Abstract
For patients with necrotizing cervical tuberculous lymphadenitis (CTL) who have formed abscesses and are unwilling to undergo surgery, early and accurate assessment of drug therapy should be performed to guide subsequent clinical adjustments. This study investigated 22 patients with necrotizing CTL who underwent chemotherapy at our hospital from February 2020 to December 2022. They were diagnosed based on the positive results of pathogen detection methods (acid-fast bacillus smear, mycobacteria culture, Gene X-pert, and next-generation sequencing). Based on the 6-month treatment outcomes, the relationship among prechemotherapy ultrasound features, pus Mycobacterium tuberculosis (MTB) load, and treatment efficacy was assessed. In this study, the maximum lymph node (LN) area, maximum necrotic area, and pus MTB load in patients with necrotizing CTL were associated with poor prognosis and showed significant differences between the effective and ineffective groups (P < 0.05). However, no statistical difference was observed in the maximum longitudinal diameter, short diameter, and necrosis rate between the two groups (P > 0.05). The maximum necrotic area of the LNs was not associated with the pus MTB load. Furthermore, maximum LN area, maximum necrotic area, and pus bacterial load may be potential radiological markers for predicting the therapeutic response of CTL.
Similar content being viewed by others
Introduction
Tuberculosis, an infectious disease caused by Mycobacterium tuberculosis (MTB), remains a serious public health concern worldwide1. As the most common type of extrapulmonary tuberculosis, CTL has a high incidence rate, prolonged disease duration, and atypical early clinical symptoms, making it prone to misdiagnosis and underdiagnosis2,3. In some patients, lymph nodes (LNs) undergo necrosis or even form a tuberculous abscess by the time they seek medical attention. At present, CTL is primarily treated with antituberculosis drugs, and if an abscess or sinus tract develops, interventional therapy or surgical resection may be indicated4,5,6,7. For patients with abscesses who are unwilling to undergo surgery, an early and accurate assessment of the prognosis of drug therapy is important for guiding the clinical adjustment of subsequent treatment plans.
Ultrasound has been the preferred method for diagnosing and monitoring the outcomes of antituberculosis treatment in patients with CTL6,8. Contrast-enhanced ultrasound (CEUS) has been proven to effectively improve the visualization of LN necrosis6. The extent of necrosis corresponds to MTB virulence, and the degree of extrapulmonary tuberculosis necrosis is a better predictor of MTB load than the quantity of bacteria in the lung tissue9. Moreover, noninvasive radiographic features can be assessed to determine the relationship between treatment outcomes and bacterial load in patients with pulmonary tuberculosis10. The necrotic area ratio of tuberculous lymphadenitis, as evaluated through computed tomography (CT) imaging, is correlated with the treatment response11. This suggests that ultrasound technology can be used to assess the prognosis of CTL treatment and monitor the MTB load. However, because data on the relationship among the ultrasound features of necrotizing CTL, pus MTB load, and treatment outcomes are limited, this study aimed to investigate their correlation.
Methods
Patient selection
Signed informed consent for CEUS inspection and LN puncture to obtain pus specimens was acquired from all patients or their guardians. The study protocol was approved by the Human Research Ethics Committee of Hangzhou Red Cross Hospital (2022-YS-151), and the study was conducted in accordance with the Declaration of Helsinki. This study included patients with necrotizing CTL who had undergone chemotherapy from February 2020 to December 2022. The images of each patient were obtained from the PACS database in DICOM and AVI formats, and patient information was acquired from the hospital’s case management system.
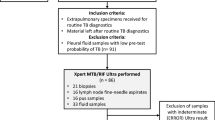
The inclusion criteria were as follows: (1) patients who had undergone ultrasound-guided puncture biopsy, with positive results in at least one pathogen detection method, including acid-fast bacillus (AFB) smear, mycobacteria culture, Gene X-pert, or next-generation sequencing (NGS); (2) those who were ≥ 18 years old, with no history of severe allergies and major diseases; (3) those who had undergone conventional ultrasound and CEUS prior to treatment, which indicated the presence of nonenhancing necrotic areas, and ultrasound-guided puncture for pus collection; (4) those whose follow-up results were available after 6 months of chemotherapy; and (5) drug susceptibility testing (DST), GeneXpert, and NGS tests revealed no resistance to rifampicin, isoniazid, pyrazinamide, and ethambutol.
The exclusion criteria were as follows: (1) recurrent CTL; (2) poor coagulation; (3) interruption of antituberculosis medication during the 6-month period or patients who opted for surgical treatment; (4) HIV-positive status; (5) development of drug resistance during the course of treatment; and (6) presence of sinus tracts at the time of the initial treatment.
All patients were administered with the standard 6-month treatment regimen for drug-susceptible tuberculosis containing four first-line drugs in the 2-month initiation phase in accordance with the WHO recommendations: 0.3 g of isoniazid once daily, 0.45 g of rifampicin once daily, 0.5 g of pyrazinamide three times/day, and 0.75 g of ethambutol once daily, for weight of ≥ 50 kg; the dosages of rifampicin and ethambutol were adjusted to 0.6 g once daily and 1.0 g once daily, respectively8,12,13.
DST, Gene X-pert, and NGS were used to assess the sensitivity of MTB to antituberculosis drugs. For patients with negative mycobacterial cultures, traditional DST was not feasible owing to insufficient bacterial growth. To address this limitation, the resistance profile of MTB was determined using molecular techniques, such as Gene X-pert and NGS. The NGS results revealed no resistance genes, including those related to rifampicin, isoniazid, pyrazinamide, and ethambutol.
The treatment outcomes after 6 months were as follows:
-
1.
Cure: complete remission of all symptoms, disappearance of LNs, or reduction of the largest LN by 1 cm.
-
2.
Improvement or stability: symptomatic relief, reduction in the size and number of LNs, and maximum residual size of ± 1 cm from baseline.
-
3.
Deterioration: appearance and persistence of new symptoms, new LNs, and/or increase in the size of the maximum LN by 2 cm from baseline8,14.
The first and second outcomes were defined as the effective group, whereas the third outcomes and the cases transferred to surgery were defined as the ineffective group.
Ultrasound image acquisition
The Philips iU-Elite ultrasound system (Washington, USA), with an L12-5 linear array transducer (frequency range of 5–12 MHz) and L9-3 linear array transducer (frequency range of 3–9 MHz), and Mindray Resona 7S (Shenzhen, China), with an L14-5 linear array transducer (frequency range of 5–14 MHz) and L9-3U linear array transducer (frequency range of 3–9 MHz), were used for the whole procedure.
Conventional ultrasound: the suspected LNs were selected as the object of observation, while the maximum longitudinal diameter (L) and maximum short diameter (S) of the LNs were recorded in the largest longitudinal section. Moreover, the maximum area was outlined along the outer edge of the LNs using the continuous measurement method.
CEUS: the contrast agent used was SonoVue (Milan, Italy, Bracco SpA), which was diluted with 5 mL of normal saline and shaken well before use, with a low mechanical index (0.06) pulse reverse harmonic imaging system during the examination. The dynamic images were stored on the instrument’s hard disk. The largest image of the LN without the enhancement area at the peak of enhancement was selected, while the maximum necrotic area was outlined. The absence of enhancement was defined as necrosis; if multiple necrotic areas were observed, their areas were summed, and the necrosis rate was calculated using the following formula: (maximum necrotic area at the peak of CEUS) / (maximum area of LN on conventional ultrasound) × 100%.
The maximum LN area and necrotic area were outlined independently by two ultrasonographers with more than 10 years of experience in superficial LN diagnosis. Moreover, if any disagreement arose during the outlining process, consensus was achieved through discussion. To further establish agreement, both ultrasonographers, along with two other specialists in tuberculosis, conducted a pilot read comprising 20 ultrasound images. This ensured that consensus on the criteria for LN boundaries and necrosis was achieved before the independent outlining of the lesion. An intraclass correlation coefficient (ICC) was calculated to test the intraobserver agreement of the outlined data.
Specimen collection
Prior to treatment, the sampling target was determined to be a nonenhanced area based on the CEUS model. Following routine disinfection, toweling, and subcutaneous local anesthesia, ultrasound-guided collection of 1 mL of pus from the lesion was performed to assess the MTB load.
Determination of the sample MTB load
The standard curve was established using the real-time fluorescence quantitative polymerase chain reaction (PCR) method, along with MTB bacterial fluids of different log-level colony-forming units (CFU) per mL. To prepare the samples, 1 mL of pus or standard bacterial solution was mixed with 1 mL of 4% NaOH, shaken well, and allowed to sit at room temperature for 15 min until liquefied. Subsequently, 1 mL of the liquefied solution was transferred to a 1.5 mL sterile EP tube containing glass microspheres and centrifuged at 12,000 rpm for 5 min, and the supernatant was discarded. Next, 1 mL of wash solution was added, and the mixture was vortexed thoroughly. Finally, 50 μL of the nucleic acid extraction solution was added, shaken for 5 min, placed in a metal bath at a temperature of 95 °C for 5 min, and centrifuged at 5,000 rpm for 1 min before setting aside.
The extracted standard bacterial DNA and sample DNA (2 μL) were added to the amplification reaction system, resulting in a final volume of 25 μL. Using the Roche 480 fluorescence PCR instrument, the amplification program was set up according to the kit instructions, and the FAM channel was selected for fluorescence collection. A negative control was also included. The standard curve was established based on the Ct values of the standard bacterial fluid, and the RR value was tested. Using the standard curve, the MTB load (CFU/mL) in the sample was calculated.
Statistical analysis
SPSS 25.0 statistical software (IBM) was used to analyze the data. The count data were described as “number of cases” and “composition ratio (%),” with the differences between the groups compared using the χ2 test. The measurement data with normal distribution were expressed as mean ± standard deviation, while the differences between the groups were compared using t-test. The non-normally distributed data were described as “median (interquartile range) [M(IQR)],” and the group comparisons were performed using Mann–Whitney U test. Pearson correlation coefficient and ICC were calculated. Furthermore, statistical difference was set at a P value of < 0.05.
Results
Clinical and ultrasound characteristics
Of the 22 patients with CTL, 14 (63.64%; 2 males and 12 females) were classified in the effective group; they had an age range of 18–61 years (35.64 ± 14.15 years) and a body mass index (BMI) of 20.76 ± 2.49 cm2. 8 patients (29.09%; 5 males and3 females) were classified in the ineffective group; they had an age range of 24–49 years (32.75 ± 7.44 years) and BMI of 20.88 ± 1.36 cm2. No statistically significant differences in age, sex, and BMI were observed between the two groups (P > 0.05). The maximum area of LNs and the maximum necrotic area were significantly different between the effective and ineffective groups (P < 0.05). However, no statistically significant differences in the L, S, and necrosis rates were observed between the two groups (P > 0.05). These results are presented in Table 1.
In a reliability test comparing the maximum area and necrotic area of LNs assessed by two ultrasonographers, the ICC revealed a “strong” correlation of 0.935 (95% CI: 0.786–0.975) for the maximum area and 0.879 (95% CI: 0.678–0.952) for the necrotic area.
Microbiological characteristics
Table 2 summarizes the culture status, AFB smear, Gene X-pert, DST, and NGS results for each patient.
The AFB smear results were positive in 4 out of 22 patients (18.18%), along with negative results in the remaining patients. The Gene X-pert results were positive for MTB in 20 of 22 patients (90.91%), all of which were sensitive to rifampicin. Furthermore, the remaining two patients had negative results.
Of the 22 patients, only 3 (13.64%) were culture-positive, while 19 (86.36%) were culture-negative. For the culture-positive patients, the DST confirmed susceptibility to rifampicin, isoniazid, pyrazinamide, and ethambutol. For the culture-negative cases, DST was unavailable, and sensitivity was assessed using molecular methods, such as Gene X-pert and NGS, to assess the resistance markers.
NGS was conducted on all patients, detecting the MTB complex in each case. No resistance genes associated with rifampicin, isoniazid, pyrazinamide, or ethambutol were identified, suggesting a cohort predominantly composed of patients with drug-susceptible tuberculosis.
Pus MTB load
A statistically significant difference in the pus MTB loads was observed between the effective and ineffective groups (202 [33.5–3812.75] CFU/mL vs. 1872 [1270–3906.75] CFU/mL) (P < 0.05). Table 2 shows the specific MTB load data for the 22 patients, while Fig. 1 shows the MTB load standard curve.
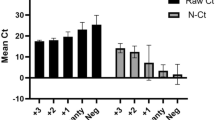
Bacterial load standard curve. The initial concentration of the bacterial solution was 2.1 × 105 CFU/L. A tenfold serial dilution was performed to obtain a range of bacterial concentrations. DNA was extracted from each diluted sample, and 2μL of the extracted DNA was used for real-time fluorescence PCR analysis. The experiment was repeated twice to calculate the average Ct value. By plotting the log10 (CFU) values on the x-axis and the average Ct values on the y-axis, a linear relationship was observed (y = − 3.5299x + 36.326, R2 = 0.9817). This indicates a strong correlation between the Ct value and the logarithmic value of the bacterial load.
Correlation analysis between the maximum necrotic area of LNs and the pus MTB load
The maximum necrotic area of LNs in the 22 patients showed no correlation with the pus bacterial load (r = 0.218, P = 0.329). Furthermore, no correlation was observed between the necrotic area of LNs and the pus MTB load in both the effective and ineffective groups, indicating correlations of r = 0.517 (P = 0.190) and r = − 0.087 (P = 0.767), respectively (Fig. 2). Figure 3 shows the ultrasound images and pus MTB load of a patient.
A 30-year-old man with left cervical tuberculous lymphadenitis. (a) Conventional ultrasound revealed enlarged lymph node (arrow) with hypoechoic to anechoic internal echoes, accompanied by disrupted lymphatic gates. The maximum longitudinal diameter of lymph nodes was 2.8 cm. The maximum short diameter of lymph nodes was 1.1 cm. The maximum area of lymph nodes was 2.131 cm2. (b) Contrast-enhanced ultrasound revealed nonenhancement, which indicated the area of lymph node necrosis (arrow). The maximum necrotic area was 1.197 cm2. (c) Ultrasound-guided sampling identified targets as nonenhanced areas. The removal of approximately 1 mL of pus sample. (d) The Ct value for pus MTB burden was 16.77.
Discussion
Extrapulmonary tuberculosis is a significant component of tuberculosis, accounting for 11%–20% of all cases15. LNs are the most commonly involved sites, accounting for 35% of all extrapulmonary tuberculosis cases, and are commonly present in anatomical regions, such as the cervical, mediastinal, axillary, and inguinal regions2,16. Typically presents with localized LN enlargement as the main clinical manifestation and may also involve LN calcification, fusion, liquefaction, necrosis, and sinus tract formation. At present, CTL is primarily treated with systemic chemotherapy; however, the existing drug regimens for CTL are considered lengthy and cumbersome. Early assessment of response to CTL treatment can facilitate certain adjustments to the regimen, ensuring its efficacy.
This study included patients who were sensitive to antituberculosis drugs prior to the initial treatment. Drug susceptibility was assessed using DST, Genne X-pert, and NGS. However, in this study, the traditional culture only confirmed positivity in 13.64% of the patients. For the culture-negative patients, conventional DST could not be conducted, which may increase the risk of undetected primary drug resistance, particularly resistance to isoniazid. The combined use of Gene X-pert and NGS allows a comprehensive assessment of tuberculosis infection and drug resistance. The use of NGS provides additional assurance by confirming the absence of known resistance genes for rifampicin, isoniazid, pyrazinamide, and ethambutol. However, several limitations may exist in detecting low-level resistance or mutations not covered by the test, and it cannot fully rule out the presence of all potential resistance mechanisms, especially in cases with low MTB loads.
Previous studies have assessed various imaging modalities, including magnetic resonance imaging (MRI), positron emission tomography–CT (PET–CT), and ultrasound, and their effectiveness in improving the diagnostic accuracy and treatment monitoring of CTL, each having unique advantages and limitations. Yu et al.17 have shown that MRI has several advantages, such as high soft tissue contrast, multiplanar imaging, and the absence of radiation exposure. The combined use of CEUS and MRI improved the diagnostic accuracy of CTL. However, the high costs associated with MRI equipment and procedures, along with the prolonged examination times, compromised its application, particularly among patients with metal implants or pacemakers. Singh et al.18 reported that the use of PET–CT allowed a quantitative assessment of the CTL treatment response through percentage changes in the SUV-max; however, its relatively high costs and radiation exposure resulted in additional health risks to patients. Ultrasound is a convenient and cost-effective procedure that remains an important part of the clinical monitoring of CTL treatment prognosis, particularly in developing countries8. In the present study, two ultrasonographers independently outlined the maximum LN area and the necrotic area, with the results indicating a strong correlation in the reliability test, which helps to reduce observer variability.
Tuberculous lymphadenitis can have different types of lesions at different stages of the disease. The necrosis-predominant type may progress to caseous necrosis and subsequently to dissolution and liquefaction, resulting in abscess formation. The liquefied material can serve as a culture medium for MTB, allowing it to proliferate6,19. The primary ultrasonographic manifestation of necrotizing CTL is enlarged LNs with hypoechoic to anechoic internal echoes, which are often accompanied by disrupted lymphatic gates and peripheral vascular patterns in LNs6,15. CEUS could more accurately delineate the area of LN necrosis, significantly enhancing the accuracy of the puncture procedures, especially for small necrotic foci20. Zhao et al. have reported specific CEUS features for CTL, especially a distinctive “glasses-shaped appearance” characterized by a large central nonenhancement area indicative of caseous necrosis, which is surrounded by a peripheral ring-shaped enhancement. This pattern results from vascular destruction and caseation necrosis typical of tuberculosis, leading to a reduced blood perfusion in the affected LNs20. Moreover, these features may indicate more severe tissue destruction and higher infectivity.
Previous studies have reported that LN size is an influential factor in predicting the treatment response. Yu et al.8 assessed the value of multimodal ultrasound in evaluating the response of CTL to antituberculosis drug therapy and found no statistically significant difference in the maximum L between the effective and ineffective groups prior to treatment. However, Chahed et al.21 reported that an L of ≥ 3 cm was an independent risk factor for poor treatment outcomes. In our study, no significant difference in the maximum L of LNs was observed between the two groups. In addition, no difference in the results of the analysis of the maximum S of LNs was observed in this study. Joo YH et al. reported a sensitivity of 88.2% and a specificity of 74.3% for predicting nonresponse to antituberculosis chemotherapy based on LN volume11. In the present study, the maximum LN area was statistically significant in both groups and could be considered another factor in predicting the treatment response. LN area can be outlined directly on ultrasound, making it a simple, time-saving, and more convenient assessment method in primary hospitals.
Necrosis reflects high bacterial virulence, weak immunity, or a severe allergic state, and its increase is another indication of a poor response to drug therapy8. It was indicated that 32.7% of the CTL patients requiring additional surgical treatment were associated with high rates of necrosis11. However, the difference in the necrosis rate between the two groups in the present study was not statistically significant (P > 0.05), which might be attributed to the different sections selected for measurement. In the present study, the maximum necrotic area of the LNs was statistically different between the two groups, suggesting that a larger area of necrosis before treatment is associated with a greater likelihood of poor treatment prognosis. This finding could be used as one of the factors to predict prognosis and adjust the treatment regimen in CTL, which is consistent with the evidence that the degree of cavitation in the necrotic lesions of tuberculosis is associated with an increased risk of treatment failure10,22,23. LIN PL et al.24 quantified the MTB in the pus of active tuberculous lymphadenitis lesions, indicating that the levels could be as high as 107–109 CFU/mL. The follow-up treatment prognosis was not satisfactory, indicating that a high MTB load in the pus was associated with poor treatment outcomes. In 2018, the tuberculosis molecular MTB load assay was recognized by the WHO as an alternative to smear microscopy and culture for monitoring the tuberculosis treatment response25. Moreover, the advantage of this assay is that it may be more accurate and predictive of long-term adverse outcomes26. PCR analysis has the added benefit of quantifying mycobacterial DNA, allowing bacterial load assessment27. In the present study, a standard curve analysis was performed using fluorescence quantitative PCR with MTB bacterial fluids of different log levels of CFU per mL. The results revealed that although the range of pus bacterial loads in the two groups was wide, the median bacterial load in the ineffective group was nine times higher than that in the effective group. The difference between the two groups was statistically significant, revealing that a high MTB load in the pus of the ineffective group predicted an unfavorable prognosis.
The chest X-ray assessment of the extent of cavitation due to tissue necrosis in the tuberculosis lesions is correlated with the increased MTB load in the sputum28. Moreover, it can be potentially used alone to monitor the bacterial load in tuberculosis29. Further studies have found that the chest X-ray imaging features could be used to assess treatment outcomes in relation to bacterial load10. These results revealed that the MTBB load could be monitored to some extent with the help of noninvasive imaging features.
For patients with CTL, the three commonly available LN specimens are pus, tissue, and puncture samples, with the pus specimens being closer to the sputum, easier to handle, and having the highest diagnostic accuracy30. In the present study, the pus was collected via ultrasound-guided puncture. Although both the maximum necrotic area of LNs and the pus MTB load could reflect the prognosis of chemotherapy, no correlation between the maximum necrotic area of LNs and the pus MTB load was observed in all patients as well as in the effective and ineffective groups. The nonenhanced area of CEUS in CTL includes pus and caseous necrosis, which makes it generally larger than the pus alone8. Therefore, the correlation between the pus bacterial load and the easily available maximum necrotic area of LNs requires further evaluation with a larger sample size. Moreover, ultrasound imaging can preliminarily differentiate TB-associated lesions from other types of abscesses. For example, bacterial abscesses often show vascularity and hyperemia on ultrasound, whereas tuberculosis abscesses tend to be more “hypoechoic” and avascular. Multilocular low densities with peripheral enhancement and a large confluent low density with a lesser degree of fat plane obliteration than a pyogenic abscess are suggestive features of advanced CTL on CT31. Moreover, certain features such as localized temperature changes, vascularity, and hyperemia serve as valuable differential markers in clinical practice. However, ultrasound remains limited in its ability to distinguish between bacterial and aseptic fluid collections.
This study had several limitations. First, this was a single-center study and had a small sample size, limiting the generalizability of the results to other populations and clinical settings. Therefore, larger sample sizes and multicenter studies are needed to provide a more robust statistical power and increase the generalizability of the findings. Second, the study employed a 6-month follow-up period, which neglected long-term follow-up for all cases, potentially overlooking patients with slower progression. Consequently, there is a degree of error in the follow-up results. Third, the accuracy of the ultrasound assessments may be influenced by various factors. Although the study attempted to reduce interobserver variability by involving two ultrasonographers and obtaining consensus opinions, some degree of subjectivity in the interpretation of the ultrasound images can be expected. Fourth, this study primarily focused on ultrasound imaging and did not compare its diagnostic performance with other imaging techniques, such as CT and MRI. Fifth, although this study followed the 6-month regimen recommended by the WHO, potential limitations on the dosage of isoniazid for the Chinese population who predominantly exhibit fast acetylator status may still exist. This may require the consideration of higher dosing to achieve optimal therapeutic effects, which was not within the scope of this study. Future studies should consider evaluating the efficacy of higher isoniazid dosages in fast acetylator populations as well as personalized dosing strategies to further optimize the treatment outcomes for Chinese patients with tuberculosis. Sixth, this study did not investigate changes in the ultrasound indicators and MTB load in pus before and after treatment. We plan to include longitudinal comparisons before and after treatment in future studies to more comprehensively assess the changes in these ultrasound indicators and MTB load in pus in response to tuberculosis treatment.
In conclusion, the ultrasound assessment of the maximum area of LNs and necrotic area before treatment in patients with CTL as well as the detection of pus bacterial load can be used to evaluate the prognosis of chemotherapy. This provides a concept for the early and noninvasive clinical prognosis assessment. However, evidence to confirm the relationship between the maximum necrotic area of LNs and pus MTB load is limited.
Data availability
Datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
W, H. O. Global Tuberculosis Report 2023. (Geneva: World Health Organization, 2023).
Gautam, H., Agrawal, S. K., Verma, S. K. & Singh, U. B. Cervical tuberculous lymphadenitis: Clinical profile and diagnostic modalities. Int. J. Mycobacteriol. 7, 212–216. https://doi.org/10.4103/ijmy.ijmy_99_18 (2018).
Mandal, N., Anand, P. K., Gautam, S., Das, S. & Hussain, T. Diagnosis and treatment of paediatric tuberculosis: An insight review. Crit. Rev. Microbiol. 43, 466–480. https://doi.org/10.1080/1040841x.2016.1262813 (2017).
Lekhbal, A. et al. Treatment of cervical lymph node tuberculosis: When surgery should be performed? A retrospective cohort study. Ann. Med. Surg. (Lond.) 55, 159–163. https://doi.org/10.1016/j.amsu.2020.05.006 (2020).
Kim, B. H. et al. Conservative treatment for cutaneous fistula resulted from abscess formation in patients with tuberculous cervical lymphadenitis. Auris Nasus Larynx 45, 1061–1065. https://doi.org/10.1016/j.anl.2018.01.006 (2018).
Zhang, Y., Yu, T., Su, D., Tang, W. & Yang, G. Value of contrast-enhanced ultrasound in the ultrasound classification of cervical Tuberculous lymphadenitis. Front Med. (Lausanne) 9, 898688. https://doi.org/10.3389/fmed.2022.898688 (2022).
Smaoui, S. et al. Tuberculosis lymphadenitis in a southeastern region in Tunisia: Epidemiology, clinical features, diagnosis and treatment. Int. J. Mycobacteriol. 4, 196–201. https://doi.org/10.1016/j.ijmyco.2015.04.004 (2015).
Yu, T. et al. The value of multimodal ultrasonography in evaluating therapeutic response of cervical tuberculous lymphadenitis to anti-tuberculosis drugs. Front Med (Lausanne) 10, 1177045. https://doi.org/10.3389/fmed.2023.1177045 (2023).
Palanisamy, G. S. et al. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 88, 295–306. https://doi.org/10.1016/j.tube.2007.12.003 (2008).
Te Riele, J. B. et al. Relationship between chest radiographic characteristics, sputum bacterial load, and treatment outcomes in patients with extensively drug-resistant tuberculosis. Int. J. Infect. Dis. 79, 65–71. https://doi.org/10.1016/j.ijid.2018.10.026 (2019).
Joo, Y. H., Hwang, S. H., Seo, J. H. & Kang, J. M. Treatment assessment based on computerized lymph node volume and ratio of necrotic area in tuberculous cervical lymphadenitis. Auris Nasus Larynx 39, 402–406. https://doi.org/10.1016/j.anl.2011.06.007 (2012).
Prevention, C. C. F. D. C. A. Technical Specifications for the Prevention and Control of Tuberculosis in China (2020 Edition). 20–22 (2020).
WHO. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment 2022 (World Health Organization, 2022).
Jindal, S. K. et al. Tuberculous lymphadenopathy: A multicentre operational study of 6-month thrice weekly directly observed treatment. Int. J. Tuberc Lung Dis. 17, 234–239. https://doi.org/10.5588/ijtld.12.0333 (2013).
Möller, K. et al. Comments and illustrations of ultrasound findings in extrapulmonary tuberculosis manifestations. Diagnostics 14, 706. https://doi.org/10.3390/diagnostics14070706 (2024).
Kathamuthu, G. R. et al. Filarial coinfection is associated with higher bacterial burdens and altered plasma cytokine and chemokine responses in tuberculous lymphadenitis. Front Immunol. 11, 706. https://doi.org/10.3389/fimmu.2020.00706 (2020).
Yu, X., Zhang, W., He, N., Su, D. & Zhao, Y. Diagnostic value of contrast-enhanced ultrasound combined with MRI for cervical hyperplastic, tuberculosis-infected, and metastatic lymph nodes. Pak. J. Med. Sci. 39, 950–955. https://doi.org/10.12669/pjms.39.4.7572 (2023).
Singh, A. et al. Positron-emission-tomography in tubercular lymphadenopathy: A study on its role in evaluating post-treatment response. Drug Discov. Ther. 15, 35–38. https://doi.org/10.5582/ddt.2020.03042 (2021).
Brucoli, M., Borello, G., Boffano, P. & Benech, A. Tuberculous neck lymphadenopathy: A diagnostic challenge. J. Stomatol Oral Maxillofac. Surg. 120, 267–269. https://doi.org/10.1016/j.jormas.2018.11.012 (2019).
Zhao, D. et al. Role of contrast-enhanced ultrasound guidance in core-needle biopsy for diagnosis of cervical tuberculous lymphadenitis. Clin. Hemorheol. Microcirc. 77, 381–389. https://doi.org/10.3233/ch-201038 (2021).
Chahed, H. et al. Paradoxical reaction associated with cervical lymph node tuberculosis: Predictive factors and therapeutic management. Int. J. Infect. Dis. 54, 4–7. https://doi.org/10.1016/j.ijid.2016.10.025 (2017).
Hesseling, A. C. et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int. J. Tuberc Lung Dis. 14, 560–570 (2010).
Jeon, D. S. et al. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. Int. J. Tuberc Lung Dis. 13, 594–600 (2009).
Lin, P. L. et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77, 4631–4642. https://doi.org/10.1128/iai.00592-09 (2009).
Musisi, E. et al. Accuracy of the tuberculosis molecular bacterial load assay to diagnose and monitor response to anti-tuberculosis therapy: A longitudinal comparative study with standard-of-care smear microscopy, Xpert MTB/RIF Ultra, and culture in Uganda. Lancet Microbe 5, e345–e354. https://doi.org/10.1016/s2666-5247(23)00367-1 (2024).
Ntinginya, N. E. et al. Tuberculosis molecular bacterial load assay reveals early delayed bacterial killing in patients with relapse. Clin. Infect. Dis. 76, e990–e994. https://doi.org/10.1093/cid/ciac445 (2023).
Mondal, S. et al. Role of real-time PCR (RT-PCR) in rapid diagnosis of tuberculous mycobacteria in different clinical samples. J. Indian Med. Assoc. 112, 81–84 (2014).
Perrin, F. M. et al. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int. J. Tuberc Lung Dis. 14, 1596–1602 (2010).
Heyckendorf, J. et al. Tuberculosis treatment monitoring and outcome measures: New interest and new strategies. Clin. Microbiol. Rev. 35, e0022721. https://doi.org/10.1128/cmr.00227-21 (2022).
Yu, G. et al. Application of mycobacterium tuberculosis RNA for the rapid diagnosis of lymph node tuberculosis using different specimens. Infect. Drug Resist. 16, 179–187. https://doi.org/10.2147/idr.S392045 (2023).
Lee, Y., Park, K. S. & Chung, S. Y. Cervical tuberculous lymphadenitis: CT findings. J. Comput. Assist. Tomogr. 18, 370–375. https://doi.org/10.1097/00004728-199405000-00006 (1994).
Acknowledgements
We thank all authors of this study for their valuable input and full cooperation. We would like to express our gratitude to the patients and their families.
Funding
This work was supported by the Medical Science and Technology Project of Zhejiang Province [grant number 2023KY970, 2022KY986]; the pre-research fund project of Zhejiang University [grant number ZAYY 10].
Author information
Authors and Affiliations
Contributions
Ying Zing, Gaoyi Yang and Ting Lin were responsible for project administration, conceptualization, wrote the main manuscript text. Yuehui Yu, Xinyi Yan, Ting Lin, Peijun Chen and Yuanyuan Chen were responsible for the data creation and analysis. Hao Li, Yuanyuan Chen and Ying Zhang prepared Figures 1-3 and Tables 1-2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Lin, T., Li, H. et al. Relationship among ultrasound features, pus Mycobacterium tuberculosis load, and efficacy of antituberculosis drugs in patients with necrotizing tuberculous lymphadenitis. Sci Rep 14, 30413 (2024). https://doi.org/10.1038/s41598-024-82444-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82444-1