Abstract
This study evaluated the effectiveness of intraovarian platelet-rich plasma (PRP) injection in improving ovarian response and embryo quality in IVF patients with poor embryo quality in previous controlled ovarian hyperstimulation (COH) cycles. 74 patients participated, with 30 in the control group and 44 in the PRP group. PRP was injected during the follicular phase for the PRP group. The control group completed two COH cycles, while the PRP group underwent COH cycles before and after the PRP injection. In the first COH cycle, there were no significant differences between groups. However, in the second COH cycle, the PRP group showed significant improvements: the number of fertilized oocytes increased (5.2 ± 3.6 vs. 3.3 ± 3.5, p = 0.011), total blastocysts (1.7 ± 1.5 vs. 0.5 ± 0.7, p < 0.0001) and good quality blastocysts (0.6 ± 0.8 vs. 0 ± 0.2, P < 0.0001). The total blastocyst rate (35 ± 31% vs. 13 ± 24%, p = 0.001) and good quality blastocyst rate (14 ± 22% vs. 1 ± 3%, p < 0.0001) were also higher in the PRP group. The most notable benefits occurred when COH was conducted one to two months post-PRP injection.
Similar content being viewed by others
Introduction
With the increase in late marriages and delayed childbearing, the rate of infertility is rising. As women grow older, their ovarian reserve diminishes, resulting in fewer and lower-quality eggs. The decline is further exacerbated in young women experiencing decreased ovarian reserve or unexplained poor egg and embryo quality. These factors result in a low pregnancy rate, even with assisted reproductive technology (ART)1.
The quantity of viable blastocysts plays a vital role in the success of ART, especially with the rising adoption of the “freeze-all” approach, frozen embryo transfers, and preimplantation genetic testing for aneuploidy (PGT-A). The quality of blastocysts dictates how many embryos can undergo the PGT-A and cryopreservation, significantly impacting the pregnancy rates2.
Following the introduction of the POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) guidelines in 20163, numerous research efforts have concentrated on enhancing ART outcomes for patients exhibiting poor ovarian response (POR)4,5,6,7. A diminished ovarian reserve and suboptimal response to gonadotropins lead to a reduced number of oocytes, which restricts the number of embryos and lowers both pregnancy and cumulative live birth rates8. Thus, increasing the number of embryos and blastocysts is critical for ART success.
In addition to age-related aneuploid in the oocytes, ooplasm abnormalities, including abnormalities in metabolism, structure proteins such as microtubules, mitochondrial function and transcription factors, also affect the embryo development9. To date, numerous studies have explored various methods to improve the quality of eggs and embryos, including physical activity, acupuncture, and pre-treatment such as testosterone, dedydroepiandrosterone (DHEA), coenzyme Q10, vitamin D, and growth hormone10,11,12,13. However, the results of these studies have been inconsistent, and no definitive conclusions have been reached4. Additionally, using different protocols14,15,16, choosing different trigger medications17,18, changing the embryo culture environment19,20 and even autologous stem cell transplantation21 had also produced varied results. As a result, there is currently no method with sufficient evidence to improve the embryo quality and increase the number of blastocysts.
Platelet-rich plasma (PRP), obtained from the patient’s blood, contains a platelet concentration that is a 4–5 times higher22. It contains more than 800 types of proteins, cytokines and growth factors23,24,25,26,27,28. These factors can stimulate cell differentiation, proliferation, migration, and angiogenesis, promoting tissue regeneration25. PRP has been widely used in orthopedics, ophthalmology, surgery, wound healing29, and obstetrics and gynecology, including treatments for sexual dysfunction and pelvic floor disorders30.
Our earlier research demonstrated that administering PRP through intrauterine infusion and hysteroscopic injection increased endometrial thickness and improved pregnancy outcomes23. Several studies have shown that injecting PRP directly into the ovaries of patients with POR increased the number of retrieved oocytes, improved embryo quality, and led to higher pregnancy rate30,31,32,33,34. However, these studies did not evaluate whether PRP could increase the number of blastocysts.
Therefore, this study aims to investigate whether the intraovarian PRP injection can increase the blastocyst rate. The primary outcomes are the number of total and good quality blastocysts, while the secondary outcomes are the total and good quality blastocyst rates.
Results
Patient characteristics
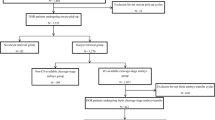
This research involved 74 participants, divided into 30 in the control group and 44 in the PRP group. The mean age of patients in the control and PRP groups was 39.8 ± 3.2 and 40.2 ± 3.2 years, respectively (p = 0.918). The groups showed no notable variations in terms of partner’s age, body mass index (BMI), anti-Mullerian hormone (AMH) levels, length of infertility, total previous IVF cycles, or infertility causes (all p > 0.05) (see Table 1).
First controlled ovarian hyperstimulation
Both groups underwent the first COH cycle and egg retrieval. In this phase, no notable distinctions were observed between the control and PRP groups regarding basal levels of FSH, LH and E2, the number of stimulation days, the total gonadotropin dosage, the count of retrieved and fertilized oocytes, or the total and good quality blastocysts (all p > 0.05). The maturation and fertilization rates, along with the total and good quality blastocyst rates, were similar between the groups (Table 2).
Second controlled ovarian hyperstimulation
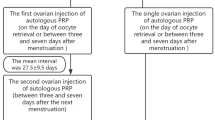
After receiving the intraovarian PRP injection during the follicular phase, patients in the PRP group underwent a second COH cycle using the same protocol. The average duration between the PRP injection and the second egg retrieval was 41.1 ± 16.7 (range: 11–82 days). The control group did not receive PRP injection and proceeded directly to the second COH cycle. Notable enhancements were noted in the PRP group following the second COH cycle when contrasted with the control group. The PRP group had a notably greater count of fertilized oocytes (5.2 ± 3.6) than the control group (3.3 ± 3.5), with a p-value of 0.011. Furthermore, the PRP group exhibited a greater count of total blastocyst (1.7 ± 1.5 vs. 0.5 ± 0.7, p < 0.0001) and good quality blastocyst (0.6 ± 0.8 vs. 0 ± 0.2, p < 0.0001). The total blastocyst rate (35 ± 31% vs. 13 ± 24%, p = 0.001) and good quality blastocyst rate (14 ± 22% vs. 1 ± 3%, p < 0.0001) were also significantly increased in the PRP group (Table 3).
Comparison within groups
The control group showed a consistent ovarian response, as there were no notable differences in the results of the first and second COH cycles (see Table 4). Nonetheless, in the PRP group, the second COH cycle following the PRP injection demonstrated notable enhancements over the first COH cycle regarding the number of total blastocysts (p < 0.0001) and good quality blastocysts (p < 0.0001), the total blastocyst rate (p = 0.001), and good quality blastocyst rate (p < 0.0001) (see Table 5).
The interval between PRP injection and subsequent COH cycle
The interval between PRP injection and subsequent COH cycle significantly impacted the outcomes. Patients who underwent COH cycles one and two months after PRP injection showed notable improvements in number of total blastocysts and good quality blastocysts, as well as the total and good quality blastocyst rates. However, for those who underwent COH three months post-injection, the results showed no statistically significant differences compared to pre-injection outcomes (Table 6).
In the PRP group, 30 patients received two consecutive COH cycles after PRP injection. In the first COH cycle following injection, which typically spans 38.7 ± 17.5 days from PRP injection to oocyte retrieval, there were notable rises in the number of total blastocysts and good quality blastocysts, as well as in the rates of total and good quality blastocyst. Nevertheless, during the second COH cycle following the injection, which 78 ± 28.2 days from PRP injection to oocyte retrieval, there was an observable trend of enhancement compared to pre-injection results, though the changes were not statistically significant (see Table 7).
PGT-A results and pregnancy outcomes
In the control group, two patients each had one blastocyst available for PGT-A after the second COH cycle. One of these blastocysts was euploid and led to pregnancy after transfer, but it resulted in an early miscarriage with no live birth. In the PRP group, after receiving PRP injections, 22 patients had blastocysts available for PGT-A following the first COH cycle. A total of 34 blastocysts were tested, resulting in an euploidy rate of 17.6% (6/34) and a mosaic rate of 20.6% (7/34). After the second COH cycle, 14 patients had blastocysts available for PGT-A, with 21 blastocysts tested, showing an euploidy rate of 9.5% (2/21) and a mosaic rate of 20.6% (7/34). In the PRP group, nine patients received embryo transfers involving both euploid and mosaic embryos, with an average of 1.6 ± 0.7 embryos transferred per individual. Seven individuals became pregnant, resulting in a 29% pregnancy rate.
Discussion
To our knowledge, this is the first study to investigate the use of intraovarian PRP injections in patients who have failed to produce good quality blastocysts in at least two previous IVF attempts, and its potential to improve both the quantity and quality of blastocysts. Our findings demonstrate that PRP injections directly into the ovaries can significantly enhance ovarian response and embryo quality in these patients. This includes an increase in the number of total blastocysts, the number of good quality blastocysts, the total blastocyst rate, and the rate of good quality blastocyst following COH. Significant improvements were observed with COH was performed one and two months after PRP injection. Although consecutive COH cycles also showed improvement, the second cycle after PRP injection only exhibited a trend of improvement without reaching statistical significance.
The underlying mechanisms of PRP’s efficacy in improving ovarian response and embryo quality are likely due to its rich concentration of growth factors such as PDGF and VEGF, which promote the angiogenesis and then enhance folliculogenesis at all stages of follicular growth and stimulate the oogonial stem cells (OSCs) achieving postnatal oogenesis to form new primordial follicles35. However, the existence of OSCs in the ovaries is still a subject of debate and lacks consensus36.
In 2017, Hosseini et al. showed that PRP could promote the growth of isolated human primordial and primary follicles and their development into preantral follicles37. Pantos et al. described an experimental study in 2018 in which PRP was transvaginally injected into bilateral ovaries of eight peri-menopausal women. After 1–3 months, the ovaries developed follicles on their own, and successful egg collection for all patients was achieved after egg retrieval38. Later, Pantos et al. injected PRP into the ovaries of three menopausal women and found that all three women menstruated one to two months following the injection. Within six months, all three women conceived naturally39, indicating that intraovarian PRP injection could promote follicle growth and restore natural ovulatory function. The growth factors in PRP were believed to be key drivers in promoting ovarian tissue regeneration. In 2018, Sills et al. performed the intraovarian PRP injection for four POR patients with an average age of 42 ± 4. IVF was performed 59–110 days later, and each patient successfully obtained at least one blastocyst for freezing40. Melo and colleagues suggested that injecting PRP directly into the ovaries could either promote the growth of preantral follicles or stop their atresia, leading to an increased count of eggs retrieved during IVF and a greater number of high- and medium-quality embryos, indicating that PRP might support embryo development post-fertilization41. The regenerative effect resulted in improvements in the hormone profile, ovarian reserve and the quality of oocytes retrieved during COH cycles, aligning with the findings of this current study. These results support the hypothesis that intraovarian PRP injections may rejuvenate ovarian tissue and enhance reproductive outcomes. The positive impact of PRP appeared to be short-lived, with significant increases in the total and good quality blastocyst counts, as well as their respective rates, observed within two months post-injection. By the third month, the regenerative effects seemed to diminish, likely due to the natural degradation and absorption of growth factors and cytokines present in PRP. Therefore, the timing of PRP injection and COH is crucial for optimizing its efficacy. Further studies are needed to explore the underlying causes of this outcome.
In contrast to our findings, some studies had reported less favorable outcomes with PRP treatment. For instance, Barad et al. found that intraovarian PRP injection did not improve hormone profiles or IVF outcomes in POR patient in Poseidon group 3 and 442. Additionally, two randomized controlled trials (RCT) conducted by Herlihy et al.43 and Barrenetxea et al.34 showed that PRP did not enhance IVF outcomes in POR patients younger than 38 years or those classified under POSEIDON groups 3 and 4. These discrepancies may be attributed to differences in patient populations, PRP preparation methods, and study designs.
Our study had some limitations. First, it was not randomized; patients chose whether to undergo intraovarian PRP injection, which may introduce selection bias. Second, the control group did not receive a sham procedure, so the observed effects could be partially attributed to the mechanical impact of the injection itself, potentially disrupting the Hippo signaling pathway34,36. Third, the sample size for embryo transfer and pregnancy outcomes was small, limiting the robustness of these findings. Additionally, long-term data on ovarian condition, as well as the fetal and neonatal outcomes, were lacking. Furthermore, since different studies used various methods to prepare PRP, the composition and concentration of growth factors may also vary. Most studies did not analyze the components of PRP, and the dosage and injection sites differed, making it difficult to compare results across studies. Lastly, this research involved a varied group of participants who had completed at least two COH and PGT-A cycles without producing good quality blastocysts. Numerous factors can influence embryo quality, and future research should focus on specific group to identify those who would most benefit from intraovarian PRP injections.
Conclusion
In summary, injecting PRP directly into the ovaries shows promise in enhancing ovarian response and embryo quality for patients with unsatisfactory outcomes from previous IVF attempts. Patients who underwent COH one and two months after PRP injection demonstrated significant improvements in both the number and quality of blastocysts, and in the corresponding rates. However, further research is needed to understand the underlying mechanisms, determine the optimal timing and frequency of injections, and evaluate long-term safety and efficacy.
Materials and methods
Patient selection
This study was a prospective case–control study. Individuals receiving controlled ovarian hyperstimulation (COH) for PGT-A from July 2020 to January 2024 at Lee Women’s Hospital in Taichung, Taiwan, were assessed for inclusion. Each participant had already undergone at least two COH and PGT-A cycles without producing of any good quality blastocyst. After thorough discussion and explanation, it was up to the patients to decide whether to receive intraovarian PRP injection. According to the Gardner grading system44, a good quality blastocyst is defined as an expanded blastocyst with grades AA, AB, BA, and BB45. The criteria for exclusion encompassed endometriosis, being under 20 years of age, blood disorders with platelet counts below 150,000/μL, or anemia with hemoglobin levels under 11 g/dL. The reasons for PGT-A included advanced maternal age, recurrent miscarriage, and repeated failures in embryo implantation46.
Ethics declarations
The Institutional Review Board of Chung Shan Medical University Hospital (CS2-20,037) approved this research, and all participants provided written informed consent to join the study. All methods were performed in accordance with the relevant guidelines and regulations.
Autologous platelet-rich plasma (PRP) preparation and intraovarian injection
PRP was prepared with 60 mL whole blood without anticoagulant into two Acti-PRP tubes (Aeon Biotherapeutics Corp., Taipei, Taiwan) as described23. During the follicular phase, PRP was injected into both ovaries under intravenous anesthesia. Using transvaginal sonography as a guide, a single-lumen ovum aspiration needle (18GA, Kitazato; Fuji, Japan) was used to inject 1 mL of PRP into the cortex of each ovary at four different locations.
Regimen for controlled ovarian stimulation (COH)
This research employed progestin-primed ovarian stimulation (PPOS) using medroxyprogesterone acetate (Provera®; Pfizer, Milan, Italy). Follitropin alfa (Gonal-f®; Merck-Sereno, Darmstadt, Germany) and hMG (Menopur®; Ferring, Pymble, NSW, Australia) were given starting from the second or third day of the menstrual cycle and continued until the leading follicles attained a size of 17 mm. Final oocyte maturation was triggered using recombinant human chorionic gonadotropin (hCG) (Ovidrel®; Merck-Sereno, Darmstadt, Germany) and triptorelin (Decapeptyl®; Ferring, Pymble, NSW, Australia), with oocyte retrieval occurring 34–36 h later.
Embryo culture, biopsy and the preimplantation genetic testing for aneuploidy (PGT-A)
A tri-gas incubator, with 5% oxygen, 5% carbon dioxide, and 90% nitrogen, set at 37 °C, was used to maintain the eggs and embryos. Fertilization was achieved either through traditional insemination or intracytoplasmic sperm injection (ICSI), based on the quality of the sperm. At first, embryos were grown in the cleavage medium (Quinn’s Advantage™, SAGE Biopharma®; Cambridge, MA, USA), and shifted to the blastocyst medium (Quinn’s Advantage™, SAGE Biopharma®; Cambridge, MA, USA), approximately 70–72 h after fertilization. The Gardner grading system44 was employed to evaluate embryos, and solely expanded blastocysts with grades AA, AB, AC, BA, BB, BC, CA or CB were chosen for PGT-A. The PGT-A procedure closely resembled the earlier account46. Biopsied blastocysts were vitrified using the Cryotech system (Cryotech®; Tokyo, Japan). Two experienced technicians utilized BlueFuse Multi Software (Illumina, San Diego, CA, USA) to divide the blastocysts into three groups: euploid, mosaic, and aneuploid blastocysts, depending on the mosaicism levels47,48,49.
Endometrial preparation for frozen embryo transfer (FET)
Hormone replacement therapy (HRT) was utilized in this study, as previously described50. Patients started taking oral estradiol valerate (Estrade®, Synmosa, Taipei, Taiwan) since the second to third day of menstrual cycle until the cycle day 11–13, and endometrial (EM) thickness was checked using a transvaginal sonography. When the EM was trilaminar-patterned and the EM thickness was above 7 mm, 10 mg of oral dydrogesterone (Duphaston®, Abbott, Hong Kong, China), three times a day, and vaginal progesterone gel (Crinone®, Merck-Serono, Darmstadt, Germany), twice a day, were given. Embryos were transferred 5 days later, and only euploid and mosaic embryos were chosen for transfer in this study. The cancellation criteria included cases where EM thickness less than 7 mm, the presence of intrauterine fluid, progesterone levels exceeding 1.5 ng/mL before progesterone administration, any patient discomfort, or if the patient requested cancellation.
Outcomes measurement
The maturation rate was calculated by dividing the number of metaphase II (MII) oocytes by the total number of oocytes collected. The fertilization rate was calculated by dividing the count of zygotes with two pronuclear (2PN) by the number of MII oocytes that underwent insemination. The total blastocyst rate was calculated by dividing the total number of all blastocysts, irrespective of their quality, by the number of 2PN zygotes. The good quality blastocyst rate was calculated by dividing the count of good quality blastocysts by the number of 2PN zygotes. The good quality blastocysts are characterized as expanded and graded AA, AB, BA, or BB (≥ 4BB)45. We examined the COH outcomes in both the control and PRP groups, considering the data from before and after the PRP injection. We also analyzed the outcomes of COH cycles initiated in one, two, and three months after PRP injection, comparing them to pre-injection results to assess whether the timing of PRP administration influenced outcomes., Additionally, we examined the results of two consecutive COH cycles following PRP injection to determine whether the effects of intraovarian PRP injection were sustained.
The pregnancy rate was calculated by dividing the number of women with serum β-HCG levels exceeding 100 mIU/mL by the total number of women who underwent embryo transfer. The implantation rate was calculated by dividing the number of gestational sacs by the number of embryos transferred. A miscarriage was characterized as the loss of a pregnancy before reaching 24 weeks, while a live birth was described as a full delivery of a living baby after 24 weeks.
Data analysis
Data analysis was performed using SPSS software version 20.0 (IBM Corporation, Armonk, New York, USA). A p-value of < 0.05 was considered statistically significant. Continuous variables were presented as mean ± standard deviation, while the categorical variables were expressed as frequency and percentage. Variations in the data were assessed using The Mann–Whitney U test, Wilcoxon Signed-Rank test and/or ANOVA. For categorical variables, the chi-squared test was applied.
Data availability
The data underlying this study will be available on reasonable request to the corresponding author.
Abbreviations
- 2PN:
-
Two pronuclear
- AMH:
-
Anti-Mullerian hormone
- ART:
-
Assisted reproductive technology
- BMI:
-
Body mass index
- COH:
-
Controlled ovarian hyperstimulation
- DHEA:
-
Dedydroepiandrosterone
- EM:
-
Endometrial
- FET:
-
Frozen embryo transfer
- hCG:
-
Human chorionic gonadotropin
- HRT:
-
Hormone replacement therapy
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- MII:
-
Metaphase II
- OSC:
-
Oogonial stem cell
- PGT-A:
-
Preimplantation genetic testing for aneuploidy
- POR:
-
Poor ovarian response
- POSEIDON:
-
Patient-oriented strategies encompassing individualized oocyte number
- PPOS:
-
Progestin-primed ovarian stimulation
- PRP:
-
Platelet-rich plasma
- RCT:
-
Randomized controlled trial
References
Gelety, T. J. The future of elective oocyte cryopreservation in extending women’s reproductive potential. Biomed. J. Sci. Tech. Res. https://doi.org/10.26717/bjstr.2023.50.008023 (2023).
Morbeck, D. E. Blastocyst culture in the era of PGS and FreezeAlls: Is a ‘C’ a failing grade?. Hum. Reprod. Open https://doi.org/10.1093/hropen/hox017 (2017).
Alviggi, C. et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil. Steril. 105, 1452–1453. https://doi.org/10.1016/j.fertnstert.2016.02.005 (2016).
Orvieto, R. Pretreatment: Does it improve quantity or quality?. Fertil. Steril. 117, 657–663. https://doi.org/10.1016/j.fertnstert.2022.01.029 (2022).
Vuong, L. N. Alteration of final maturation and laboratory techniques in low responders. Fertil. Steril. 117, 675–681. https://doi.org/10.1016/j.fertnstert.2022.01.028 (2022).
Hart, R. J. Stimulation for low responder patients: Adjuvants during stimulation. Fertil. Steril. 117, 669–674. https://doi.org/10.1016/j.fertnstert.2022.01.027 (2022).
Cedars, M. I. Managing poor ovarian response in the patient with diminished ovarian reserve. Fertil. Steril. 117, 655–656. https://doi.org/10.1016/j.fertnstert.2022.02.026 (2022).
Esteves, S. C. et al. The POSEIDON criteria and its measure of success through the eyes of clinicians and embryologists. Front. Endocrinol. https://doi.org/10.3389/fendo.2019.00814 (2019).
Krey, L. C. & Grifo, J. A. Poor embryo quality: The answer lies (mostly) in the egg. Fertil. Steril. 75, 466–468. https://doi.org/10.1016/s0015-0282(00)01778-7 (2001).
Zhu, F. et al. TEAS, DHEA, CoQ10, and GH for poor ovarian response undergoing IVF-ET: A systematic review and network meta-analysis. Reprod. Biol. Endocrinol. https://doi.org/10.1186/s12958-023-01119-0 (2023).
Fichera, M. et al. Vitamin D, reproductive disorders and assisted reproduction: evidences and perspectives. Int. J. Food Sci. Nutr. 71, 276–285. https://doi.org/10.1080/09637486.2019.1661978 (2020).
Noventa, M. et al. Testosterone therapy for women with poor ovarian response undergoing IVF: A meta-analysis of randomized controlled trials. J. Assist. Reprod. Genet. 36, 673–683. https://doi.org/10.1007/s10815-018-1383-2 (2019).
Naik, S. et al. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD009749.pub3 (2024).
Lambalk, C. B. et al. GnRH antagonist versus long agonist protocols in IVF: A systematic review and meta-analysis accounting for patient type. Hum. Reprod. Update 23, 560–579. https://doi.org/10.1093/humupd/dmx017 (2017).
Welp, A. M., Williams, C. D., Smith, L. P., Purcell, S. & Goodman, L. R. Oral medroxyprogesterone acetate for the use of ovulation suppression in in vitro fertilization: a cohort trial. Fertil. Steril. 121, 806–813. https://doi.org/10.1016/j.fertnstert.2024.01.026 (2024).
Huang, P., Tang, M. & Qin, A. Progestin-primed ovarian stimulation is a feasible method for poor ovarian responders undergoing in IVF/ICSI compared to a GnRH antagonist protocol: A retrospective study. J. Gynecol. Obstet. Hum. Reprod. 48, 99–102. https://doi.org/10.1016/j.jogoh.2018.10.008 (2019).
Zhou, C. et al. Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum. Reprod. 37, 1795–1805. https://doi.org/10.1093/humrep/deac114 (2022).
Haas, J. et al. GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: A double-blinded, randomized controlled study. Hum. Reprod. 35, 1648–1654. https://doi.org/10.1093/humrep/deaa107 (2020).
Kaser, D. J. et al. Randomized controlled trial of low (5%) versus ultralow (2%) oxygen for extended culture using bipronucleate and tripronucleate human preimplantation embryos. Fertil. Steril. 109, 1030-1037 e1032. https://doi.org/10.1016/j.fertnstert.2018.02.119 (2018).
Brouillet, S. et al. Biphasic (5–2%) oxygen concentration strategy significantly improves the usable blastocyst and cumulative live birth rates in in vitro fertilization. Sci. Rep. https://doi.org/10.1038/s41598-021-01782-6 (2021).
Herraiz, S. et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 110, 496-505 e491. https://doi.org/10.1016/j.fertnstert.2018.04.025 (2018).
Streit-Cieckiewicz, D. et al. Platelet rich plasma in gynecology-discovering undiscovered-review. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph19095284 (2022).
Yu, T.-N. et al. Intrauterine infusion and hysteroscopic injection of autologous platelet-rich plasma for patients with a persistent thin endometrium: A prospective case-control study. J. Clin. Med. https://doi.org/10.3390/jcm13102838 (2024).
Efendieva, Z. et al. Hysteroscopic injections of autologous endometrial cells and platelet-rich plasma in patients with thin endometrium: a pilot randomized study. Sci. Rep. 13, 945. https://doi.org/10.1038/s41598-023-27982-w (2023).
Amable, P. R. et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4, 67. https://doi.org/10.1186/scrt218 (2013).
Russell, S. J., Kwok, Y. S. S., Nguyen, T. T. N. & Librach, C. Autologous platelet-rich plasma improves the endometrial thickness and live birth rate in patients with recurrent implantation failure and thin endometrium. J. Assist. Reprod. Genet. 39, 1305–1312. https://doi.org/10.1007/s10815-022-02505-0 (2022).
Agarwal, M. et al. Management of a thin endometrium by hysteroscopic instillation of platelet-rich plasma into the endomyometrial junction: A pilot study. J. Clin. Med. https://doi.org/10.3390/jcm9092795 (2020).
de Miguel-Gomez, L. et al. Comparison of different sources of platelet-rich plasma as treatment option for infertility-causing endometrial pathologies. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2020.07.053 (2020).
Zadehmodarres, S., Salehpour, S., Saharkhiz, N. & Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist. Reprod. 21, 54–56. https://doi.org/10.5935/1518-0557.20170013 (2017).
Shrivastava, J., More, A., Shrivastava, V., Choudhary, N. & Shrivastava, D. Enhancement of ovarian reserve and oocyte quality after platelet-rich plasma instillation in a woman with diminished anti-Mullerian hormone. Cureus 16, e53474. https://doi.org/10.7759/cureus.53474 (2024).
Najafian, A., Alyasin, A., Aghahosseini, M., Hosseinimousa, S. & Kazemi, S. N. Beneficial effects of intraovarian injection of platelet-rich plasma in women with poor ovarian response. Clin. Exp. Reprod. Med. 50, 285–291. https://doi.org/10.5653/cerm.2023.06086 (2023).
Fattahi Meybodi, N., Eftekhar, M. & Gandom, B. Intrauterine autologous platelet-rich plasma treatment in women with at least two implantation failures: A retrospective cohort study. Int. J. Reprod. Biomed. 22, 9–16. https://doi.org/10.18502/ijrm.v22i1.15236 (2024).
Safarova, S. et al. Does platelet-rich plasma treatment increase in vitro fertilization (IVF) success in the infertile population?. Cureus 15, e47239. https://doi.org/10.7759/cureus.47239 (2023).
Barrenetxea, G. et al. Intraovarian platelet-rich plasma injection and IVF outcomes in patients with poor ovarian response: a double-blind randomized controlled trial. Hum. Reprod. 39, 760–769. https://doi.org/10.1093/humrep/deae038 (2024).
Farimani, M., Heshmati, S., Poorolajal, J. & Bahmanzadeh, M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol. Biol. Rep. 46, 1611–1616. https://doi.org/10.1007/s11033-019-04609-w (2019).
Atkinson, L., Martin, F. & Sturmey, R. G. Intraovarian injection of platelet-rich plasma in assisted reproduction: Too much too soon?. Hum. Reprod. 36, 1737–1750. https://doi.org/10.1093/humrep/deab106 (2021).
Hosseini, L. et al. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Biomed. Online 35, 343–350. https://doi.org/10.1016/j.rbmo.2017.04.007 (2017).
Pantos, K. et al. Ovarian rejuvenation and folliculogenesis reactivation in perimenopausal women after autologous platelet-rich plasma treatment. In: Abstracts, ESHRE 32nd Annual Meeting, pp. 3–6 (2016).
Pantos, K. et al. A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Transplant. 28, 1333–1340. https://doi.org/10.1177/0963689719859539 (2019).
Sills, E. S., Rickers, N. S., Li, X. & Palermo, G. D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 34, 756–760. https://doi.org/10.1080/09513590.2018.1445219 (2018).
Melo, P., Navarro, C., Jones, C., Coward, K. & Coleman, L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J. Assist. Reprod. Genet. 37, 855–863. https://doi.org/10.1007/s10815-020-01710-z (2020).
Barad, D. H., Albertini, D. F., Molinari, E. & Gleicher, N. Preliminary report of intraovarian injections of autologous platelet-rich plasma (PRP) in extremely poor prognosis patients with only oocyte donation as alternative: a prospective cohort study. Hum. Reprod. Open https://doi.org/10.1093/hropen/hoac027 (2022).
Herlihy, N. S. et al. Effect of intraovarian platelet-rich plasma injection on IVF outcomes in women with poor ovarian response: The PROVA randomized controlled trial. Hum. Reprod. (Oxford, England) https://doi.org/10.1093/humrep/deae093 (2024).
Gardner, D. K., Lane, M., Stevens, J., Schlenker, T. & Schoolcraft, W. B. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril. 73, 1155–1158. https://doi.org/10.1016/s0015-0282(00)00518-5 (2000).
Hsieh, C.-E. et al. Early blastulation (EB) of day 4 embryo is predictive of outcomes in single embryo transfer (SET) cycles. Taiwan. J. Obstet. Gynecol. 57, 705–708. https://doi.org/10.1016/j.tjog.2018.08.016 (2018).
Lee, C. I. et al. Embryo morphokinetics is potentially associated with clinical outcomes of single-embryo transfers in preimplantation genetic testing for aneuploidy cycles. Reprod. Biomed. Online 39, 569–579. https://doi.org/10.1016/j.rbmo.2019.05.020 (2019).
Munne, S. et al. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum. Reprod. (Oxford, England) 32, 743–749. https://doi.org/10.1093/humrep/dex031 (2017).
Desai, N., Goldberg, J. M., Austin, C. & Falcone, T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy?. Fertil. Steril. 109, 665–674. https://doi.org/10.1016/j.fertnstert.2017.12.025 (2018).
Munne, S. & Wells, D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil. Steril. 107, 1085–1091. https://doi.org/10.1016/j.fertnstert.2017.03.024 (2017).
Chen, H. H. et al. Optimal timing of blastocyst vitrification after trophectoderm biopsy for preimplantation genetic screening. PLoS One 12, e0185747. https://doi.org/10.1371/journal.pone.0185747 (2017).
Funding
This study was generously supported by a grant from the National Science and Technology Council of Taiwan (MOST 110-2314-B-040-005-).
Author information
Authors and Affiliations
Contributions
T.N.Y.: literature review, clinical procedures, results interpretation, manuscript design, manuscript writing, manuscript edition. M.J.C.: results interpretation, manuscript edition. T.H.L.: study design, results interpretation. Y.C.C.: enrollment, clinical procedures, data collection, statistical analysis. E.H.C.: study design, results interpretation. C.C.H.: study design, clinical procedures. C.I.C.: clinical procedures. C.I.L.: study design. M.S.L.: study design, results interpretation. P.Y.L.: literature review, study design, clinical procedures, results interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, TN., Chen, MJ., Lee, TH. et al. Intraovarian platelet-rich plasma injection significantly improves blastocyst yield and quality in IVF patients. Sci Rep 15, 1301 (2025). https://doi.org/10.1038/s41598-024-82630-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82630-1
Keywords
This article is cited by
-
Intraovarian platelet-rich plasma (PRP) infusion appeared to benefit low prognosis IVF patients; however, when compared to controls, no significant benefit could be confirmed
Journal of Assisted Reproduction and Genetics (2025)