Abstract
Our study aimed to investigate the relationship between δ-catenin expression and whole-brain small-world network in breast cancer patients before chemotherapy using rs-fMRI. The study was based on the hypothesis that different δ-catenin expression levels correspond to distinct brain imaging characteristics. A total of 105 pathologically confirmed breast cancer patients were collected and categorized into high δ-catenin expression (DH, 52 cases) and low expression (DL, 53 cases) groups. Additionally, 36 age-matched healthy women were enrolled as a healthy control group (HC). The results demonstrated differences in several network topology attributes among the three groups. Furthermore, in addition to differences in nodal efficiency, betweenness, and degree centrality metrics observed between the patient groups and HCs across multiple brain regions, significant alterations were also identified between the DL and DH groups, particularly in the supramarginal gyrus and inferior frontal gyrus. Correlation analysis revealed associations between cognitive and memory-related brain regions, such as the caudate nucleus and frontal lobe, and scores on cognitive and verbal memory scales (all P < 0.05). This study concludes that high and low expression levels of δ-catenin in breast cancer patients are associated with distinct whole-brain network topology patterns, and that these differences in network segregation and integration functions are associated with alterations in cognition and verbal memory.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignant tumor among women. Previous studies have shown that the impact of related cognitive impairment is even more serious than tumor recurrence and metastasis in the long-term survival of breast cancer patients1. Other studies2,3 have indicated that breast cancer patients may suffer from cognitive impairment after chemotherapy, with memory loss being the most frequently reported side effect. However, further research suggests that cancer can impact cognitive function in breast cancer survivors4. This underscores the need for further investigation into the mechanisms of cognitive impairment in these patients and the development of strategies to mitigate long-term cognitive deficits.
δ-catenin is a member of a subfamily characterized by ten Armadillo-repeats. High expression of δ-catenin has been associated with the development of tumors5,6,7. Moreover, studies have found that elevated δ-catenin expression levels in lung cancer patients8 are closely related to poor prognosis, providing a solid experimental basis for targeting δ-catenin in cancer therapies of significant clinical significance.
P120 catenin (P120ctn) is an intracellular signal transducer and cell adhesion molecule that plays an important biological role in cell proliferation, adhesion, and migration9. As a member of the p120ctn family, δ-catenin was initially found to be expressed in the central nervous system, mainly in the dendrites of mature neurons, and especially concentrated at the postsynaptic membrane, Knockout of δ-catenin expression leads to impaired dendrite development, providing evidence for its physiological mechanism10. However, numerous studies have found abnormal expression of δ-catenin in patients with malignant tumors, such as prostate cancer11, colorectal cancer12, and lung cancer8. Moreover, the abnormal upregulation of δ-catenin expression is closely related to poor prognosis. Therefore, it is reasonable to hypothesize that δ-catenin is involved in both physiological and pathological mechanisms.
Even though breast cancer is the most common malignant tumor in women, there are few studies on the relationship between δ-catenin and breast cancer. A limited number of studies13,14 have reported increased δ-catenin expression in breast cancer, which was associated with poor prognosis in those cases. The association between δ-catenin and breast cancer, along with the involvement of δ-catenin in neuronal development and maintenance, suggests that high δ-catenin expression in breast cancer patients may contribute to cognitive dysfunction. Therefore, investigating the association between δ-catenin and brain alterations in breast cancer patients before chemotherapy is of significant clinical value.
Rs-fMRI investigates central neuronal activity by detecting Blood Oxygen Level Dependent (BOLD) changes in blood oxygenation level. When neural activity increases, the corresponding brain region experiences an increase in blood supply and oxygen consumption, leading to alterations in the BOLD signal. The organization and efficiency of neural networks can be quantified using graph theory15. To better describe the synergistic coupling of brain neural networks in terms of functional integration and differentiation, the concept of a small-world network is introduced16. The brain’s intrinsic networks are organized as small-world networks, characterized by an economical architecture with a higher clustering coefficient (Cp) and a shorter characteristic path length (Lp)17. Typically, a shorter Lp suggests that information can be transmitted rapidly across distant nodes, indicating stronger functional integration, while Cp reflects the prevalence of clustering connections around a single node on average and the efficiency of short-distance information transfer. This organization enables efficient information transmission and processing at both local and global levels18,19. Previous studies have shown that the topology of brain small-world networks may be altered by disease20,21, with these changes potentially reflecting underlying pathophysiological mechanisms. Consequently, measuring these network characteristics could potentially be used as biomarkers for early detection, diagnosis, and treatment evaluation.
MRI-based BOLD imaging is thus a powerful tool for studying brain network differences in breast cancer patients with different expressions of δ-catenin. Numerous studies22,23,24 have demonstrated that chemotherapy can alter brain structure and functional networks, particularly in the frontal lobe and other regions, and that post-chemotherapy cognitive impairment may be associated with damage to specific brain regions. However, it remains unclear whether high δ-catenin expression in breast cancer patients affects neuronal survival and accelerates or delays the onset of cognitive impairment. Currently, no studies have explored the properties of brain regions and networks in relation to different δ-catenin expression states in breast cancer patients. Therefore, this study aims to investigate the relationship between different δ-catenin expression statuses and the topological properties of small-world whole-brain network in breast cancer patients before chemotherapy by rs-fMRI, hypothesizing that different δ-catenin expression levels are associated with distinct brain imaging characteristics.
Materials and methods
Patients
This prospective study was approved by the Ethics Committee of the Affiliated Hospital of Chifeng University (approval number: fsyy202136) and each participant provided written informed consent before the study. A total of 105 patients with breast cancer confirmed by pathology without any treatments were prospectively collected, including 52 patients with high expression of δ-catenin (DH) and 53 patients with low expression of δ-catenin (DL). Inclusion criteria were as follows: (1) newly diagnosed breast cancer patients without anti-estrogen therapy, chemotherapy, and radiotherapy; (2) the general cognitive function was normal and right-handed; (3) the physical status score of Eastern Cooperative Oncology Group (ECOG) was 0–1; (4) no contraindications of MRI scanning. Exclusion criteria were: (1) the presence of cachexia, pernicious anemia, or brain metastasis; (2) preoperative anti-estrogen therapy or radiotherapy; (3) drug or alcohol dependence, severe neurological or mental illness; (4) history of brain trauma, cerebral infarction, cerebral tumor or congenital brain malformations; (5) patients older than 50 of age and with diabetes, small cerebral vascular disease (CSVD) and other medical history affecting brain cognition. Thirty-six age-matched healthy females were enrolled as controls (HC). Clinical data of all subjects, including age, education level, estrogen receptor (ER), progesterone receptor (PR), pathological grade, and HER-2 index, were collected.
Neuropsychological testing
Neuropsychological testing was performed by a trained neurologist (attending physician with 13 years of work experience) in a quiet environment. The Chinese version of the Assessment of Cognitive Function in Cancer Therapy (FACT-Cog)25 was used for evaluating patients’ cognitive status. It includes four categories, i.e., CogPCI (perceived cognitive impairments), CogQOL (impact of perceived impairments on quality of life), CogOth (comments from others), CogPCA (perceived cognitive abilities), which have good reliability and validity for Chinese breast cancer patients. In addition, the objective cognitive function of patients was assessed by the Mini-mental State Examination (MMSE26) and Montreal Cognitive Assessment Scale (MoCA27). Additionally, the Chinese Auditory Verbal Learning Test (AVLT28) was used to evaluate clinical memory, including short-term memory (AVLT-short), long-term memory (AVLT-long), and delayed recognition memory tests (AVLT-delay), The higher the score, the better the performance. Clinical memory evaluation was also tested by the Wechsler memory scale29,30, which included the Digital Symbol Substitution Test (DSST) and Trail Making Test A and B (TMT A-B).
MRI parameters
All subjects underwent MRI scans using a GE HDXT 3.0T MR scanner with an 8-channel head coil and were instructed to keep their eyes closed but remain awake during the scan. MR Protocols included conventional MRI sequences, 3D-T1 weighted images, and rs-fMRI. The scanning baseline was parallel to the former union plane. In addition, routine MRI images were used to exclude patients with other central nervous system disorders, including transverse T1WI, T2WI, DWI, and T2-FLAIR sequences.
Structural data (high-resolution T1-weighted images) were acquired using a three-dimensional fast spoiled gradient echo (FSPGR) sequence with the following parameters: TR = 6.7 ms, TE = 2.4 ms, FOV = 350 mm × 350 mm, voxel size = 1.0 × 1.0 × 1.0 mm3, scanning layers = 188, and scanning time was 3min. Rs-fMRI data were obtained using echo-planar imaging (EPI) sequence with the following parameters: TR = 3000 ms, TE = 40 ms, FOV = 240 mm × 240 mm, voxel size = 3.44 × 3.44 × 3.0 mm3, slice thickness = 4 mm, slice spacing = 1 mm. A total of 200-time points were scanned for 10 min.
rs-fMRI data processing
Rs-fMRI data were performed by the Data Processing Assistant for Resting-State fMRI Advanced Edition (DPARSFA, Version 4.3, http://rfmri.org/DPARSF) toolbox in MATLAB R2016b (MathWorks, Natick, MA, USA). The DICOM format image was converted to NIFTI format. The data of the first 10 time points were deleted to eliminate the non-equilibrium effect of magnetization. Slice timing and realignment were performed to correct time differences and head motion (displacement or rotation less than 3 mm or 3°) (No. 18, 23, 31, 32, and 38 patients were eliminated in HC group; NO. 32, 81 and 109 patients were excluded in DH and DL groups). DARTEL was used to register T1 images, and regression was used to remove nuisance covariates, including Friston 24 head motion parameters, cerebrospinal fluid signals, and white matter signals. The corrected images were then spatially normalized to the standard Montreal Neurological Institute (MNI) space. Each voxel was re-sampled to 3 × 3 × 3mm3. Finally, low-frequency drift and high-frequency signals, such as respiration and heartbeat, were removed.
After that, the graph theory network and measures analysis were constructed using the GRETNA toolbox (https://www.nitrc.org/projects/gretna/). The pre-processed rs-fMRI images were used to construct a whole-brain functional network using an anatomical auto-labeling template (AAL). First, the whole brain was segmented into 90 brain regions; each brain region was defined as a node, and the time series of all voxels within each brain region were weighted and averaged to obtain the average time series of the 90 brain regions. Then, a 90 × 90 correlation matrix was generated by calculating the Pearson correlation coefficient between the average time series of each two brain regions for each subject, which is the brain functional connectivity matrix. The range of sparsity was set to 0.05–0.50 to ensure that: (1) all nodes of the brain functional network of the two groups of subjects were in a fully connected state, i.e., the network had no breakpoints; (2) the small-world properties of the HC group had an estimable maximum value. The step size was set as 0.01 and compared with 500 pre-generated random networks to obtain the specific parameter values of the brain network attributes under 46 sparsities, including small-worldness (σ), normalized characteristic path length (λ), normalized clustering coefficient (γ), clustering coefficient (Cp), characteristic path length (Lp), global efficiency (Eg), local efficiency (Eloc), nodal efficiency (NE), betweenness centrality (BC) and degree centrality (DC). The area under the curve (AUC) values for all sparsities were also calculated to provide scalar quantities that were not dependent on the choice of a particular threshold. The whole procedure is illustrated in Fig. 1.
Immunohistochemical analysis of δ-catenin
All postoperative tumor tissues of breast cancer patients were fixed by neutral formalin and embedded in paraffin. After that, tissue chip wax blocks were made into 4 μm thick specimens by LEICARM2235, Immunohistochemical hypersensitive two-step method was used for staining. The δ-catenin (sc-81793) antibody was purchased from Santa Cruz Inc. in a 1:50 dilution. The immunohistochemical test kit and DAB chromogenic reagent were purchased from Beijing Zhongshan Jinqiao Biological Company.
Two experienced pathologists blindly and independently scored all breast cancer patient’s immunostained specimens. At low magnification, the uniformly stained areas were first identified, then, brown particles in the cytoplasm of tumor cells were counted as a positive signal at high magnification(×400). The scoring criteria of δ-catenin were as follows: immunohistochemical staining intensity was divided into 4 levels: 0: negative; 1: weak staining; 2: medium staining; 3: strong staining. The percentage of positive cells was divided into 5 grades: 0: absent; 1:1%~25%; 2:26%~50%; 3:51%~75%; 4: ≥76%. The total score of each specimen was calculated by multiplying the staining intensity of the tumor cells and the positive percentage of the tumor cells, ranging from 0 to 12 scores. Among them, the cases with 0 ~ 7 scores were considered as DL group and those with 8 ~ 12 scores as DH group8. In addition, healthy tissues more than 2 cm away from the tumor tissue in breast cancer specimens were selected for scoring to represent the δ-catenin expression in the HC group, given the extreme difficulty in obtaining tissue sections from healthy individuals. Figure 2 demonstrates examples of pathological immunohistochemistry of breast cancer patients and healthy breast tissue with different δ-catenin expression status.
Pathological immunohistochemical examples of breast cancer patients with different expression statuses of δ-catenin and healthy breast tissues. In a case of breast cancer patients with low expression, under low-power microscopy (A), δ-catenin positive tumor cells accounted for approximately 25–50% of all tumor cells, with a score of 2 for the number of positive cells. Under high-power microscopy (×400) (B), the staining intensity of δ-catenin was weak, with a score of 1 for the staining degree. The final score was 2. In a case of breast cancer patients with high expression, under low-power microscopy (C), δ-catenin positive tumor cells accounted for ≥ 76% of all tumor cells, with a score of 4 for the number of positive cells. Under high-power microscopy (×400) (D), the staining intensity of δ-catenin was strong, with a score of 3 for the staining degree. The final score was 12. Healthy tissues more than 2 cm away from the tumor tissue in breast cancer sections were selected for scoring to represent the δ-catenin expression in the healthy control group, and the final scores of the two subjects presented were both 0 (E and F).
Statistical analysis
SPSS26.0 software was used for the statistical analysis of clinical data among the three groups. An independent sample t-test or nonparametric test was used for measurement, and a chi-square test was used for categorical data, all of which were two-tailed tests. GRETNA software was used to make a statistical analysis of obtained graph theory network measures. Age, education duration, and HER-2 index were entered as covariates. A covariance analysis was used to compare the three groups using a One-Way ANOVA, after which the differences among the groups were determined by pair-wise post-hoc comparisons. A Pearson’s correlation analysis was used to explore the correlations between the indicators. The statistical significance threshold was set at P < 0.05.
Results
Comparison of demographic, clinical and neuropsychological characteristics
The comparison results of demographic, clinical and neuropsychological characteristics are shown in Table 1. Increased HER-2 expression levels were observed in the DH group compared to the DL group. In addition, there were significant differences in MMSE, MoCA, and AVLT among the three groups (P < 0.05). There were no differences in between-group comparisons in the remaining indicators (P > 0.05).
Comparison of network topology properties
Results of small-world differences and AUC differences in all sparsity among the three groups of patients (Fig. 3). The Lp values in the DL group were significantly lower than those in the HC group, and the Eg values were significantly higher than those in the HC group. The σ and γ values in the DH group were significantly higher than those in the HC group. Also, these differences were still statistically significant at most of the sparsities. The λ and Eloc values significantly differed among the three groups at some sparsities; however, the AUC values of these indices were not significantly different among the three groups. There was no significant difference in other attributes among the three groups.
Comparison of network topology properties. A Reduced Lp and elevated Eg were observed in the DL group, compared to the HC group. And the σ and γ values in the DH group were significantly higher than those in the HC group. B Differences in these properties were still statistically significant at most of the sparsities. In addition, differences in λ and Eloc are also observed in a small number of sparsities among the three groups. *P < 0.05; DH: high δ-catenin expression group in patients with breast cancer; DL: low δ-catenin expression group in patients with breast cancer; HC: healthy control group.
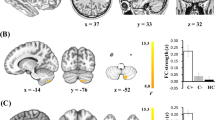
The significant differences in NE values among the three groups are shown in Fig. 4A. Compared with the HC group, NE values in the right orbital superior frontal gyrus, the right opercular part of the inferior frontal gyrus, bilateral caudate nucleus, right putamen, and bilateral thalamus were increased in the DL group. Compared with the HC group, NE values were increased only in the right opercular part of the inferior frontal gyrus and the right caudate nucleus in the DH group. As for the patient group, the NE value of the right supramarginal gyrus was elevated in the DH group compared to the DL group.
The significant differences in DC values among the three groups are shown in Fig. 4B. Compared with the HC group, the DC values of the left inferior parietal angular gyrus, left paracentral lobule, and left superior temporal gyrus of the temporal pole were decreased in the DL group, while the DC values of the right caudate nucleus and the left transverse temporal gyrus were decreased in DH group. The DC value of the bilateral caudate nucleus and bilateral thalamus in the DL group was higher than that in the HC group, but there was no brain region with increased DC value in the DH group. As for the patient group, only the DC value of the right supramarginal gyrus was decreased in the DH group compared with DL.
The significant differences in BC values among the three groups are shown in Fig. 4C. Compared with the HC group, BC values of the left anterior cingulate and paracingulate gyrus and left paracentral lobule were decreased, and the right caudate nucleus was increased in the DL group, while the BC value of the right medial cingulate and paracingulate gyrus was decreased, and right caudate nucleus was increased in DH group compared with HC. As for the patient group, only the BC value of the right triangular part of the inferior frontal gyrus was increased in the DH group compared with DL.
Brain regions with significant between-group differences in nodal efficiency (A), degree (B) and betweenness centrality (C) (P < 0.05, Bonferroni corrected). CAU: caudate nucleus; THA: thalamus; ORBsup: orbital part of superior frontal gyrus; IFGoperc: opercular part of inferior frontal gyrus; SMG: supramarginal gyrus; PCL: paracentral lobule; TPOsup: temporal pole of superior temporal gyrus; IPL: inferior parietal, but supramarginal and angular gyri; HES: heschl gyrus; ACG: anterior cingulate and paracingulate gyri; DCG: median cingulate and paracingulate gyri; IFGtriang: triangular part of inferior frontal gyrus; L: left; R: right; DH: high δ-catenin expression group in patients with breast cancer; DL: low δ-catenin expression group in patients with breast cancer; HC: healthy control group.
Correlation between network attributes changes and neuropsychological results
Correlation were analysed for each of the two patient groups between the network measures and the cognitive scores, and the results are shown in Fig. 5. In the DL group (Fig. 5A), NE in the right caudate nucleus was negatively correlated with MoCA (r = -0.038, P = 0.038), DSST (r = -0.280, P = 0.042), AVLT-short-memory (r = -0.302, P = 0.028), AVLT-delayed-memory (r = -0.313, P = 0.023) and HER-2 (r = -0.280, P = 0.042), and positively correlated with TMT-B (r = 0.300, P = 0.029), FACT-Cog_CogQOL (r = 0.381, P = 0.005) and FACT-Cog-SUM (r = 0.305, P = 0.026); NE in the left caudate nucleus was negatively correlated with DSST (r = -0.288, P = 0.036), AVLT-short-memory(r = -0.278, P = 0.044), and positively correlated with FACT-Cog-SUM (r = 0.312, P = 0.023). NE in the right orbital part of the inferior frontal gyrus was negatively correlated with FACT-Cog-SUM (r = -0.277, P = 0.045). NE in the right middle frontal gyrus was positively correlated with FACT-Cog_CogOth (r = -0.289, P = 0.036). Eloc was negatively correlated with TMT-B (r = -0.379, P = 0.005).
In the DH group (Fig. 5B), σ (r = -0.311, P = 0.025) and γ (r = -0.317, P = 0.022) attributes were negatively correlated with AVLT-long-memory, while NE in the orbital part of Inferior frontal gyrus (r = 0.298, P = 0.032) was positively correlated with AVLT -long-memory. NE in the right opercular part of the inferior frontal gyrus was negatively correlated with MMSE (r = -0.347, P = 0.012). Cp (r = 0.495, P < 0.001), NE in the right middle frontal gyrus (r = 0.334, P = 0.016), and Eloc (r = 0.326, P = 0.018) were positively correlated with AVLT-delayed-memory. NE in the right opercular part of the inferior frontal gyrus was positively correlated with TMT-A (r = 0.302, P = 0.029).
Heat maps for correlation analysis of low (A) and high (B) δ-catenin expression patients with breast cancer. MMSE: Mini-mental State Examination; MoCA: Montreal Cognitive Assessment Scale; AVLT: the Chinese Auditory Verbal Learning Test; DSST: the digital symbol substitution test; TMT: Trail Making Test; CogQOL: impact of perceived impairments on quality of life; CogOth: comments from others; FACT-Cog: the chinese version of the Assessment of Cognitive Function in Cancer Therapy; NE: nodal efficiency; MFG: middle frontal gyrus; ORBinf: orbital part of inferior frontal gyrus; CAU: caudate nucleus; IFGoperc: opercular part of inferior frontal gyrus; ORBinf: orbital part of inferior frontal gyrus; L: left; R: right.
Discussion
To the best of our knowledge, this is the first study that used rs-fMRI to investigate the differences in whole-brain network topological properties in breast cancer patients with different expressions of δ-catenin protein.
In the present study, rs-fMRI was used to investigate the relationship between different δ-catenin expression statuses and the topological properties of small-world whole-brain network in breast cancer patients before treatment. Given that HER-2 status was the only variable differing between the two patient groups, HER-2 was included as a covariate in the analysis. Before treatment, the Eg index of the DL group was higher compared to the HCs, while the Lp index was lower, suggesting a slightly enhanced functional integration ability of the network in the early stages of cancer. Moreover, network metrics revealed differences between the two breast cancer groups, indicating that different δ-catenin expression statuses were uniquely associated with the right supramarginal gyrus and triangular part of the right inferior frontal gyrus. Importantly, no cognitive decline was observed in either group before treatment, suggesting that brain network changes induced by δ-catenin precede cognitive dysfunction, and early brain damage induced by high expression of δ-catenin may be involved in executive function.
Neural plakophilin-related armadillo protein (NPRAP or δ-catenin) is a neuronal-specific protein, which was first discovered by German scientists in 1997 through brain cDNA sequencing31. δ-catenin is a component of the cadherin-catenin cell adhesion complex, and recent studies have increasingly demonstrated its upregulation in certain epithelial tumors5,6,7,8. Given its multiple structural domains and central role in molecular signaling pathways, δ-catenin is involved in various mechanisms underlying disease progression32,33,34. It has been speculated that the increased expression of δ-catenin gene level in tumors may be related to several potential mechanisms32,33,34,35,36,37: (1) δ-catenin can regulate the expression of apoptosis-related proteins, such as Bcl-2L, survivin, and cleaved caspase 3, thereby promoting the proliferation of tumor cells and inhibiting apoptosis; (2) δ-catenin may stimulate the secretion of vasoactive factors within tumor tissues and participate in neovascularization, while also modulating the activity of small GTPases; (3) δ-catenin overexpression may be associated with the transcriptional activator E2F1 or co-expression of transcriptional repressor Hes1; (4) δ-catenin mediated FAK/Src/Rac1 signaling pathway can promote tumor development.
HER-2 is a transmembrane protein crucial for regulating cell growth, apoptosis, and differentiation, with its overactivity linked to the malignant biological behaviors of the mammary gland38. In this study, HER-2 was the only clinical parameter differing between the DH and DL groups, and the results also confirmed HER-2’s significant role in breast cancer. To control the influence of HER-2 on patients, HER-2 was taken as covariable in the statistical analysis, ensuring the observed effects were specifically attributable to δ-catenin. Studies have suggested that the synergistic effect between δ-catenin and HER-2 may have an important role in the progression of tumor invasion. By promoting the expression of Cyclin D1 and Cdc34 and enhancing HER-2 phosphorylation, δ-catenin could promotes cell mitosis, thereby exerting a tumor-promoting effect39. In this study, the HER-2 index was significantly higher in the DH group compared to the DL group, indirectly suggesting that high δ-catenin expression in breast cancer patients is associated with more aggressive tumor behavior. Given that δ-catenin levels in healthy breast tissue were considered negligible or zero, we inferred that the δ-catenin expression in the HC group was negative.
Lp and Eg are functional integration indicators, and the functional integration ability is measured by estimating the ease of communication between brain regions, which is usually based on the concept of path. Generally, a shorter Lp corresponds to stronger functional integration ability. Eg is the average value of node efficiency in the whole brain, which measures the information transfer ability between nodes in the network. Cp and Eloc assess the local information transmission capability of the network. In this study, the AUC values of Lp, Eg, σ, and γ were different among the three groups under sparsity conditions. Compared to the HC group, DL group exhibited shorter Lp, and higher Eg, indicating slightly stronger functional integration in the early stages of the DL group. No significant differences were observed between the DH and HC groups in terms of Lp and Eg values. However, Only the σ and γ values in the DH group were marginally higher than those in the HC group.
In this study, we observed elevated NE, BC, and DC in certain brain regions of both the DH and DL groups. Elevated NE was concentrated in the right superior frontal gyrus, inferior frontal gyrus, bilateral caudate nucleus, bilateral thalamus, and right putamen. The BC value of the right caudate nucleus in the DH and DL groups was higher than that in the HC group. The DC value of the bilateral caudate nucleus and bilateral thalamus in the DL group was higher than that in the HC group. From the results, we speculate that certain brain regions in the DH and DL groups may exhibit a compensatory increase in resting-state activity, particularly in the frontal lobe40,41, cingulate gyrus, caudate nucleus42,43, thalamus44,45 and other brain areas related to language and high-level cognitive functions. As these regions are also important components of the default mode network (DMN)46,47, the results suggest that more neuronal synergy is needed to maintain cognitive function as much as possible. DMN regions are typically characterized by high resting cerebral blood flow (CBF), and several studies48,49,50,51 have demonstrated that brain regions with high resting CBF tend to have increased low frequency amplitude and regional homogeneity. Furthermore, the weakened function caused by neuronal injury can be compensated by increasing neuronal recruitment and activation52,53,54,55, which is regarded as a manifestation of neuronal compensatory response.
Despite the presence of compensatory mechanisms, our study identified several differences in small-world network properties between the two patient groups with different expressions of δ-catenin. Specifically, the NE and DC differed between the DH and DL groups in the right supramarginal gyrus, a part of the parietal lobule and an important part of the frontoparietal network. This region integrates nerve tracts of the parietal, temporal, and occipital lobes56,57, playing a crucial role in cognitive control and processing of working memory. Additionally, The BC showed differences in the triangular part of the right inferior frontal gyrus. This sub-region of the prefrontal lobe, the most complex and highly developed brain area, is involved in numerous cognitive functions such as memory, attention and language. It is also considered as the higher emotional center. Pathological anxiety may be related to its abnormal function58,59. These findings suggest that the expression of δ-catenin were uniquely associated with these two brain regions, which belong to the prefrontal cortex, and are associated with the frontal lobe-striatum frontal, parietal network. Both of these are involved in cognitive and memory processing. This is consistent with our observed differences in local efficiency in neuronal processing as well as a statistical difference in memory performance between the two groups. The study further demonstrates that part of the function of the prefrontal lobe changes in the early stage of cancer. Correlation analyses revealed both positive and negative correlations between frontal lobe and various cognitive scale scores, suggesting that δ-catenin protein may be involved in brain regions related to executive brain function.
In this study, the memory scores of the DH group were higher than those of the DL group. However, correlation analysis showed that only certain brain regions had a limited correlation with cognitive function, which does not directly demonstrate a direct effect of δ-catenin on cognition. This is likely due to the presence of multiple factors affecting memory, including compensatory mechanisms resulting from pathological hyperexcitability60, the duration of the disease, and other potential variables.
Considering the BC and DC indexes, the two groups showed decreased functional areas in different brain regions. The BC value of the left Anterior cingulate gyrus, para cingulate gyrus, and the left paracentral lobule in the DL group were lower than the HC group. The DC value of the left inferior parietal supramarginal and angular gyrus, left parietal lobule and superior temporal gyrus in the DL group were reduced compared to the HC group. These reduced brain areas were mostly located in the left hemisphere. In contrast, the DH group showed decreased brain areas mainly in the right hemisphere compared to the HC group, with lower BC value in the right median cingulate and paracingulate gyrus, and reduced DC value in the right caudate nucleus and left heschel gyrus. These findings suggest asymmetric brain distribution differences. In the absence of chemotherapy drugs and other indicators except for HER-2, breast cancer patients with different expressions of δ-catenin exhibit different appearances on the function of bilateral cerebral hemispheres. While there may be a certain compensatory mechanism in patients, as previously discussed, this observed difference suggests that the balance between injury and compensation may be influenced by the different expression levels of δ-catenin. This relationship is not yet fully understood and warrants further investigation.
There are several limitations to the present study. Firstly, due to the near impossibility of obtaining tissue specimens from healthy individuals, we opted to score healthy tissue located more than 2 centimeters away from the tumor tissue in breast cancer specimens as a representative of δ-catenin expression in the HC group. This does not completely replace δ-catenin expression levels in healthy human tissues. In addition, we were not able to directly obtain δ-catenin expression data in the subjects’ brain tissues. Consequently, our results cannot conclusively demonstrate that the disruption of brain network architecture is a direct consequence of δ-catenin’s influence. Secondly, this study did not assess breast cancer patients on disease course and mood scales, which could potentially affect brain networks. Thirdly, the comparison in this study was based on baseline data collected before chemotherapy. Ongoing follow-up treatments and evaluations are being carried out step by step for a longitudinal analysis of breast cancer patients with different levels of δ-catenin. Finally, although the sample size of this study met the requirements for the analysis, it was still not large enough. In addition, this was a single-center study. Increasing the sample size and conducting multi-center studies in the future may further improve the accuracy of verifying the influence of δ-catenin.
In summary, the present study examined the topological properties of the resting-state small-world whole-brain network in breast cancer patients with different δ-catenin expression levels. Our findings indicate that high and low expression states of δ-catenin in breast cancer patients have different brain network topology patterns, and are associated with alterations in cognition and memory. Future self-controlled studies in patients before and after chemotherapy are needed to prove that high expression of δ-catenin is associated with cell malignancy-related behaviors in breast cancer patients so as to use δ-catenin protein as a potential biomarker for breast cancer patients. This could have important clinical significance for the early prevention of cognitive impairment in patients.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Kesler, S. et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin. Breast Cancer. 13(4), 299–306. https://doi.org/10.1016/j.clbc.2013.02.004 (2013).
Vega, J. N., Dumas, J. & Newhouse, P. A. Self-reported chemotherapy-related cognitive impairment compared with cognitive complaints following menopause. Psychooncology 27(9), 2198–2205. https://doi.org/10.1002/pon.4796 (2018).
Alexandra, C. et al. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin. 20, 110–118. https://doi.org/10.1016/j.nicl.2018.07.010 (2018).
Cimprich, B., & Welsh R.C. et al. Prechemotherapy alterations in brain function in women with breast cancer. J. Clin. Exp. Neuropsychol. 32(3), 324–331. https://doi.org/10.1080/13803390903032537 (2010).
Lee, M. et al. Development of a multiplex bead-based method for the microquantitation of δ-catenin. J. Nanosci. Nanotechnol. 20(9), 5819–5822. https://doi.org/10.1166/jnn.2020.17673 (2020).
Lu, Q., Lanford, G. W., Hong, H. & Chen, Y. H. δ-Catenin as a potential cancer biomarker. Pathol. Int. 64(5), 243–246. https://doi.org/10.1111/pin.12156 (2014).
Shen, Y. et al. δ-Catenin participates in EGF/AKT/p21Waf signaling and induces prostate cancer cell proliferation and invasion. Int. J. Mol. Sci. 22(10), 5306. https://doi.org/10.3390/ijms22105306 (2021).
Zhang, J. Y. et al. δ-catenin promotes malignant phenotype of non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in cytoplasm. J. Pathol. 222(1):76–88. https://doi.org/10.1002/path.2742 (2010).
Pieters, T. et al. P120 catenin-mediated stabilization of e-cadherin is essential for primitive endoderm specification.PLoS Genet, 12(8):e1006243.https://doi.org/10.1371/journal.pgen.1006243 (2016).
Ligon, C. et al. δ-Catenin engages the autophagy pathway to sculpt the developing dendritic arbor. J. Biol. Chem. 295(32), 10988–11001. https://doi.org/10.1074/jbc.RA120.013058 (2020).
Shrestha, N. et al. δ-Catenin increases the stability of EGFR by decreasing c-Cbl interaction and enhances EGFR/Erk1/2 signaling in prostate cancer. Mol. Cells. 41(4), 320–330. https://doi.org/10.14348/molcells.2018.2292 (2018).
Zhang, H. et al. Overexpression of δ-catenin is associated with a malignant phenotype and poor prognosis in colorectal cancer. Mol. Med. Rep. 12(3), 4259–4265. https://doi.org/10.3892/mmr.2015.3918 (2015).
Zhang, D. & Zhang, J. Y. En-Hua Wang.δ-catenin promotes the malignant phenotype in breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 36 (2), 569–575. https://doi.org/10.1007/s13277-014-2680-8 (2015).
Huang, C. et al. AKR1B10 promotes breast cancer metastasis through integrin α5/δ-catenin mediated FAK/Src/Rac1 signaling pathway. Oncotarget 7(28), 43779–43791. https://doi.org/10.18632/oncotarget.9672 (2016).
Hallquist, M. N. & Hillary, F. G. Graph theory approaches to functional network organization in brain disorders: a critique for a brave new small-world. Netw. Neurosci. 3(1), 1–26. https://doi.org/10.1162/netn_a_00054 (2019).
Humphries, M. D., Gurney, K. & Prescott, T. J. .The brainstem reticular formation is a small world, not scale-flee, network. J. Proc. R Soc. Lond. B Biol. Sci. 273(1585), 503–511. https://doi.org/10.1098/rspb.2005.3354 (2006).
He, Y., Chen, Z. J. & Evans, A. C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex. 17(10), 2407–2419. https://doi.org/10.1093/cercor/bhl149 (2007).
Liao, X., Vasilakos, A.V., & He, Y. small-world human brain networks: perspectives and challenges. Neurosci. Biobehav. Rev. 77, 286–300. https://doi.org/10.1016/j.neubiorev.2017.03.018 (2017).
Ibrahim, G. M. et al. Mapping the network of neuropsychological impairment in children with autism spectrum disorder: a graph theoretical analysis. J. Autism Dev. Disord. 46(12), 3770–3777. https://doi.org/10.1007/s10803-016-2929-8 (2016).
Sanz-Arigita, E. J. et al. Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 5(11), e13788. https://doi.org/10.1371/journal.pone.0013788 (2010).
Tomasi, D. & Volkow, N. D. Mapping small-world properties through development in the human brain: disruption in schizophrenia. PLoS One. 9(4), e96176. https://doi.org/10.1371/journal.pone.0096176 (2014).
Li, X. et al. Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep. 8(1), 13801. https://doi.org/10.1038/s41598-018-32257-w (2018).
Michiel, B. et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum. Brain Mapp. 33(12), 2971–2983. https://doi.org/10.1002/hbm.21422 (2012).
Shen, C. Y. et al. Association of functional dorsal attention network alterations with breast cancer and chemotherapy. Sci. Rep. 9, 104. https://doi.org/10.1038/s41598-018-36380-6 (2019).
Cheung, Y. T., Lim, S. R., Shwe, M., Tan, Y. P. & Chan, A. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Health. 16(6), 1001–1013. https://doi.org/10.1016/j.jval.2013.06.017 (2013).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12(3), 189–198. https://doi.org/10.1016/0022-3956 (1975).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Craft, S. et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol. Aging. 17(1), 123–130. https://doi.org/10.1016/0197-4580(95)02002-0 (1996).
Daderwal, M. C., Sreeraj, V. S., Suhas, S., Rao, N. P. & Venkatasubramanian, G. Montreal Cognitive Assessment(MoCA) and digit symbol substitution test(DSST) as a screening tool for evaluation of cognitive deficits in schizophrenia. Psychiatry Res. 316, 114731. https://doi.org/10.1016/j.psychres.2022.114731 (2022).
Toshinobu, T., Nakashima, Y. & Tsuij, Y. Discrepancies in Wechsler Adult Intelligent Scale III profile in adult with and without attention deficit hyperactivity disorder. Neuropsychopharmacol. Rep. 40(2), 166–174. https://doi.org/10.1002/npr2.12106 (2020).
Paffenholz, R. & Franke, W. W. Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation 61(5), 293–304. https://doi.org/10.1046/j.1432-0436.1997 (1997). 6150293.x.
Ryu, T. et al. Improved memory and reduced anxiety in δ-catenin transgenic mice. Exp. Neurol. 318, 22–31. https://doi.org/10.1016/j.expneurol.2019.04.006 (2019).
Adegbola, A. et al. Disruption of CTNND2, encoding delta-catenin causes a penetrant attention deficit disorder and myopia. HGG Adv. 1(1), 1000007. https://doi.org/10.1016/j.xhgg.2020.100007 (2020).
Lu, Q., Aguilar, B. J., Li, M., Jiang, Y. & Chen, Y. H. Genetic alterations of δ-catenin/NPRAP/Neurojungin (CTNND2): functional implications in complex human diseases. Hum. Genet. 135(10), 1107–1116. https://doi.org/10.1007/s00439-016-1705-3 (2016).
DeBusk, L. M., Boelte, K., Min, Y. & Lin, P. C. Heterozygous deficiency of delta-catenin impairs pathological angiogenesis. J. Exp. Med. 207(1), 77–84. https://doi.org/10.1084/jem.20091097 (2010).
Nopparat, J. et al. δ-catenin, a Wnt/β-catenin modulator, reveals inducible mutagenesis promoting cancer cell survival adaptation and metabolic reprogramming. Oncogene 34(12), 1542–1552. https://doi.org/10.1038/onc.2014.89 (2015).
Zhou, R. et al. p300/CBP-associated factor promotes autophagic degradation ofδ-catenin through acetylation and decreases prostate cancer tumorigenicity. Sci. Rep. 9(1), 3351. https://doi.org/10.1038/s41598-019-40238-w (2019).
Wahler, J. & Suh, N. Targeting HER2 positive breast cancer with chemopreventive agents. Curr. Pharmacol. Rep. 1(5), 324–335. https://doi.org/10.1007/s40495-015-0040-z (2015).
Bose, R. et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc. Natl. Acad. Sci. USA. 103(26), 9773–9778. https://doi.org/10.1073/pnas.0603948103 (2006).
Kim, D. et al. Distinct roles of parvalbumin-and somatostatin-expressing interneurons in Working Memory. Neuron 92(4), 902–915. https://doi.org/10.1016/j.neuron.2016.09.023 (2016).
Karlsgodt, K. H. et al. Functional activation during a cognitive control task in healthy youth specific to externalizing or internalizing behaviors. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 3(2), 133–140. https://doi.org/10.1016/j.bpsc.2017.09.003 (2018).
Robinson, O. J., Cools, R., Carlisi, C. O., Sahakian, B. J. & Drevets, W. C. Ventral striatum response during reward and punishment reversal learning unmedicated major depressive disord. Am. J. Psychiatry. 169(7), 152–159. https://doi.org/10.1176/appi.ajp.2011.11010137 (2012).
Li, H. et al. The relationship between the striatal dopaminergic neuronal and cognitive function with aging. Front. Aging Neurosci. 12, 41. https://doi.org/10.3389/fnagi.2020.00041 (2020).
Sherman, S. M. Functioning of circuits connecting thalamus and cortex. Compr. Physiol. 7(2), 713–739. https://doi.org/10.1002/cphy.c160032 (2017).
Zheng, Z. S., Reggente, N., Lutkenhoff, E., Owen, A. M. & Monti, M. M. Disentangling disorders of consciousness: insights from diffusion tensor imaging and machine learning. Hum. Brain Mapp. 38(1), 431–443. https://doi.org/10.1002/hbm.23370 (2017).
Li, J. et al. Mapping the subcortical connectivity of the human default mode network. NeuroImage 245, 118758. https://doi.org/10.1016/j.neuroimage.2021.118758 (2021).
Zovetti, N. et al. Default mode network activity in bipolar disorder. Epidemiol. Psychiatr Sci. 29, e166. https://doi.org/10.1017/S2045796020000803 (2020).
Zou, Q., Wu, C. W., Stein, E. A., Zang, Y. & Yang, Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 48(3), 515–524. https://doi.org/10.1016/j.neuroimage.2009.07.006 (2009).
Chen, X. et al. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: evidence from an arterial spin labeling perfusion study. Sci. Rep. 7, 42684. https://doi.org/10.1038/srep42684 (2017).
Nudelman, K. N. et al. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLoS One. 9(5), e96713. https://doi.org/10.1371/journal.pone.0096713 (2014).
Li, Z., Zhu, Y., Childress, A. R., Detre, J. A. & Wang, Z. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PLoS One. 7(9), e44556. https://doi.org/10.1371/journal.pone.0044556 (2012).
Aust, S. et al. How emotional abilities modulate the influence of early life stress on hippocampal functioning. Soc. Cogn. Affect. Neurosci. 9(7), 1038–1045. https://doi.org/10.1093/scan/nst078 (2014).
Deng, Y., Ma, X. & Tang, Q. Brain response during visual emotional processing: an fMRI study of alexithymia. Psychiatry Res. 213(3), 225–229. https://doi.org/10.1016/j.pscychresns.2013.03.007 (2013).
Reuter-Lorenz, P. A. & Park, D. C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. J. Neuropsychol. Rev. 24(3), 355–370. https://doi.org/10.1007/s11065-014-9270-9 (2014).
Xue, S. W., Yu, Q., Guo, Y., Song, D. & Wang, Z. Resting-state brain entropy in schizophrenia. Compr. Psychiatry. 89, 16–21. https://doi.org/10.1016/j.comppsych.2018.11.015 (2019).
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E. & Buckner, R. L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100(6), 3328–3342. https://doi.org/10.1152/JN.90355.2008 (2008).
Humphreys, G. F. & Lambon Ralph, M. A. Fusion and fission of cognitive functions in the human parietal cortex. Cereb. Cortex. 25(10), 3547–3560. https://doi.org/10.1093/cercor/bhu198 (2015).
Zhou, L. Y., Wright, T. E. & Clarkson A. N. Prefrontal cortex stroke induces delayed impairment in spatial memory. Behav. Brain Res. 296, 373–378. https://doi.org/10.1016/j.bbr.2015.08.022 (2016).
Wang, X. L. et al. Neural correlates during working memory processing in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 56, 101–108. https://doi.org/10.1016/j.pnpbp.2014.08.011 (2015).
Telias, M., & Segal, M. Editorial: Pathological hyperactivity and hyperexcitability in the central nervous system. Front. Mol. Neurosci. 12(15):955542. https://doi.org/10.3389/fnmol.2022.955542 (2022).
Acknowledgements
This research was Funded by: (1) Scientific Research Project of Higher Education Institutions of Inner Mongolia Autonomous Region (No: NJZY22190). (2) Health Science and Technology Project of Inner Mongolia Health Commission (No: 202202336).
Author information
Authors and Affiliations
Contributions
This work was performed by all authors. M.T.X. and Y.W.M. conceived and designed this study. M.T.X. performed the experiments, obtained the data and wrote the manuscript. J.J.G. is responsible for collecting breast cancer patients. Neurologist X.J.X. is responsible for scoring the cognitive scale of breast cancer patients. W.D. processed and analyzed the data, J.J.C. counted some data. Pathological immunohistochemistry of patients analyzed by D.Y. and J.Y.Z., Y.H.J. and D.S. helped discuss the results. L.Z.X. helped polish the English language of the thesis. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xue, M., Du, W., Cao, J. et al. Relationship between δ-catenin expression and whole-brain small-world network in breast cancer patients before chemotherapy. Sci Rep 14, 31119 (2024). https://doi.org/10.1038/s41598-024-82391-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82391-x