherm as per the IUPAC terminology35. This type of plot shows the mesopores and macropores within the material36. The calculated area of 2.073 m2/g is observed for the BCF1 sample while for the BCF3 sample, the specific surface area is increased to 3.352 m2/g. The study indicates that with the increase in the dopant content, there is a rise in area of the material. The Fig. S1 illustrates the pore size distribution for the prepared Ba1-xCoxFe12O19 (x = 0.00 and 0.04) samples. It is evident from the data that the pore diameters of BCF1 and BCF3 hexaferrites are primarily centered at 2.2 nm and 2.4 nm, respectively. This shift in pore size distribution suggests that introducing cobalt ions into the barium hexaferrite structure causes an increase in the average pore diameter. Also indicates that doping in barium hexaferrites increases the mesoporosity of the samples.
Numerous studies demonstrate that doping increased surface area37. The increased surface area with doping concentration aids in the photocatalysis by providing more active sites due to increased surface to volume ratio and absorb more contaminates, which improve photocatalysis38.
Magnetic study
The magnetic property of produced hexaferrites is evaluated at ambient temperature by VSM at 20 kOe. The M-H curves for each specimen can be observed in the Fig. 12(a). These curves provided the essential magnetic characteristics like coercivity (Hc), saturation magnetization (Ms), magnetic moment (ŋB), retentivity (Mr), and squareness ratio (Mr/Ms) as tabulated in Table 5. The Mr/Ms and the ŋB of the specimens were computed by using formula given below:
where M is molecular weight, 5585 is the conversion factor, Mr is retentivity. The plots indicate that the hexaferrites shows the characteristic behavior of hard ferromagnetic nanomaterials. The zoom view of the M-H graphs is demonstrated in Fig. 12(b). The effect of cobalt concentration on Ms, Hc, and Mr of developed hexaferrites was demonstrated in Fig. 12(c). The magnetic traits exhibited by hexaferrites are predominantly affected by spatial arrangement of iron ions. It is established that the barium hexaferrite possesses the five distinct substitutional sites for the “Fe” within its hexagonal configuration. The 2b, 2a, and 12 k sites exhibit an up-spin orientation, whereas 4f2 and 4f1 sites demonstrate a down- spin orientation. Because cobalt cover both the octahedral 4f2 and tetrahedral 4f1 positions, operating under the assumption that the cobalt ions exhibit minimal incorporation into the 2a sites. Consequently, due to the elevated levels of cobalt substitution, the Ms is observed to be marginally lower than that of the BaFe12O19. As a result, the pure M-type hexaferrite shows largest Ms, which subsequently decreases as the cobalt content rises, from 60.40 to 46.19 emu/g39. Additionally, The M versus 1/H2 graph is generated to find the Ms, as demonstrated in Fig. 12(d).
However, coercivity (Hc) depends on values of magnetocrystalline anisotropy (Ha), as discussed in other studies40. The undoped sample is found to have the highest coercivity. Because of high anisotropy towards c-axis, the barium hexaferrite has a Hc of 4320.32 Oe. As the doping content increases, the Hc decreases significantly, dropping from 4320.32 to 3088.25 Oe. This finding is indicative of a decrease in the crystal’s anisotropy field, due to a shift in the preferred axis. The calculated SQR data fall in between 0.529 to 0.521, denoting the presence of uniaxial anisotropy in the synthesized hexaferrites. Due to high coercivity and magnetization, the developed hexaferrites are highly beneficial for the permanent magnets and photocatalysis processes.
Photocatalytic degradation study
The current study investigated the photocatalytic decomposition process of CR dye under the natural sunlight exposure to evaluate the degradation efficiency by use of undoped and doped barium hexaferrites. Optimizing essential parameters like pH and dosage is crucial for maximizing the elimination of organic toxins41,42.
Effect of pH
The pH level is a vital component in breakdown of dyes and it’s influencing the removal rate. In acidic conditions, the degradation process is notably faster in case of CR dye. This heightened degradation efficiency because of anionic behaviour of the CR, which facilitates its breakdown in an acidic environment. In contrast, in alkaline environments, the degradation process slows down significantly. The deceleration observed is ascribed to the repulsion existing among the catalyst and the particles of dye, consequently impeding the progress of the reaction43. At (pH = 1), the dye degrades most effectively because the sulphonic groups on the dye molecules are ionized and resulting in the formation of a soluble CR dye anion. This ionization enhances the interaction between dye and Ba1-xCoxFe12O19 catalysts and thus, promoting a more efficient degradation process. The findings are illustrated in the Fig. 13. Several studies have corroborated these findings and indicating that the acidic media generally yield the higher degradation rates for the CR dye43,44,45.
Effect of catalyst dose
The study investigated the impact of varying levels of produced hexaferrites dosages on the elimination of CR in a liquid setting. The quantity of catalyst used ranged from 0.02 to 0.08 g, and it was noted that the breakdown of CR dye escalated as the catalyst dosage reached 0.04 g. Beyond this dosage, there were no alterations in removal rate as demonstrated in Fig. 14. Consequently, 0.04 g was determined to be optimal dosage for further experiments. The enhancement in the CR dye degradation with increasing catalyst dosage is ascribed to the accessibility of additional active sites and leading to a higher number of superoxide and hydroxyl radicals because of large surface area of the catalyst. On the other hand, light penetration to the catalyst surface, which is essential for the reaction is hampered when the catalyst dose is higher than optimum concentration46. The data indicate no change in the decomposition efficiency of CR dye beyond the 0.04 g catalyst dosage.
Kinetics study
This study investigates the photodegradation efficiency of CR dye using the Ba1-xCoxFe12O19 catalysts. Four different hexaferrites were synthesized and their degradation performance was measured. The removal percentage of CR dye reaches 87.90%, 90.73%, 91.86% and 94.88% for the prepared BCF1, BCF2, BCF3, and BCF4 samples, respectively within the 2 h. The results are demonstrated in the Fig. 15. The photodegradation kinetics were evaluated using a pseudo-1st-order kinetic47. This model suggested that the rate of degradation is proportional to the amount of CR at any given time as demonstrated in the Fig. 15. The kinetic equation used is mentioned below48.
where, \({\text{C}}_{{\text{t}}}\) is the amount of CR dye at t, \({\text{C}}_{\text{O}}\) is the original amount of dye, and \({\text{K}}_{\text{app}}\)is the rate constant41. The utilization of this model is appropriate for elucidating the photodegradation mechanism of CR dye utilizing these ferrites. The \({\text{K}}_{\text{app}}\) for the photocatalytic reactions were determined from the slopes of the plots of \(\text{ln}({\text{C}}_{\text{O}}/{\text{C}}_{\text{t}})\) against “t”. The rate constant of CR dye degradation was achieved 0.016 min−1, 0.019, 0.021 and 0.023 min−1 for the prepared BCF1, BCF2, BCF3, and BCF4 samples, respectively.
The decomposition process through photocatalysis has been extensively documented by the numerous researchers. Photocatalysis involves the absorption of light energy by a photocatalyst with a frequency surpassing the Egof material49. The illustration of this process can be observed in Fig. 16. The electrons in an excited state undergo a reaction with oxygen molecules to produce superoxide radicals, while positive holes react with H2O molecules to create hydroxyl radicals. Subsequently, these highly reactive oxygen species, encompassing superoxide and hydroxyl radicals, engage with dye molecules, causing the breakdown of the dye and a alteration in its initial configuration. The sequence of chemical reactions culminates in the decomposition of CR dye molecules, as documented in the prior research studies44,50,51. Table S1 shows the comparison table of photocatalytic degradation of present work with the reported studies.
Reusability study
One of the key aspects in evaluating a catalyst’s performance and durability is its ability to be reused. To evaluate this factor, the reusability potential of prepared BCF1, BCF2, BCF3, and BCF4 samples were studied over the six consecutive experimental cycles. The findings of this experiment are demonstrated in Fig. 17. Results illustrated that, there is slight decrease in the degradation efficiency of CR dye. After six successive cycles, the BCF1, BCF2, BCF3, and BCF4 samples showed slight reductions of 8.11%, 9.81%, 7.92%, and 6.79%, respectively. The reduction in photocatalytic performance is due to the loss of catalyst while the washing, drying processes, and magnetic separation52,53,. The results demonstrate that Ba0.04Co0.06Fe12O19 hexaferrite retains its high degradation efficiency and exhibits the excellent stability and reusability over several cycles and making it a viable contender for the environmental remediation.
Conclusion
In this current study, pure and cobalt doped Ba1-xCoxFe12O19 (x = 0–0.06) were fabricated by SC approach to degrade the CR dye. XRD data revealed the hexagonal crystal structure without any impurities. FESEM results confirm the hexagonal structure with the grain size 264.51–198.84 nm. As the concentration of the cobalt increase, the crystallite and the grain reduce. The BCF1 sample exhibit the highest Ms, Mr, and Hc. The Eg of the pure barium hexaferrite obtained to be 2.9 eV but, as the cobalt concentration increases, the optical band gap decreases to 1.64 eV. The degradation percentage of CR dye achieves 87.90%, 90.73%, 91.86% and 94.88% for the BCF1, BCF2, BCF3, and BCF4 hexaferrites respectively within the 2 h of natural sunlight irradiation. The reusability potential of prepared BCF1, BCF2, BCF3, and BCF4 samples were also studied over six consecutive experimental cycles. The results demonstrate that the Ba0.04Co0.06Fe12O19 hexaferrite retains its high degradation efficiency and exhibits excellent stability and reusability over six cycles and making it a viable contender for practical uses for ecological cleanup.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
References
Liu, W. et al. Different Pathways for Cr(III) oxidation: implications for cr(vi) reoccurrence in reduced chromite ore processing residue. Environ. Sci. Technol. 54, 11971–11979. https://doi.org/10.1021/acs.est.0c01855 (2020).
Effective Extraction of Cr(VI) from Hazardous Gypsum Sludge via Controlling the Phase Transformation and Chromium Species | Environmental Science & Technology, (n.d.). https://pubs.acs.org/doi/abs/https://doi.org/10.1021/acs.est.8b02213 (accessed November 10, 2024).
Cai, Z. et al. An overview of nanomaterials applied for removing dyes from wastewater. Environ. Sci. Pollut. Res. 24, 15882–15904. https://doi.org/10.1007/s11356-017-9003-8 (2017).
Processes | Free Full-Text | Investigation of Congo Red Toxicity towards Different Living Organisms: A Review, (n.d.). https://www.mdpi.com/2227-9717/11/3/807 (accessed July 18, 2024).
Katheresan, V., Kansedo, J. & Lau, S. Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 6, 4676–4697. https://doi.org/10.1016/j.jece.2018.06.060 (2018).
Zafar, S., Bukhari, D. A. & Rehman, A. Azo dyes degradation by microorganisms – An efficient and sustainable approach. Saudi J. Biol. Sci. 29, 103437. https://doi.org/10.1016/j.sjbs.2022.103437 (2022).
Nasron, A. N., Azman, N. S., Rashid, N. S. S. M. & Said, N. R. Degradation of congo red dye in aqueous solution by using advanced oxidation processes. J. Acad. 6, 1–11 (2018).
Soh et al. Valorization of spent brewery yeast biosorbent with sonication-assisted adsorption for dye removal in wastewater treatment. Environ. Res. https://doi.org/10.1016/j.envres.2021.112385 (2024).
Rattanaburi, P., Nuengmatcha, P., Pimsen, R. & Porrawatkul, P. Photocatalytic degradation of organic dyes on magnetically separable barium hexaferrite as photocatalyst under conditions of visible light irradiation. Environ. Sci. Pollut. Res. 30, 68969–68986. https://doi.org/10.1007/s11356-023-27331-4 (2023).
Thang, P. D. et al. Mn-doped (Ba, Y)Fe12O19 hexaferrites: Crystal structure and oxidation states of Mn and Fe. Curr. Appl. Phys. 20, 1263–1267. https://doi.org/10.1016/j.cap.2020.08.018 (2020).
Bibi, I. et al. Enhanced visible light-driven photocatalytic degradation of crystal violet dye using Cr doped BaFe12O19 prepared via facile micro-emulsion route. J. Saudi Chem. Soc. 26, 101533 (2022).
Bibi, I. et al. Effect of doping on dielectric and optical properties of barium hexaferrite: Photocatalytic performance under solar light irradiation. Ceram. Int. 47, 31518–31526. https://doi.org/10.1016/j.ceramint.2021.08.030 (2021).
Valero-Luna, C., Palomares-Sanchéz, S. A. & Ruíz, F. Catalytic activity of the barium hexaferrite with H2O2/visible light irradiation for degradation of Methylene Blue. Catal. Today 266, 110–119. https://doi.org/10.1016/j.cattod.2015.08.049 (2016).
Jasrotia, R. et al. Photocatalytic degradation of malachite green pollutant using novel dysprosium modified Zn–Mg photocatalysts for wastewater remediation. Ceram. Int. 48, 29111–29120 (2022).
Khakwani, N. U. A. et al. Tailoring the physical, optical, and structural properties of bismuth oxide to enhance its anionic, cationic, and phenol dye degradation activities. Ceram. Int. 50, 33333–33344. https://doi.org/10.1016/j.ceramint.2024.06.143 (2024).
Jasrotia, R. et al. Photocatalytic dye degradation efficiency and reusability of Cu-substituted Zn-Mg spinel nanoferrites for wastewater remediation. J. Water Process Eng. 48, 102865 (2022).
Sözeri, H., Mehmedi, Z., Kavas, H. & Baykal, A. Magnetic and microwave properties of BaFe12O19 substituted with magnetic, non-magnetic and dielectric ions. Ceram. Int. 41, 9602–9609. https://doi.org/10.1016/j.ceramint.2015.04.022 (2015).
El Shater, R. E., El-Ghazzawy, E. H. & El-Nimr, M. K. Study of the sintering temperature and the sintering time period effects on the structural and magnetic properties of M-type hexaferrite BaFe12O19. J. Alloys Compd. 739, 327–334. https://doi.org/10.1016/j.jallcom.2017.12.228 (2018).
Dhage, V. N., Mane, M. L., Babrekar, M. K., Kale, C. M. & Jadhav, K. M. Influence of chromium substitution on structural and magnetic properties of BaFe12O19 powder prepared by sol–gel auto combustion method. J. Alloys Compd. 509, 4394–4398. https://doi.org/10.1016/j.jallcom.2011.01.040 (2011).
Bsoul, I. & Mahmood, S. H. Magnetic and structural properties of BaFe12−xGaxO19 nanoparticles. J. Alloys Compd. 489, 110–114. https://doi.org/10.1016/j.jallcom.2009.09.024 (2010).
Thakur, N. et al. Improvement in the structural, magnetic and electromagnetic behaviour of barium hexaferrites with yttrium doping for EMI shielding. J. Alloys Compd. 976, 173042. https://doi.org/10.1016/j.jallcom.2023.173042 (2024).
Gulbadan, S. et al. Investigation of crystal structure, photoluminescence, and Raman studies of Sm–Co substituted Ba–Sr M-type hexaferrites. Ceram. Int. 49, 18076–18083. https://doi.org/10.1016/j.ceramint.2023.02.176 (2023).
Effect of Drying Temperature on the Magnetic and Microstructural Properties of BaFe12O19 Synthesized by Coprecipitation Method - Sanda - 2021 - Advances in Materials Science and Engineering - Wiley Online Library, (n.d.). https://onlinelibrary.wiley.com/doi/https://doi.org/10.1155/2021/5884308 (accessed July 18, 2024).
Fatima, G. et al. Mn-doped BaFe12O19 nanoparticles synthesis via micro-emulsion route: solar light-driven photo-catalytic degradation of CV, MG and RhB dyes and antibacterial activity. Mater. Res. Bull. 168, 112491 (2023).
Mustafa, G. et al. Zn and Ni doping effect on barium hexaferrite ferroelectric, optical properties and photocatalytic activity under visible light irradiation. Phys. B Condens. Matter 663, 415006 (2023).
Priya, O., Singh, R. K., Das, S. B., Kumar, V. & Farozan, S. Optimization of the structural, optical, and magnetic properties of sol-gel derived La3+ substituted nanostructured barium hexaferrites. Phys. Scr. 98, 075920 (2023).
Yadav, S. K., Pabba, D. P., Soosairaj, A. & Divya, K. A A A, L R Asirvatham, V S Manikandan, M Navaneethan, A Thirumurugan, Study on the structural, magnetic, and magnetodielectric properties of M-type BaFe12O19 and SrFe12O19 hexaferrite nanoparticles. Surf Interfaces 52, 104956. https://doi.org/10.1016/j.surfin.2024.104956 (2024).
Ijaz, M., Ullah, H., Al-Hazmi, G. A., Althomali, R. H. & Asif, S. U. Impact of cobalt substitutions on optical, magnetic, dielectric, and structural, properties of BaFe11. 6-xAl0. 4CoxO19 hexaferrites prepared by Co-precipitation process followed by rapid sonochemical synthesis. Mater. Chem. Phys. 321, 129504. https://doi.org/10.1016/j.matchemphys.2024.129504 (2024).
Polley, K., Kundu, R. & Bera, J. Adsorption and sunlight-induced photocatalytic degradation of methyl blue by BaFe12O19 ferrite particles synthesised through co-precipitation method. Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2021.1887165 (2021).
Kishor, G., Bhowmik, R. N., Kaushik, S. D. & Babu, P. D. Investigation of structural phase stability, modified magnetic spin order and low temperature spin glass-like phase transitions in Sc-doped M-type BaFe12O19 hexaferrite. Phys. B Condens. Matter 685, 416034. https://doi.org/10.1016/j.physb.2024.416034 (2024).
Synthesis of Low Coercive BaFe12O19 Hexaferrite for Microwave Applications in Low-Temperature Cofired Ceramic | Journal of Electronic Materials, (n.d.). https://link.springer.com/article/https://doi.org/10.1007/s11664-012-2398-6 (accessed July 17, 2024).
Kumar, S. et al. Lattice strain mediated dielectric and magnetic properties in La doped barium hexaferrite. J. Magn. Magn. Mater. 473, 312–319 (2019).
Dudziak, S. et al. Pseudo-superparamagnetic behaviour of barium hexaferrite particles. RSC Adv. 10, 18784–18796. https://doi.org/10.1039/D0RA01619E (2020).
Jinendra, U. et al. Template-free hydrothermal synthesis of hexa ferrite nanoparticles and its adsorption capability for different organic dyes: comparative adsorption studies, isotherms and kinetic studies. Mater. Sci. Energy Technol. 2, 657–666 (2019).
Almessiere, M. A. et al. A study on the spectral, microstructural, and magnetic properties of Eu–Nd double-substituted Ba0 5Sr0. 5Fe12O19 hexaferrites synthesized by an ultrasonic-assisted approach. Ultrason. Sonochem. 62, 104847 (2020).
Almessiere, M. A. et al. Sm–Dy co-substituted Sr hexaferrite microspheres: an investigation on their structural, magnetic, optical, and porosity characteristics. Ceram. Int. 47, 25131–25141 (2021).
Aldrdery, M. et al. Synergistic role of indium doping and g-C3N4 reinforcement in boosting the visible light-triggered rhodamine B and diclofenac sodium salt degradation over rare earth molybdate. Surf. Interfaces 54, 105232. https://doi.org/10.1016/j.surfin.2024.105232 (2024).
Sun, L. et al. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 174, 71–78. https://doi.org/10.1016/j.cherd.2021.08.002 (2021).
Chavan, V. C., Shirsath, S. E., Mane, M. L., Kadam, R. H. & More, S. S. Transformation of hexagonal to mixed spinel crystal structure and magnetic properties of Co2+ substituted BaFe12O19. J. Magn. Magn. Mater. 398, 32–37. https://doi.org/10.1016/j.jmmm.2015.09.002 (2016).
Structural, magnetic and microwave absorption properties of doped Ba-hexaferrite nanoparticles synthesized by co-precipitation method - ScienceDirect, (n.d.). https://www.sciencedirect.com/science/article/pii/S0304885314012724#s0025 (accessed July 19, 2024).
Boutra, B., Güy, N., Özacar, M. & Trari, M. Magnetically separable MnFe2O4/TA/ZnO nanocomposites for photocatalytic degradation of Congo Red under visible light. J. Magn. Magn. Mater. 497, 165994 (2020).
Thomas, M., Naikoo, G. A., Sheikh, M. U. D., Bano, M. & Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. Chem. 327, 33–43 (2016).
Ali, N. et al. Photocatalytic degradation of congo red dye from aqueous environment using cobalt ferrite nanostructures: development, characterization, and photocatalytic performance. Water. Air. Soil Pollut. 231, 50. https://doi.org/10.1007/s11270-020-4410-8 (2020).
Bessy, T. C. et al. Efficient antibacterial activities and photocatalytic degradation of congo red dye by CuxMg0.8-xFe2O4 nanoparticles synthesized by combustion method. J. Mater. Sci. Mater. Electron. 34, 518. https://doi.org/10.1007/s10854-023-09906-6 (2023).
Dinesh, A. et al. Sol–gel combustion synthesis and photocatalytic dye degradation studies of rare earth element Ce substituted Mn–Zn ferrite nanoparticles. J. Mater. Res. Technol. 18, 5280–5289 (2022).
Jasrotia, R., Kumari, N. & Kumar, R. Mu Naushad, P Dhiman, G Sharma, Photocatalytic degradation of environmental pollutant using nickel and cerium ions substituted Co0.6Zn0.4Fe2O4 nanoferrites. Earth Syst. Environ 5, 399–417. https://doi.org/10.1007/s41748-021-00214-9 (2021).
Kour, S. et al. Improving photocatalytic efficiency of MnFe2O4 ferrites via doping with Zn2+/La3+ ions: photocatalytic dye degradation for water remediation. Environ. Sci. Pollut. Res. 30, 71527–71542. https://doi.org/10.1007/s11356-021-13147-7 (2021).
Alburaih, H. A. et al. Multifunctional Fe and Gd co-doped CeO2-RGO nanohybrid with excellent solar light mediated crystal violet degradation and bactericidal activity. Synth. Met. 287, 117093. https://doi.org/10.1016/j.synthmet.2022.117093 (2022).
Abu-Elsaad, N. I. & Nawara, A. S. Sm-substituted Co-Mn nanoferrites: synthesis, characterization, magnetic properties and photocatalytic degradation of methylene blue. Phys. Scr. 98, 115983 (2023).
Boulahbel, H. et al. Enhanced photodegradation of Congo red dye under sunlight irradiation by pn NiFe2O4/TiO2 heterostructure. Inorg. Chem. Commun. 154, 110921 (2023).
Kumar, A. et al. Impact of Gd3+ doping on structural, electronic, magnetic, and photocatalytic properties of MnFe2O4 nanoferrites and application in dye-polluted wastewater remediation. Environ. Sci. Pollut. Res. 30, 18820–18842. https://doi.org/10.1007/s11356-022-23420-y (2022).
Kaur, G. et al. Photocatalytic dye degradation by recyclable ZnCo magnetic ferrites at ambient conditions. Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2022.2161900 (2023).
Yelagandula, S., Eragari, R. R., Putta, C. S., Kotha, L. R., Adulla, P. R. Ni and La Substitution of Cobalt Nanoferrites Preparation: Structural, Magnetic, Dielectric Studies and Photocatalytic Activity for Waste Water Treatment, Magn. Dielectr. Stud. Photocatalytic Act. Waste Water Treat. (n.d.). https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4606425 (Accessed June 5, 2024).
Acknowledgements
The authors express their deep gratitude to the Researchers Supporting Project number (RSP2025R391) at King Saud University, Riyadh, Saudi Arabia, for their valuable support.
Funding
King Saud University, RSP2025R391.
Author information
Authors and Affiliations
Contributions
H. wrote the whole manuscript and performed the data investigation, S.G., P.K.P., MD.R., and S. provided the resources, J.A., C.C.K, and N.L. performed the review and editing, A.V. performed the data curation, R.J. supervised the whole work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Himanshi, Ganesan, S., Pathak, P.K. et al. Robustic and hybrid cobalt doped BaFe12O19 hexaferrites for the photocatalytic degradation of Congo Red for wastewater treatment. Sci Rep 14, 31051 (2024). https://doi.org/10.1038/s41598-024-82273-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82273-2
Keywords
This article is cited by
-
Insights into the microstructural, optical and magnetic characteristics of cobalt and dysprosium co-doped BaFe12O19 M-type hexagonal ferrites
Journal of Sol-Gel Science and Technology (2025)