Abstract
Magnetoliposomes containing magnetite, soy lecithin, stigmasterol, and beta-sitosterol of the mean size minor than 160 nm were obtained by a scalable and green process using autoclave and sonication without organic solvents. The formation, size of the liposome, linkage, and encapsulation of the magnetite were evaluated by Cryo-TEM. The stability of magnetoliposomes after storage for 6 months at 4 °C was improved by liposome size, the ability of soy lecithin to preserve the magnetite phase against oxidation, pH, polydispersity index, and zeta potential. The iron oxide phase stability was assessed using no conventional X-ray diffraction (high-resolution transmission electron microscopy), energy loss electron spectroscopy, and selected area electron diffraction) in time zero (fresh sample) and 6 months. The high zeta potential measured for magnetoliposomes, │53│ mV, indicated a low tendency to agglomerate. Lip-Fe3O4@lecithin with concentrations of 0.58 mg mL−1 of liposome showed high cell viability and are potential candidates for drug delivery and hyperthermia treatments in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays.
Similar content being viewed by others
Introduction
Magnetic nanoparticles (MNPs) as magnetite (Fe3O4) and maghemite (Ⓒ-Fe2O3) have been used in clinical diagnosis and medical therapies because of their superparamagnetic behavior, high saturation magnetization, high magnetic susceptibility, low toxicity, and heating under the action of a magnetic field which is used for hyperthermia treatment of cancer1,2. These MNPs are especially interesting due to the possibility of long circulation time in the human body and scalable production3. Prolonged circulation time optimizes the biodistribution and pharmacokinetics of drugs in target tissues, such as those compromised by cancer4.
Although such characteristics make magnetite a strong candidate for creating new therapies against cancer and other diseases5, some inherent features of MNPs, such as their tendency to agglomerate and oxidation in the air6, can be challenging. The predisposition to agglomeration has been superated by coating of MNPs with surfactants7, polymeric materials8, silica1,2, and liposomes3. Liposomes are hollow containers limited by a lipid bilayer9. The liposome structure enables the encapsulation of hydrophilic or hydrophobic substances10 in their hydrophilic aqueous compartment or lipophilic limiting membrane, respectively10. Liposomes are especially interesting due to their biocompatibility11,12, adjustability in composition and size13, easy modification of their surface properties14,15,16, and their physicochemical properties can make it easier to reach diseased tissue. MNPs encapsulated in liposomes are denominated magnetoliposomes3.
The magnetoliposomes system allows the benefits of the liposomes and the MNPs in a unique drug delivery that can be used in the synergic action between hyperthermia and chemotherapy treatment of diverse diseases. The liposome protects the iron phase and reduces the required medicine for the treatment due to its effectiveness in reaching the target. The stability of liposomes and magnetoliposomes depends on their physicochemical parameters17,18,19, and the physicochemical parameters depend on the composition and methodology of preparation. Frequently, the stabilizing of the liposomes is realized by compositional change of liposomal bilayers by incorporating sterols. Cholesterol is traditionally used to increase the packing density of phospholipids in bilayers by introducing the liquid-ordered phase that conserves the fluid state of the membranes. However, cholesterol is associated closely with cardiovascular diseases. Another option, phytosterols, are known as “heart-friendly” and reduce the absorption of ingested cholesterol and an anticancer20.
Various conventional techniques have produced liposomal formulations, including Bangham, detergent depletion, ether/ethanol injection, reverse phase evaporation, and emulsion methods21. Conventional methods generally involve producing a lipid solution using an organic solvent before dispersion in the aqueous phase. However, there are some drawbacks in these traditional techniques linked to scale-up issues and to the frequent use of toxic organic solvents such as ethers (isopropyl ether and diethyl ether), methyl alcohol, or chlorinated compounds such as chloroform22 and non-scalable methods. In a previous study3, we described for the first time in the literature the preparation by a new, green, and scalable methodology with excellent capacity of encapsulation, without using an organic solvent, and a detailed physicochemical characterization and magnetic parameters of magnetoliposomes, based on soy lecithin and their antibacterial activity.
In the current work, the objectives are to evaluate magnetoliposomes of soy lecithin for (1) the confirmation by an image of the formation of liposomes, size, and encapsulating of the magnetite phase by Cryo-TEM, (2) to study the stability of the magnetite phase of magnetoliposomes (Lip- Fe3O4@lecithin) samples in time of 6 months using “alternative technique” selected area electron diffraction (SAED), high-resolution transmission electron microscopy (HRTEM), and electron energy loss spectroscopy (EELS); (3) to study the influence on the stability of liposome formulations by parameter zeta potential, pH, particle size, and polydispersity index (PI) performed on freshly prepared samples due the addition of stigmasterol, β-sitosterol; (4) characterized the composition of lecithin and Lip by 1H NMR and (5) to study the cytotoxicity of liposomes (Lip) and magnetoliposomes (Lip-Fe3O4@lecithin) samples through cell cytotoxicity assays in cultures of human breast cancer cells (MGSO-3) on the freshly magnetoliposomes prepared samples.
Material and methods
Material
The chemicals used in the synthesis of magnetoliposomes were ammonium iron(II) sulfate hexahydrate (Synth, Brazil), iron(III) chloride hexahydrate (Vetec, Brazil), ammonium hydroxide PA (Synth, Brazil), and soy lecithin (CRQ, Brazil). For cell culture assays, Dulbecco modified Eagle’s medium (DMEM, Sigma-Aldrich, USA), fetal bovine serum (FBS, Gibco, USA), penicillin–streptomycin (Pen-Strep, Sigma-Aldrich, USA), phosphate-buffered saline (PBS, Gibco, USA), trypsin–EDTA (Gibco, USA), MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (Thermo Fisher Scientific, USA), sodium dodecyl sulfate (SDS, Sigma-Aldrich, USA), hydrochloric acid (HCL, Sigma-Aldrich, USA). All reagents were used without any purification.
Synthesis of bare liposome (Lip)
Bare liposome was prepared as previously described by Costa e Silva3. Soy lecithin (100 mg mL−1) was hydrated in ultra-purified water using an autoclave ((CS Prismatec Autoclave, Brazil) for 15 min at 120 °C. The size of the bare liposome was reduced for sonication by on-tip ultrasound (Disruptor Unique Model DES 500, Brazil). Four cycles of 4 min with intervals of 1 min were realized at 95% power (500 W), and the sample temperature was controlled below 75 °C. Then, the sample was centrifugated, and the supernatant was suspended in buffer PBS 10X, stabilizing the pH at 7.3 ± 0.1. The bare liposome sample was denoted Lip and maintained at 4 °C.
Synthesis of lecithin-coated magnetite (Fe3O4@lecithin)
Fe3O4@lecithin was prepared using a co-precipitation method3 in a one-pot reaction: ammonium iron(II) sulfate hexahydrate (0.0459 mol) and iron(III) chloride hexahydrate (0.08879 mol) was added to ultra-purified degassed water. Ammonium hydroxide solution (200 mL, 28% w/v) was added under magnetic stirring and maintained for 5 min. The previously prepared Lip (100 mg mL−1) was added, and the mixture was kept under stirring for 40 min. Fe3O4@lecithin formed was filtered and washed against ultra-purified degassed water until pH 7.0. The sample was lyophilized (Terroni Lyophilizer model LS 3000) and stored at 0 °C.
Preparation of the magnetoliposomes sample (Lip-Fe3O4@lecithin) and phytosterols samples
The previously prepared freeze-dried Fe3O4@lecithin (88% w/w of Fe3O4) was added to the Lip sample and ultra-purified degassed water in the 12:6:4 (w/w) proportion. The mixture was sonicated, centrifuged, and stabilized with PBS buffer (pH 7.3 ± 0.1) as described in the bare liposome (Lip) preparation. The sample was denominated Lip-Fe3O4@lecithin. The concentration of Fe3O4 in Lip-Fe3O4@lecithin is 10 mg mL−1.
Adding phytosterols (Stigmasterol and β-sitosterol) to the Lip sample was done using the same methodology for preparing the Lip- Fe3O4@lecithin.
The Lip-Fe3O4@lecithin sample utilized in the Cryo-TEM technique has been prepared in dilution of 0.2 mg mL−1 of Fe3O4@lecithin for 10 mg mL−1 of Lip to observe the interaction of magnetite with the liposome structure.
The Lip-Fe3O4@lecithin sample utilized in the total reflection X-ray fluorescence (TXRF) technique was not centrifuged and stabilized with PBS to evaluate the possibility of contamination due to the sonication by on-tip ultrasound.
Physical–chemical characterization of samples
The Cryo-Transmission Electron Microscopy (Cryo-TEM) technique allowed the evaluation of the formation of liposomes and encapsulation of magnetite. A droplet (3 μL) of the specimen was loaded in a pre-glow discharged ultrathin lacey carbon coated Cu-TEM (EMS) within the Leica GM2 Plunge Freezer under humidity 90%. Posteriorly, the specimen was plunged frozen in liquid ethane, and kept in liquid nitrogen until Cryo-TEM imaging. The sample was mounted in a Fischione M2550 TEM cryo holder and imaged in an FEI Tecnai Spirit at 120 kV. Cryo sample preparation and Cryo-TEM were performed at the Center of Microscopy at the Universidade Federal de Minas Gerais.
Conventional transmission electron microscopy images (TEM) were used to determine the morphology and size of the MNPs. The crystallographic phase stability of the MNPS was evaluated by SAED, HRTEM, and EELS. The TEM samples were prepared by placing a drop of each diluted sample in different holey carbon-coated TEM Cu-grids (300 mesh) (EMS) and let dry until analysis. The TEM sample preparation and analysis were performed in the Center of Microscopy at the Universidade Federal de Minas Gerais. The thermionic LaB6-Tecnai G2-20 SuperTwin FEI microscope was operated at 200 kV. The SAED patterns were interpreted with the help of JEMS software (version 3.4922U2010). The total reflection X-ray fluorescence (TXRF) was used for elemental determination of Lip-Fe3O4@lecithin components using an S2 PICOFOX ™ Portable Spectrometer (Bruker Nano GmbH, Karlsruhe, Germany). The equipment uses a Mo tube with an excitation source Kα 17.5 keV (600 μA, 50 kV, 50 W), a multilayer monochromator, and an active area silica detector of 30 mm2. Gallium was used as the internal standard in these analyses. Zeta potential analyses (ζ) were recorded in triplicate at 25.0 °C for freshly prepared and after 6 months of storage Lip and Lip-Fe3O4@lecithin samples (Zetasizer Nano ZS equipment). These samples’ particle size and polydispersity index (PI) were evaluated under the same conditions and apparatus using dynamic light scattering (DLS).
Cell lineage
Cells from the human primary breast cancer line MGSO-3 were previously obtained and characterized from mammary tumor fragments by Correa et al.23 The cell line was grown in Dulbecco-modified Eagle’s medium with 10% fetal bovine serum, 100 μg mL−1 streptomycin, and 100 U mL−1 penicillin. The cells were maintained in a 5% CO2 humidified incubator at 37 °C and detached from the bottle for the experiments when they reached 90% confluency using 0.25% trypsin–EDTA.
Cell viability assay
Sample viability was evaluated by the assay with 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl-2H-tetrazolium bromide (MTT). The MTT assay quantifies the mitochondrial activity by reducing the tetrazolium salt in formazan crystals by living cells. MGSO-3 cells were seeded in 48 well plates (5 × 104 cells/well). After cell cultivation for 24 h, the cells were treated with Lip 0.58 mg mL−1 and Lip-Fe3O4@lecithin in different liposome/Fe3O4 concentrations (0.58 mg mL−1/100 μg mL−1, 1.16 mg mL−1/200 μg mL−1 and 2.32 mg mL−1/400 μg mL−1) diluted from stock solutions prepared in 1 × PBS, in addition to the negative control, which received only 1× PBS. After treatment, the cell groups were evaluated at 1 and 3 days. Sequentially, the medium was removed, and 130 μL of DMEM and 100 μL of MTT (5 mg mL−1)/well were added. After 2 h, the formazan crystals were visualized under a light microscope and were dissolved in 130 μL of 10% SDS in 0.01 mol L−1 HCl. The culture plates were maintained at 37 °C during the experiment in a 5% CO2 humidified atmosphere. After 18 h, 100 μL of the above solution was transferred to a 96-well plate, and the optical density reading was performed at 595 nm. The whole biological assay was performed in triplicate.
Stability study
The stability study compares the results of the characterization of the freshly prepared Lip-Fe3O4@lecithin samples with the samples stored in Falcon tubes at 4 °C, after 6 months of storage. The stability parameters studied were liposome size distribution, polydispersity index (PI), pH, and zeta potential, and the identification of the iron oxide phase present in the samples was done by HRTEM, SAED, and EELS.
Statistical analysis
Statistical analysis for cell viability assays was performed using one-way ANOVA followed by Tukey’s post-test. The GraphPad Prism 5 software was used, and the reported percentage values are expressed as the mean of the cellular viability normalized by the mean viability of the control group. The reported values were expressed as mean ± S.D, and results with p ≤ 0.05 were considered statistically significant.
Results and discussion
Characterization of samples as prepared and after storage by sixty months
The physical–chemical data of Table 1 shows the mean size, PI, zeta potential, and pH values for all samples as-prepared and mean size, PI, zeta potential, and pH values for Lip and Lip-Fe3O4@lecithin samples after storage by 6 months.
Liposome mean sizes were evaluated by DLS techniques before and after adding magnetite, β-sitosterol, and stigmasterol or combinations of these. The average sizes are consistent with those described in the literature24. The addition of β-sitosterol (Lip-Βeta@lecithin) reduced the liposome size from 123 ± 1 to 117 ± 1 nm. In comparison, adding stigmasterol (Lip-Stigma@lecithin, Figure S1) increased the liposome size to 160 ± 4 nm. The molar ratios between Lip and Stigma and Lip and β-sitosterol were experimentally determined by 1H NMR (attached). The molar ratio between Lip and Stigma was 1.3:1.0, corresponding to 43.48 mol% of Stigma, while the molar ratio between Lip and β-sitosterol was 1:1.5, corresponding to 60 mol% of β-sitosterol. The increase in the mean size of liposomes containing stigmasterol corroborates the result described by Tai et al.25. The authors observed the reduction in the average size of liposomes of lecithin from 233 ± 11 to 197 ± 4 nm and 194 ± 4 nm due to addiction of 20 and 33 mol% of β-sitosterol, respectively, while for the addition of 50 mol% of β-sitosterol, an increase in liposome size was observed from 233 ± 11 to 383 ± 11 nm20. The larger average liposome sizes obtained by Tai et al.26 than those described in this study can be explained by the origin and composition of the lecithin used or the liposome preparation methodology.
However, the mean size of the liposome containing β-sitosterol (60 mol%) contradicts the authors’ theory since the liposome mean size was reduced even at high concentrations of β-sitosterol.
Considering only the structures of stigmasterol and β-sitosterol, one explanation could be given by the presence of a double bond at carbon C-22 of stigmasterol, the only difference between the structures of the two phytosterols studied. The presence of the double bond at C-22 makes the structure of stigmasterol more rigid and, therefore, reduces packing in the liposomal structures. Another factor could be that a new type of structure would be formed by exceeding the molar proportion of 50% of β-sitosterol27. The drastic reduction in the average diameter of empty lecithin liposomes (211 ± 1.1 nm) to 12.9 ± 1.2 nm, when added diclofenac sodium, an amphiphilic molecule, in the proportion 1:1 and 20.5 ± 3.3 nm described by Schutze and Muller-Goymann, corroborates the results of this work. The study also corroborates with Tai et al. (2019) when observing the increase by adding smaller proportions of diclofenac (16:1, 220.6 ± 5.5 nm) and reduction in liposome diameter for other proportions (8:1, 194.0 ± 4.5; and 2:1, 155.8 ± 0.9 nm)27.
The careful evaluation of the 1H NMR spectrum of Lip using the same parameters established for quantification of the phospholipids: phytosterols ratio resulted in the observation of the presence of phytosterols between 9 and 19 mol% in the soy lecithin samples (attached) and in the liposomes considered empty (Lip). The result found was unexpected; however, it can be easily explained by the origin of soy lecithin, which naturally contains phytosterols, and probably the separation process of phospholipids and soy lecithin was inefficient.
The average Lip size by DLS and TEM (Figure S1) in the experimental corroborates the results of Song et al.28, whose average liposome size is approximately 100 nm for liposomes formed by egg lecithin and Tween-80 with up to 20% sterols (stigmasterol, beta-sitosterol, or cholesterol). Song et al.28 also observed a greater increase in the average sizes of liposomes when stigmasterol was added in relation to beta-sitosterol and cholesterol in the same proportion of sterols in the range between 0 and 40% of each sterol29.
Regarding the polydispersity index (PI), there was a slight tendency for PI to increase due to the addition of phytosterols. The polydispersity index (PI) measures the size distribution of the nanoparticle population. On a scale from 0 to 1, values of 0.2 and below might be associated with a mono-dispersion in the particle population, whereas high PI values suggest a broad size distribution or even several populations. The PI of samples (Table 1) was around 0.2, which suggests homogenous monodispersed size characteristics29,30,31,32,33 and agrees with the variation and average size of liposomes determined by the DLS technique.
Song et al.28 also observed increased PI due to increased sterols, mainly stigmasterol and beta-sitosterol. However, the increase in the Song et al. experimental was approximately between 0.2 and 0.8, which resulted in the polydisperse liposome. The PI variation of Song et al. can be explained by the composition of formulation that resulted in the reduced Zeta potential29.
The Zeta potential of Lip, Lip-Fe3O4@lecithin, Lip-Stigma@lecethin, Lip-Βeta@lecethin, Lip-Stigma-Fe3O4@lecithin, Lip-Βeta-Fe3O4@lecithin samples were greater than │49│ mV. Zeta potential modules greater than│60│ mV result in excellent physical stability, and greater than │30│ mV present good physical stability29,34,35,36,37,38. Therefore, the zeta potential of all samples enjoys excellent stability.
However, adding Fe3O4@lecethin, Stigmasterol, or β-sitosterol considerably reduced the zeta potential values. The simultaneous addition of β-sitosterol and Fe3O4@lecethin or Stigmasterol and Fe3O4@lecethin resulted in a reduction of up to 50% less in the zeta potential compared to the addition of phytosterols alone.
Considering the results observed for the freshly prepared samples and studies at 14 and 21 days (not shown), the posterior addition of Stigmasterol and β-sitosterol did not show a significant increase in the stability evaluated by DLS and zeta potential or other physicochemical parameters, corroborating with not stability of addiction of sterols in the formulations describes by Tai et al.20. The author shows results with an increase of up to 30% in the size of lecithin liposomes containing β-sitosterol in 21 days20. Therefore, only the Lip and Lip-Fe3O4@lecithin samples were evaluated using the other techniques after storage for 6 months. The pH of formulations was maintained at pH 7.3 ± 0.1 after 6 months of storage.
The slight variation of sizes, pH in the Lip-Fe3O4@lecithin sample, high zeta potential, and PI values at 6 months confirmed the stability of liposome formulations containing soy lecithin. The dual use of soy lecithin (coating and encapsulation of the MNPs in magnetoliposomes) increased the modulus value of the zeta potential from − 19 ± 1 mV (data not shown, Costa e Silva3) to − 53 ± 1 mV (Fe3O4@lecithin and Lip-Fe3O4@lecithin, respectively). The coating reduced the possibility of aggregation due to high electrostatic repulsion among particles.
The encapsulation of curcumin in soy lecithin liposomes produced in a single step did not show encapsulation efficiency over time20. Shilova et al. described the coating of approximately 4 nm magnetite nanoparticles with Cetyltrimethylammonium Bromide to increase the encapsulation capacity of magnetite in phosphatidylcholine liposomes. The average diameter of the liposomes formed was 173 nm. However, the authors did not achieve an increase in the stability of the liposomes. The authors attributed the instability to the simplicity of the formulation39.
Cryo-Transmission Electron Microscopy technique confirmed the formation of liposomes (Fig. 1a) and the interaction of magnetite by encapsulation or external linkage in the surrounding of the liposome (Fig. 1b). The typical image corroborated with the literature24. The diameter of the Lip sample was (73 ± 14) nm, and the Lip-Fe3O4@lecithin sample was (96 ± 16) nm measured by Cryo-TEM (Table 1). The mean sizes calculated by images produced by Cryo-TEM in the fresh sample (Table 1) show variations in the Lip and Lip-Fe3O4@lecithin and the mean sizes calculated by DLS techniques. The variation of mean size between techniques can be explained by the characteristics of the measurement of technique type40 and the variation of concentration of MNPs and Lip, which are described in the methodologies for the different techniques. However, liposomes with diameters less than 200 nm are ideal for drug delivery and pharmaceutical applications3.
The diameter of liposomes is a limiting factor in drug delivery systems. Liposomes with a diameter of less than 50 nm tend to aggregate due to low thermodynamic stability27, while liposomes with diameters greater than 200 nm have a circulation time of less than 20 min41. Therefore, drug delivery systems generally involve a diameter of liposomes between 50 and 200 nm, which allows the drug to last longer in the body and the possibility of entry into blood vessels and tissues with small interstitial spaces42,43,44. Figure 1b shows the magnetite in the center of the liposome or adhered to the outer surface of the lipidic surface. The preference for the center or external surface of liposomes can be explained by the attraction of charges of magnetite and the polar head of liposomes localized in the center and extremities of structures.
Therefore, the methodology enables the formation and encapsulation of liposomes of adequate size for drug delivery applications.
The total reflection X-ray fluorescence (TXRF) technique was used to determine possible contamination by titanium or aluminum if there was an erosion of the ultrasound tip during exposure while sonicating the sample. The elemental composition of the Lip-Fe3O4@lecithin sample obtained by TXRF is shown in Table 2. The literature reports that long-term exposure may lead to tip wear and contamination of the medium with titanium and aluminum metals45,46. The liposomes showed no titanium and aluminum contamination. The hydration step by autoclave and the reduction of the size of liposomes earlier reduced the ultrasound time and possible contamination by erosion of the ultrasound probe.
Phase characterization of Lip-Fe3O4@lecithin samples
Iron oxide phases in different nanoregions of Lip-Fe3O4@lecithin samples at both times, fresh and after 6 months of storage, were analyzed by HRTEM, SAED, and EELS. In the previous study, we identified the iron phases by X-ray diffraction and compared them with the SAED and HRTEM techniques. In this work, we used HRTEM, SAED, and EELS techniques as alternatives to X-ray diffraction due to the necessity of lower quantities of samples and reduction of analysis techniques and time.
Figure 2 shows TEM and HRTEM images of the Lip-Fe3O4@lecithin sample. After 6 months, the Lip-Fe3O4@lecithin sample showed almost no change in morphology and homogeneity compared to the as-fresh sample. The mean diameters of MNPs in the Fe3O4@lecithin (data not shown) and Lip-Fe3O4@lecithin samples were calculated from several TEM images using the ImageJ software. The values found were 13 ± 2 and 14 ± 2 nm, respectively. Therefore, the coverage with Lip (Fe3O4@lecithin sample) and encapsulation in the liposomes (Lip-Fe3O4@lecithin sample) did not significantly change the diameter of the MNPs.
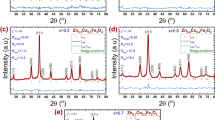
The magnetite phase was confirmed for samples at both times by HRTEM and SAED, whose measurements of the dhkl-spacings are shown in Table 3. Figure 3 shows SAED patterns of the Lip- Fe3O4@lecithin sample fresh and after 6 months of storage. To clarify the information on the SAED patterns, we have performed their line profiles (Fig. 3) with the help of the DiffTools suite47,48 on Gatan Digital Micrograph software. The profiles were obtained from the SAED patterns employing their rotational average and by integrating the reflection intensities along the radius direction. The simulated electron diffraction profiles were done with the help of JEMS software using as references the magnetite (a = 8.3967 Å, space group *Fd-3 m) and maghemite (a = 8.33 Å, space group *Fd-3 m) crystal structures.
(At the top) SAED patterns with the respective line profiles of the sample Lip- Fe3O4@lecithin samples fresh and at the 6 months of storage. (At the bottom) Simulated electron diffraction line profiles for maghemite and magnetite, and the experimental ones for Lip- Fe3O4@lecithin samples fresh and at the 6 months of storage.
Besides likely dynamical effects on electron diffraction, the reflections on the SAED patterns match well with the magnetite (Fe3O4) phase for both Lip-Fe3O4@lecithin fresh and after 6 months of storage. The intensities of the experimental SAED pattern closely follow the simulated ones. The excellent match of peak positions of SAED images between these two profiles indicates that the Lip-Fe3O4@lecithin sample is composed of the pure Fe3O4 crystal phase. Therefore, there was no formation of maghemite (γ-Fe2O3) and no other further phase transformation due to the 6 months of storage. This result shows the capacity of lecithin coverage to protect the magnetite against oxidation in an aqueous medium at a temperature of 4 °C. This fact is consistent with the literature that uses polymer coatings known to preserve the phase magnetite and prevent it from being degraded during or after the preparation of the hybrid systems8. The covering reduced the possibility of aggregation due to high electrostatic repulsion among particles and avoided the oxidation of magnetite, thus making the samples physically stable under storage conditions for at least 6 months.
Electron energy-loss spectroscopy (EELS) (Fig. 4) analysis also confirms the presence of the same iron oxide phase in both Lip-Fe3O4@lecithin fresh and after 6 months. The technique provides information on the electronic state and local chemical composition. The onset at 532 eV refers to the line at the ionization K-edge of the oxygen element, and for iron oxides, the pre-peak at 530 eV refers solely to the bond between oxygen and iron atoms.
The iron Fe L2,3-edges, onset at 708 eV, denotes the transition from Fe 2p63d5 (2p63d6) to Fe 2p53d6 (2p53d7). Confirmation of the iron oxide phase present was done by determining the white-line ratio of the peak intensities in Fe L2,3-Edges (Fe L3:Fe L2), where the L3 is the highest peak with the onset at about 708 eV, and the L2 is the peak with the onset at 718 eV. The white-line ratios found for Lip-Fe3O4@lecithin were 5.5 for the sample fresh and 5.3 for the sample after 6 months of storage using the EELS tools software suite47,48 in the Gatan Digital Micrograph. The data were compared to the literature49,50, which reports a white-line ratio of 5.2 ± 0.3, distinct from maghemite 5.8 ± 0.3. The EEL spectra (Fig. 4) were identified as referring to the magnetite phase51,52, corroborating our results from HRTEM and SAED analysis.
These results indicate that the lecithin covering on sample Lip-Fe3O4@lecithin was sufficient to avoid oxidation of magnetite and confirm the results of stability physical-chemistry of magnetoliposomes under the storage conditions for 6 months.
Cell viability evaluation
The cell viability evaluation was performed through an MTT assay to determine the occurrence of cytotoxicity of tumor cells treated by Lip 0.58 mg mL−1 and Lip-Fe3O4@lecithin in different liposome/Fe3O4 concentrations (0.58 mg mL−1/100 μg mL−1, 1.16 mg mL−1/200 μg mL−1 and 2.32 mg mL−1/400 μg mL−1).
Figure 5 illustrates the effects of these formulations on the viability of human breast cancer cells in situ (MGSO-3). As far as we know, no other study has evaluated the concentration-dependent toxicity of liposomal formulations based on soy lecithin in human breast cancer cells in situ thus far. Analyses were performed in one day to assess an acute effect observed early in the first cell cycle after treatment and a more chronic toxicity effect, with three days when cells had already reached an average of three cell cycles. The Lip 0.58 mg mL−1 sample showed a non-toxic profile at one and three days compared to the control. This non-toxicity presented by Lip in the first 24 h was also observed in 2011 by Abbasalipourkabir in breast cancer cells using lecithin-based liposomal formulations53. For the comparison, the exposition to the magnetoliposomes of the same concentration (Lip-Fe3O4@lecithin 0.58 mg mL−1 sample) also showed no toxicity in 1 and 3 days. A pronounced toxicity profile was observed in sample Lip-Fe3O4@lecithin in the concentrations 1.16 and 2.32 mg mL−1 that demonstrated a significant reduction in tumor cell viability as early as the first day of the analysis (68% and 87%), as well as more aggressive 3-day toxicity (95% and 92%). Thus, it can be concluded that the magnetoliposomes proposed in this study present cytotoxic activity in concentrations 1.16 and 2.32 mg mL−1. Similar dosages were tested in a toxicity assay with dextran-coated superparamagnetic nanoparticles, which showed lower toxicity in high concentrations in LO2 and HepG2 cells35. A possible explanation for the pronounced cytotoxicity observed by Lip-Fe3O4@lecithin in the concentrations 1.16 and 2.32 mg mL−1 is that there is a high internalization of Fe3O4@lecithin in the intracellular environment, which may activate the endocytic pathway of cells and cause an exhaustive process of intracellular digestion and subsequent reduction in cell metabolism, causing the cells to enter a death path. Thus, future studies providing a deeper characterization of the endocytic mechanisms of MGSO-3 cells (for example, incorporation, clathrin-mediated coating, and transport) and the degradation of Fe3O4@lecithin nanoparticles by lysosomes could help to understand the endocytosis of these better to understand the endocytosis of these nanoparticles better and correlate it with the reduction of cellular metabolism.
MGSO-3 cell viability determined by an MTT assay for Lip and Lip-Fe3O4@lecithin. Mean cell viability was normalized by the mean viability of the control group. Error bars denote ± SD. Stars indicate statistical significance at the p ≤ 0.05 (*) level as determined by one-way ANOVA, followed by a Tukey’s post-test. The experiments were performed in biological triplicates (n = 3).
Conclusions
The production of liposomes and magnetoliposomes by autoclave and sonication techniques showed the capacity for scalable production of solvent-free liposomes with the stability of phase magnetite, size, Zeta potential, and pH for 6 months of storage. Sonication has not produced inorganic contaminations (Al and Ti) in the liposomes frequently observed by techniques that use sonication due to the wear of tips due to excess sonication. Cryo-TEM images indicated the formation of typical liposomes and the linkage of magnetite in the liposome’s intern and extern polar regions.
The Lip and Lip-Fe3O4@lecithin samples with a concentration of 0.58 mg mL−1 showed high cell viability and are potential candidates for drug delivery in hyperthermia treatments and chemotherapy of diverse diseases.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (R.M.F.C.S).
References
Andrade, A. L., Domingos Fabris, J., Zacarias Domingues, R. & Pereira, M. C. Current status of magnetite-based core@ shell structures for diagnosis and therapy in oncology short running title: Biomedical applications of magnetite@ shell structures. Curr. Pharmaceut. Des. 21(37), 5417 (2015).
Andrade, A. L., Fabris, J. D., Pereira, M. C., Domingues, R. Z. & Ardisson, J. D. Preparation of composite with silica-coated nanoparticles of iron oxide spinels for applications based on magnetically induced hyperthermia. Hyperfine Interact. 218, 71 (2013).
da Costa e Silva, R. M. F. et al. Preparation of magnetoliposomes with a green, low-cost, fast and scalable methodology and activity study against S. aureus and C. freundii bacterial strains. J. Braz. Chem. Soc. 29, 2636 (2018).
Rocas, P. et al. Improved pharmacokinetic profile of lipophilic anti-cancer drugs using ανβ3-targeted polyurethane-polyurea nanoparticles. Nanomed. Nanotechnol. Biol. Med. 14(2), 257 (2018).
Mejía-López, J. & Mazo-Zuluaga, J. Energy contributions in magnetite nanoparticles: computation of magnetic phase diagram, theory, and simulation. J. Nanopart. Res. 13, 7115 (2011).
Sodipo, B. K. & Aziz, A. A. Non-seeded synthesis and characterization of superparamagnetic iron oxide nanoparticles incorporated into silica nanoparticles via ultrasound. Ultrason. Sonochem. 23, 354 (2015).
Kommareddi, N. S. et al. Synthesis of superparamagnetic polymer−ferrite composites using surfactant microstructures. Chem. Mater. 8(3), 801 (1996).
de Oliveira Machado, V. et al. A novel hybrid nanoparticle based on Fe3O4/TMAOH/poly (l-co-D, l lactic acid-co-trimethylene carbonate) prepared through the solvent displacement method. Hyperfine Interact. 240, 1 (2019).
Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers Nature reviews. Drug Discov. 4(2), 145 (2005).
Walde, P. & Ichikawa, S. Enzymes inside lipid vesicles: Preparation, reactivity and applications. Biomol. Eng. 18(4), 143 (2001).
Tan, Yn. et al. Lecithin in mixed micelles attenuates the cytotoxicity of bile salts in Caco-2 cells. Toxicol. In Vitro. 27(2), 714 (2013).
Gándola, Y. B. et al. Mitogenic effects of phosphatidylcholine nanoparticles on MCF-7 breast cancer cells. BioMed Res. Int. 2014, 1–13 (2014).
Fenske, D. B. & Cullis, P. R. Entrapment of small molecules and nucleic acid-based drugs in liposomes. In Methods in Enzymology 7 (Elsevier, 2005).
Allen, T. & Chonn, A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 223(1), 42 (1987).
Al-Jamal, W. T., Al-Jamal, K. T., Bomans, P. H., Frederik, P. M. & Kostarelos, K. Functionalized-quantum-dot–liposome hybrids as multimodal nanoparticles for cancer. Small 4(9), 1406 (2008).
Takeuchi, H., Kojima, H., Yamamoto, H. & Kawashima, Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Controll. Release 75(1–2), 83 (2001).
Clares, B. et al. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharmaceut. Biopharmaceut. 85(3), 329 (2013).
Clares, B., Gallardo, V., Medina, M. & Ruiz, M. Multilamellar liposomes of triamcinolone acetonide: Preparation, stability, and characterization. J. Liposome Res. 19(3), 197 (2009).
Phan, T. T. V. et al. Coating chitosan thin shells: A facile technique to improve dispersion stability of magnetoliposomes. J. Nanosci. Nanotechnol. 18(1), 583 (2018).
Tai, K. et al. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 293, 92–102 (2019).
Meure, L. A., Foster, N. R. & Dehghani, F. Conventional and dense gas techniques for the production of liposomes: A review. Aaps Pharmscitech. 9, 798 (2008).
Magnan, C., Badens, E., Commenges, N. & Charbit, G. Soy lecithin micronization by precipitation with a compressed fluid antisolvent—Influence of process parameters. J. Supercrit. Fluids 19(1), 69 (2000).
Correa, C., Bertollo, C. & Goes, A. Establishment and characterization of MACL-1 and MGSO-3 cell lines derived from human primary breast cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 17(10), 473 (2009).
Taladrid, D. et al. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 100, 541–550 (2017).
Tai, K. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food chem. 293, 92–102 (2019).
Bot, F., Cossuta, D. & O’Mahony, J. A. Inter-relationships between composition, physicochemical properties and functionality of lecithin ingredients. Trends Food Sci. Technol. 111, 261–270 (2021).
Schütze, W. & Müller-Goymann, C. C. Phase transformation of a liposomal dispersion into a micellar solution induced by drug-loading. Pharmaceut. Res. 15, 538 (1998).
Song, F., Chen, J., Zheng, A. & Tian, S. Effect of sterols on liposomes: Membrane characteristics and physicochemical changes during storage. Lwt 164, 113558 (2022).
Chen, L.-C. et al. Development and characterization of lecithin-based self-assembling mixed polymeric micellar (sa MPMs) drug delivery systems for curcumin. Sci. Rep. 6(1), 37122 (2016).
Fatouros, D. G. & Antimisiaris, S. G. Effect of amphiphilic drugs on the stability and zeta-potential of their liposome formulations: A study with prednisolone, diazepam, and griseofulvin. J. Colloid Interface Sci. 251(2), 271 (2002).
Campardelli, R. et al. Efficient encapsulation of proteins in submicro liposomes using a supercritical fluid assisted continuous process. J. Supercrit. Fluids 107, 163 (2016).
Sebaaly, C., Charcosset, C., Stainmesse, S., Fessi, H. & Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 138, 75 (2016).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10(2), 57 (2018).
Riddick, T. M. Zeta-meter Manual (Zeta-Meter Inc., 1968).
Peng, M. et al. Dextran-coated superparamagnetic nanoparticles as potential cancer drug carriers in vivo. Nanoscale 7(25), 11155 (2015).
Chen, Y. et al. Baicalin loaded in folate-PEG modified liposomes for enhanced stability and tumor targeting. Colloids Surf. B: Biointerfaces 140, 74 (2016).
Honary, S. & Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharmaceut. Res. 12(2), 265 (2013).
Pérez, S. E. et al. Formulation strategies, characterization, and in vitro evaluation of lecithin-based nanoparticles for siRNA delivery. J. Drug Deliv. 2012, 986265 (2012).
Shilova, E. V., Koltakov, I. A., Kannykin, S. V. & Artyukhov, V. G. Inclusion of magnetite nanoparticles stabilized with cetyltrimethylammonium bromide in soy lecithin-based liposomes. Biophys. 67(3), 435–439 (2022).
Pippa, N. et al. Studying the colloidal behavior of chimeric liposomes by cryo-TEM, micro-differential scanning calorimetry and high-resolution ultrasound spectroscopy. Colloids Surf. A: Physicochem. Eng. Asp. 555, 539 (2018).
Batista, C. M., Carvalho, C. MBd. & Magalhães, N. S. S. Lipossomas e suas aplicações terapêuticas: Estado da arte. Rev. Brasileira Ciências Farm. 43, 167 (2007).
Harashima, H., Sakata, K., Funato, K. & Kiwada, H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharmaceut. Res. 11, 402 (1994).
Woodle, M. C., Engbers, C. M. & Zalipsky, S. New amphipatic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjug. Chem. 5(6), 493 (1994).
Immordino, M. L., Dosio, F. & Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 1(3), 297 (2006).
Ali, F. et al. Effect of sonication conditions: Solvent, time, temperature and reactor type on the preparation of micron sized vermiculite particles. Ultrason. Sonochem. 21(3), 1002 (2014).
Betts, J. N., Johnson, M. G., Rygiewicz, P. T., King, G. A. & Andersen, C. P. Potential for metal contamination by direct sonication of nanoparticle suspensions. Environ. Toxicol. Chem. 32(4), 889 (2013).
Mitchell, D. R. DiffTools: Electron diffraction software tools for DigitalMicrograph™. Microsc. Res. Tech. 71(8), 588 (2008).
Mitchell, D. & Schaffer, B. Scripting-customised microscopy tools for Digital Micrograph™. Ultramicroscopy 103(4), 319 (2005).
Chen, S.-Y. et al. Electron energy loss spectroscopy and ab initio investigation of iron oxide nanomaterials grown by a hydrothermal process. Phys. Rev. B 79(10), 104103 (2009).
Colliex, C., Manoubi, T. & Ortiz, C. Electron-energy-loss-spectroscopy near-edge fine structures in the iron-oxygen system. Phys. Rev. B 44(20), 11402 (1991).
Golla-Schindler, U., Hinrichs, R., Bomati-Miguel, O. & Putnis, A. Determination of the oxidation state for iron oxide minerals by energy-filtering TEM. Micron 37(5), 473 (2006).
Lins, U., Keim, C. N., Evans, F. F., Farina, M. & Buseck, P. R. Magnetite (Fe3O4) and greigite (Fe3S4) crystals in multicellular magnetotactic prokaryotes. Geomicrobiol. J. 24(1), 43 (2007).
Abbasalipourkabir, R., Salehzadeh, A. & Abdullah, R. Cytotoxicity effect of solid lipid nanoparticles on human breast cancer cell lines. Biotechnology 10(6), 528 (2011).
Acknowledgements
The authors thank the Center of Microscopy at UFMG for the available open facility and the Profa. Izabel Cristina Riegel Vidotti and Prof. Rilton Alves de Freitas by the availability of the Laboratory of Chemistry in the Universidade Federal do Paraná (UFPR).
Author information
Authors and Affiliations
Contributions
These authors contributed equally. R.M.F.C.S.: Investigation and Writing, Original Draft, Supervision and Review & Editing; A.L.A., E.T.F.F., T.M.V., L.R.S.L, D.A.M., J.L.L. C.C.N.: Investigation and Formal analysis and Writing H.O.S., A.M.G. and R.Z.D.: Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
da Costa e Silva, R.M.F., Andrade, Â.L., Freitas, E.T.F. et al. Formation and stability of green and low-cost magnetoliposomes of the soy lecithin, stigmasterol, and β-sitosterol for hyperthermia treatments. Sci Rep 15, 2831 (2025). https://doi.org/10.1038/s41598-024-82480-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82480-x