Abstract
Ice wine is produced from concentrated grape juice obtained by the natural freezing and pressing of grapes. The high sugar content of this juice has an impact on fermentation. To investigate the impact of the initial sugar concentration on the fermentation of ice wine, the initial sugar concentration of Vidal ice grape juice was adjusted to 370, 450, 500 and 550 g/L by the addition of glucose. The fermentation process was conducted at a temperature of 18 °C with Zymaflore ST yeast. To ensure optimal fermentation, the yeast assimilable nitrogen (YAN) in the grape juice was adjusted to 350 mg/L using a commercial nitrogen source. The initial sugar concentrations (370, 450, 500, and 550 g/L) were significantly negatively correlated with the number of viable yeast during active fermentation, the total consumption of sugar and YAN, and ethanol production (130.26, 117.83, 106.14, and 90.85 g/L) but positively correlated with the production of acetic acid (0.82–2.86 g/L) and acetaldehyde. Fermentation with initial sugar concentrations ≥ 500 g/L (500 and 550 g/L) produced ethanol (106.14 and 90.85 g/L) and excessive acetic acid (2.14 and 2.86 g/L), inhibited the esterification of n-butanol and n-hexanol, and promoted the production of isopentanol, isoamyl acetate, diethyl succinate, ethyl hexanoate and ethyl octanoate while increasing the production of 2-phenylethanol and isobutanol. The initial sugar concentration affected the production of glycerol, isobutanol, ethyl acetate, and diethyl succinate, but it did not affect the final contents of the ice wine. This study examined fermentation dynamics, including substrate consumption, yeast cell production, and the production of important metabolites such as ethanol, acetic acid, higher alcohols, and esters. Appropriate initial concentrations of YAN and sugar (below 500 g/L) and a relatively high ratio of glucose to fructose (between 1:1 and 1.7:1) is preferable for effective ice wine fermentation.
Similar content being viewed by others
Introduction
The growth and metabolism of yeast in wine fermentation are affected by a variety of compounds present in grape juice, which ultimately affect the composition and quality of the wine. Yeast converts sugar from grapes into alcohol and relies on the materials in grapes to complete their growth and reproduction before declining. Therefore, the sugar content directly affects the ability of yeast to reproduce and transform. If the sugar level in the raw grapes is too low, the resulting wine may not meet the quality requirements for alcohol content and may not be suitable for storage. Ice wine is produced from naturally frozen grapes, in which chemical components such as sugars, acids, aromas, and flavor compounds are concentrated, leading to a rich, balanced, and intense flavor1,2,3.
The sugar concentration in the grape juice used to make ice wine varies with cultivar, viticulture conditions, the weather after grapes reach maturity, the treatment of grapes after harvest, and so on4,5. Ice wine fermentation is usually performed with high sugar concentrations ranging from 32 to 50 °Bx6,7,8; the process is usually completed at 28–40 °Bx in industrial ice wine production9. The upper limit of the juice concentration for wine fermentation was predicted to be 52.5 °Bx by Pigeau et al.8; at concentrations greater than this, yeasts no longer ferment sugar to produce ethanol. Wine is produced by the addition of white sugar, resulting in grape juices with varying initial sugar contents. An increase in the initial sugar content from 230 g/L to 450 g/L resulted in a notable prolongation of the fermentation period, extending it from 7 days to 50 days. Additionally, this change reduced the ethanol content from 13.9 to 9%, whereas the volatile acid content increased from 0.25 g/L to 1.6 g/L. Furthermore, the glycerol content notably increased from 8.1 g/L to 11 g/L10.
In ice wine fermentation under high osmotic stress, yeast cells overproduce glycerol, increase the production of acetic acid and titratable acidity, and decrease the accumulation of ethanol and biomass. Additionally, these conditions may result in the production of ice wine with a poor flavor2,11,12. On the other hand, a specific type of ice wine has a unified standard in terms of the contents of sugar, ethanol, glycerol, total acidity, volatile acidity and so on. Therefore, winemakers must modify the composition of the must, including the contents of sugar, nitrogen, or other elements, to a certain extent. Bisson et al.13, proposed that for each 1 °Bx increase in soluble solids above a sugar concentration of 200 g/L, the required YAN should be increased by 25 mg/L. Consequently, an increased level of YAN is necessary during the fermentation process of ice wine. Ice wine fermentation performed at high sugar concentrations and low temperatures often leads to stuck and sluggish fermentation. Measures to prevent potential problems usually involve selecting adequate yeast strains, supplying sufficient nutrition, including nitrogen sources, and lowering the ratio of fructose in the must fermented by Saccharomyces cerevisiae (S. cerevisiae)14,15,16.
To our knowledge, previous investigations on the effects of sugar concentration on ice wine fermentation have focused mainly on sugar consumption, microbial growth, acidity, and limited metabolites, including ethanol, glycerol, and acetic acid, at sugar levels below 46 °Bx8,17,18. However, reports concerning the fermentation dynamics of YAN utilization, as well as the changes in critical volatile compounds at various initial sugar concentrations, are limited.
This study supplied sufficient YAN and different initial sugar concentrations (370–550 g/L) to frozen Vidal grape juice by supplementing the juice with a commercial nitrogen source and glucose to prevent stuck and sluggish fermentation. The fermentation dynamics of yeast growth were investigated in terms of the performance of ice wine fermentation at relatively high initial sugar concentrations; the consumption of reducing sugars and YAN; and the production of ethanol, acetic acid, acetaldehyde, glycerol, and important volatile compounds to provide a reference for the quality control of ice wine fermentation and to determine the extreme sugar concentration of ice wine fermentation.
Materials and methods
Microorganism and inoculum preparation
Active dried Zymaflore ST yeast, provided by Wunvshan Milan Wine Ltd. Co. (Benxi, China), was employed for ice wine fermentation. The dried yeast was activated by gradually increasing the sugar concentration and lowering the temperature. Three grams of dry yeast were rehydrated with 30 mL of 40 °C sterile deionized water for 15 min, activated with 30 mL of twice-diluted ice grape juice at 25 °C for 1 h, and finally activated with 30 mL of ice grape juice at 20 °C for 2 h2.
Grape juice preparation and fermentation
Vidal grapes naturally frozen on vines were collected and squeezed into grape juice by a basket press at the workshop of Wunvshan Milan Wine Ltd. Co. (Benxi, China). The grape juice was stored at -20 °C. Before brewing ice wine, the frozen grape juice was thawed and clarified for 24 h at 4 °C by adding 0.8 g/L bentonite swollen in 50 °C water. The clarified juice with an initial sugar concentration of 370 g/L was used as a control and adjusted to sugar concentrations of 450, 500, and 550 g/L with glucose and deionized water (glucose: fructose ratios of 1:1, 1.43:1, 1.7:1, and 1.97:1 at initial sugar concentrations of 370, 450, 500, and 550 g/L, respectively). All the grape juice mixtures with various initial sugar concentrations were supplemented with a 250 mg/L commercial nitrogen source (Thiazote®; Laffort, France) to yield YAN concentrations of 350 mg/L (carbon: YAN ratios of 100:0.237, 100:0.195, 100:0.175, and 100:0.159 at initial sugar concentrations of 370, 450, 500, and 550 g/L, respectively).
The various ice wine juices prepared as described above were transferred into 1 L glass Bordeaux bottles, with 550 mL of juice in each bottle. In each bottle, potassium metabisulphite (120 mg/L) and pectinase (30 mg/L) were added before 12 mL of the yeast culture activated as above was inoculated (i.e., 0.4 g/L inoculum of the dried yeast), and the solution was mixed well after every addition. Fermentation was conducted in fermentation bottles with airlocks at 17–19 °C for 50 days. The final ice wine was stabilized by adding 320 mg/L potassium metabisulphite and 200 mg/L potassium sorbate.
Fermentation monitoring
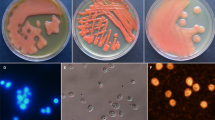
Samples measuring 15 mL were collected every five days during fermentation. The sealed fermentation bottles were shaken at 75 r/min for 3 min prior to aseptic sampling. The viable yeast were counted microscopically using the alkaline methylene blue staining method19. The supernatant was obtained by centrifuging the samples at 11,000 × g, and the product was maintained at -20 °C until further analysis of the wine compound contents.
The reduced sugar concentration was determined using the DNS method20. The glycerol concentration was determined using an enzymatic test kit from Megazyme (Bray, Co. Wicklow, Ireland). The YAN concentration was measured using the formaldehyde method21. The glucose and fructose concentrations were measured via HPLC22.
Gas chromatography
Acetaldehyde, ethyl acetate, ethanol, ethyl butyrate, n-propanol, isobutanol, isoamyl acetate, n-butanol, isopentanol, ethyl hexanoate, ethyl lactate, n-hexanol, ethyl octanoate, acetic acid, ethyl phenylacetate, diethyl succinate, and 2-phenylethanol (in increasing order of retention times) were selected for volatile compound analysis. Static headspace gas chromatography (HS-GC) was employed. A sample (10 mL) and solid NaCl (2 g) were stirred in a 20 mL headspace bottle until the solid NaCl was completely dissolved. An Agilent 7697 A headspace sampling injector was used. Prior to injection, the samples were equilibrated at 90 °C for 30 min. A 2 µL sample was injected into the injection port at 250 °C. The quantitative loop was maintained at 100 °C; the transmit pipe temperature was 110 °C, and the sampling time was 0.5 min.
The volatile compounds were measured on an Agilent 6850 instrument equipped with a flame ionization detector (FID) and DB-FFAP column (30 m × 0.250 mm × 0.25 μm). N2 was used as the carrier gas and flowed at 10 mL/min with a column head pressure of 50 kPa. Air was injected at 300 mL/min, and H2 gas was injected at 30 mL/min. The split ratio was 10:1. The oven was programmed to start at 40 °C for 5 min, increase to 190 °C at 5 °C/min, hold for 1 min at 190 °C, increase to 230 °C at 20 °C/min, and hold for 2 min at 230 °C. The detector was maintained at 250 °C23.
Statistical analysis
All experiments were performed in triplicate, and the values are expressed as the means ± standard deviations from three independent experiments. The significance of the differences was determined by analysis of variance (ANOVA), followed by Duncan’s test with a significance threshold of p < 0.05. The data were analyzed using SPSS version 17.0 (IBM Corp., USA).
Results and discussion
Effect of the initial sugar concentration on the quantity of viable yeast during ice wine fermentation
Following the inoculation of yeast into ice grape juice, the number of active yeasts decreases rapidly. In the 27 days preceding fermentation, the higher the initial sugar concentration was, the fewer active yeasts were present during the fermentation process (Fig. 1). This occurred because the yeast had just been inoculated into a new environment with high osmotic stress and a low temperature. In the groups with relatively low sugar concentrations (370 and 450 g/L), the numbers of viable yeast increased after 1 day, reached a maximum on days 5 and 7, respectively, and decreased sharply from days 19 to 30 of fermentation (Fig. 1). In the groups with relatively high sugar concentrations (500 and 550 g/L), the viable yeast quantity increased slowly after 5 days, reached a maximum on day 11 and day 15, respectively, and then decreased slowly (Fig. 1). These findings indicated that when the concentration of sugar was ≥ 500 g/L, the growth of yeast was significantly impeded.
The first decline at the beginning of the fermentation period and the increase after the lag phase and the second decline after the maximal viable yeast quantity was reached were linearly correlated with the initial sugar concentration; the former was positively correlated, and the latter two were negatively correlated, with statistically significant differences (y = 0.003x − 0.9621, p < 0.05, R2 = 0.9716). The final viable yeast quantity increased with increasing initial sugar concentration below 500 g/L. The difference between the maximal and the final viable yeast quantity for each group decreased with increasing initial sugar concentration. The maximal viable yeast quantities were linearly negatively correlated with the initial sugar concentration (y = − 0.0137x + 8.3438, p < 0.05, R2 = 0.9903). The maximum and minimum values of the quantity of viable yeast at 370 g/L initial sugar were the greatest and the lowest, respectively, among all the groups.
The stronger inhibition of yeast growth in the early stage caused a more extended lag phase and a lower maximum at higher initial sugar concentrations. The earlier, faster, and even greater growth of yeast at lower initial sugar concentrations quickly consumed the dissolved oxygen and nutrients in the ice wine juice, resulting in the rapid production of more ethanol (Fig. 3A), which caused a faster and more significant decrease in viable yeast quantities.
Effect of the initial sugar concentration on the reducing sugar and assimilable nitrogen contents during ice wine fermentation
To produce ice wine, the concentration of sugars in grape juice must exceed 320 g/L. The initial sugar concentration of grape juice is dependent on the harvesting time and grape variety. In the groups with relatively high initial sugar concentrations, the reducing sugar contents slightly changed within the first 5 days, decreased significantly from day 5 to day 19 and stabilized after 19 days (Fig. 2A). In the groups with lower sugar concentrations, the reduced sugar content decreased significantly from day 1 to day 23 and stabilized after 23 days (Fig. 2A). The total sugar consumption had a linear negative correlation with the initial sugar concentration (y = − 0.2827x + 174.48, p < 0.05, R2 = 0.988). Furthermore, the difference between the final residual sugar contents at various initial sugar concentrations was significant (p < 0.05) and was 1.4- and 2.5-fold greater than the difference between the corresponding initial sugar concentrations. The results indicated that the stronger inhibition of yeast metabolism by higher sugar concentrations delayed and slowed sugar consumption. The greater the initial sugar concentration is, the greater the final residual sugar content (110, 234, 332, and 434 g/L at initial sugar concentrations of 370, 450, 500, and 550 g/L, respectively), deviating from the standard sugar content in ice wine (140–320 g/L) at initial sugar concentrations of 370 and 550 g/L9.
The contents of reducing sugars and assimilable nitrogen during ice wine fermentation at different initial sugar concentrations. (A) Reducing sugars, (B) assimilable nitrogen; ■, initial sugar concentration of 370 g/L; ●, initial sugar concentration of 450 g/L; ▲, initial sugar concentration of 500 g/L; ▼, initial sugar concentration of 550 g/L.
During the fermentation of ice wine, higher sugar content in ice grape juice requires more YAN. We have studied the impact of different nitrogen sources and YAN concentrations on the fermentation of ice wine. The results show that different nitrogen sources used in the production of ice wine lead to differences in yeast concentration, alcohol, and acetic acid, highlighting the importance of small molecule amino nitrogen in YAN for fermentation. When studying YAN contents ranging from 250 to 800 mg/L, it was found that when YAN was below 350 mg/L, yeast growth was inhibited during the early stages of fermentation, and abnormal metabolism occurred in the yeast. As the YAN decreased, the production of higher alcohols, acetic acid, and biogenic amines increased, and there was a significant difference in residual sugar content after fermentation (P < 0.05). When YAN was above 350 mg/L, yeast fermentation proceeded normally, and there was a significant positive correlation between YAN and acetic acid production (P < 0.05). With the increase in YAN content, the production of n-propanol and n-butanol decreased. In contrast, the production of iso-butanol increased, and the content of acetic acid ethyl ester and hexanoic acid ethyl ester decreased. When YAN was between 350 and 500 mg/L, the utilization of carbon sources by yeast did not increase with the YAN content, and there was no significant difference in residual sugar and YAN consumption after fermentation. When YAN was above 500 mg/L, acetic acid production was positively correlated with YAN content. The production was excessive (exceeding the Vintners Quality Alliance VQA standards), with a significant residual of YAN after fermentation, leading to the waste of nitrogen sources and a higher content of biogenic amines in the finished wine, affecting the flavor of the ice wine. The study indicates that in the high-sugar fermentation of ice wine, YAN content positively affects yeast fermentation within a certain range. Still, beyond a certain range, the impact of YAN content on yeast fermentation is minimal and may even have a negative effect. The impact of initial sugar concentration on ice wine fermentation with a YAN content of 350 mg/L was explored (not yet published).
The YAN content initially decreased but subsequently increased faster, and was more pronounced at lower initial sugar concentrations (Fig. 2B). The net increase in the YAN content after the decline was negatively correlated with the initial sugar concentration (y = − 0.0166x + 51.942, p > 0.05, R2 = 0.7632). With increasing initial sugar concentration, the YAN contents increased by 21.97%, 16.31%, 16.42%, and 14.11%, respectively. However, the increase in the YAN content followed the decline in the quantity of viable yeast, with the greatest decrease occurring at the initial sugar concentration of 370 g/L, indicating that the increase in the YAN content was probably caused by yeast autolysis. Autolyzed yeast cells release intracellular substances, including peptides and amino acids, which can affect the metabolism of the surviving yeast cells and the flavors of the wine24. Most previous studies on the influence of the initial sugar concentration on wine fermentation did not examine YAN utilization in detail. In this study, the YAN content initially decreased due to consumption for the growth and metabolism of yeast and then increased, probably due to cell autolysis caused by the depletion of dissolved oxygen and accumulation of ethanol together with high osmotic stress. The final YAN content was linearly positively correlated with the initial sugar concentration and negatively correlated with the maximal viable yeast quantity. The consumption rate of YAN was negatively correlated with the initial sugar concentration (y = − 0.1399x + 90.349, p < 0.05, R2 = 0.9915).
The residual YAN contents in all the groups were above 250 mg/L and even exceeded 300 mg/L at initial sugar concentrations of 500 and 550 g/L (Fig. 2B); moreover, a large quantity of reducing sugars also remained, indicating that sufficient available YAN could not completely overcome the inhibition of yeast growth and metabolism at high sugar levels, which is in agreement with the findings of a previous study15. Generally, the minimum YAN content for yeast to reliably complete alcoholic fermentation is 140 mg/L, depending on the sugar concentration in must. Although a must with a high sugar concentration typically requires a large amount of nitrogen25, the ratio of the initial YAN content relative to the carbon source should be appropriate. Excess nitrogen can cause the production of undesired metabolites and a high residual YAN content in wine, which increases the danger of microbial contamination during later processing15,25. Therefore, the supplementation of YAN in must with a relatively low concentration of YAN should be appropriately controlled; moreover, it is worthwhile to measure the levels of nitrogen-containing metabolites such as biogenic amines and ethyl carbamate in ice wine fermented from must with a high nitrogen concentration.
Effect of initial sugar concentration on ethanol and acetaldehyde contents during ice wine fermentation
During fermentation, there is a noticeable difference in ethanol production at different sugar concentrations. During the first 25 days of fermentation, the rate of ethanol production by yeast was relatively fast, and the amount of ethanol generated decreased with increasing sugar concentration (Fig. 3A). The ethanol content increased faster in the first 12 days to maxima of 130.26 g/L and 121.77 g/L at initial sugar concentrations of 370 and 450 g/L, respectively, but increased in the first 17 days to maxima of 106.14 g/L and 90.85 g/L at initial sugar concentrations of 500 and 550 g/L, respectively (Fig. 3A). The ethanol content continuously increased after 17 days at initial sugar contents of 450, 500 and 550 g/L. The ethanol content significantly decreased with the increase in the initial sugar concentration throughout fermentation (y = 0.0016x − 0.1275, p < 0.05, R2 = 0.9372). The final ethanol content was linearly negatively correlated with ice wine fermentation at initial sugar concentrations greater than 370 g/L (y = − 0.2199x + 215.06, p < 0.05, R2 = 0.9389), which is consistent with the results of Pigeau et al.8. Both the ethanol production per gram of sugar consumed and the ethanol production per gram of YAN consumed were linearly positively correlated with the initial sugar concentration (y = 0.0047x − 0.8073, p < 0.05, R2 = 0.9991). Moreover, the maximal ethanol production was also positively correlated with the maximal viable yeast quantity (y = 15.475x + 82.504, p > 0.05, R2 = 0.8859).
In an earlier study on ice wine fermentation of Riesling juices8, the initial juice concentration showed a strong linear negative relationship with yeast growth, and the yeast population exhibited a slower increase for approximately 10 days in the earlier stage of fermentation, with no sharp decrease after the longer stationary phase. In addition, the ethanol production per gram of sugar consumed was not influenced by the initial sugar concentration, but the final ethanol content was lower than our results. The authors predicted that the sugar concentration that can no longer be fermented by yeast should be 52.5 °Bx. In our current study, the inoculated strain S. cerevisiae Zymaflore ST grew more rapidly, but the viable yeast quantity was significantly lower by approximately threefold, and the reducing sugars were consumed faster and more rapidly than those in the Riesling ice wine fermentation at the equivalent sugar concentrations. Furthermore, a certain amount of ethanol was produced even at an initial sugar concentration of 550 g/L. Compared with that of the wine fermented from Sauvignon and Semillon must with 350 g sugar/L and 190 mg N/L26, the cell density of S. cerevisiae Zymaflore ST in the present study reached a greater maximum later and was maintained for a shorter duration.
Glucose is generally depleted first by S. cerevisiae, and there is more fructose than glucose in the residual sugar in wine fermented with equal initial concentrations of glucose and fructose14. Riesling ice wine juice with a sugar concentration ranging from 40 to 46 °Bx was prepared by diluting concentrated juice, in which the nutrients were also diluted with the decreasing sugar concentration, and very similar amounts of glucose and fructose were present. In contrast, the ice wine juices with initial sugar contents ranging from 370 to 550 g/L and an equal initial YAN of 350 mg/L used in the present study were prepared by the addition of glucose and a commercial nitrogen source in the original ice wine juice with 370 g/L reducing sugar and 100 mg/L YAN. Perhaps, to a certain extent, the yeast growth and ethanol production in the current study can be explained by the sufficient amount of nitrogen and relatively high glucose content.
The contents of ethanol and acetaldehyde during ice wine fermentation at different initial sugar concentrations. (A) Ethanol, (B) acetaldehyde; ■, initial sugar concentration of 370 g/L; ●, initial sugar concentration of 450 g/L; ▲, initial sugar concentration of 500 g/L; ▼, initial sugar concentration of 550 g/L.
The change in acetaldehyde content showed a reverse trend to that of ethanol during fermentation. The acetaldehyde content initially rapidly increased, subsequently decreased, and finally stabilized, significantly differing across all the groups (Fig. 3B) (y = 0.1481x − 18.321, p < 0.05, R2 = 0.9855). The acetaldehyde content reached a maximum earlier with faster yeast growth at lower initial sugar contents of 370 and 450 g/L but reached a maximum later with a longer lag phase of yeast growth at higher initial sugar contents of 500 and 550 g/L. The maximal and final acetaldehyde contents were positively correlated with the initial sugar concentration (maximal, y = 0.0042x − 1.1082; p < 0.05; R2 = 0.9669). Finally, y = 0.0022x − 0.7288, p < 0.05, R2 = 0.9185) but there is a negative correlation with the maximal viable yeast quantity (y = − 6.2937x + 168.41, p > 0.05, R2 = 0.8794). Both the acetaldehyde production per gram of sugar consumed and the acetaldehyde production per gram of YAN consumed were linearly positively correlated with the initial sugar concentration (y = 0.0107x − 3.0513, p < 0.05, R2 = 0.9741).
Although the acetaldehyde content significantly increased with the increasing initial sugar level, which might impact yeast growth, ethanol production, and sugar consumption17, lower acetaldehyde production is beneficial for improving the quality and safety of ice wine.
Effect of the initial sugar concentration on glycerol and acetic acid contents during ice wine fermentation
The glycerol content increased greatly from day 12 to day 20 (Fig. 4A). Glycerol was produced after yeast growth and ethanol production, which is consistent with the results of Orlić et al.27. Finally, the maximal glycerol contents at various initial sugar levels were very close to each other, unlike the results of Pigeau et al.8, were negatively correlated with the initial sugar concentration (y = -0.0013x + 13.323, p > 0.05, R2 = 0.6079), and were positively correlated with the maximal viable yeast quantity (y = 0.0988x + 12.505, p > 0.05, R2 = 0.6263). Both the glycerol production per gram of sugar consumed (y = 0.0003x − 0.0781, p < 0.05, R2 = 0.9135) and the glycerol production per gram of YAN consumed (y = 0.0008x − 0.229, p < 0.05, R2 = 0.9612) were linearly positively correlated with the initial sugar concentration. The 370 and 450 g/L groups presented a greater number of viable cells and higher glycerol levels. However, at concentrations exceeding 500 g/L, yeast growth was inhibited, and glycerol production decreased.
The contents of glycerol and acetic acid during ice wine fermentation at different initial sugar concentrations. (A) Glycerol, (B) acetic acid; ■, initial sugar concentration of 370 g/L; ●, initial sugar concentration of 450 g/L; ▲, initial sugar concentration of 500 g/L; ▼, initial sugar concentration of 550 g/L.
As one of the major byproducts of ethanol fermentation of wine, glycerol provides wine with positive sensory attributes, slight sweetness and fullness12. The glycerol concentration in wine ranges from 1 to 11 g/L, typically ranging from 4 to 9 g/L17,27. A much greater quantity of glycerol is produced under osmotic stress than under normal conditions to maintain redox balance and prevent cell dehydration1,8,17. In the present study, the final glycerol contents did not exceed 13 g/L in any of the groups and were generally lower than those in some similar wine fermentations with higher sugar concentrations8,17,26, which was also related to glucose supplementation and higher YAN concentrations.
In high-sugar fermentation, the accumulation of glycerol results in a shortage of NADH. Acetaldehyde is converted into acetic acid, while NAD+ is reduced to NADH to restore the redox balance14,16,28. The increase in acetic acid content during the first 4 days was negatively related to the initial sugar concentration, with no significant differences between the groups (Fig. 4B). After 12 days, the higher the initial sugar concentration was, the greater the acetic acid content was. The acetic acid content increased faster after 17 days, except for the group with an initial sugar concentration of 370 g/L, which remained almost stable after 23 days and differed significantly between the groups (Fig. 4B) (y = 0.0001x − 0.0424, p < 0.05, R2 = 0.9225). The final acetic acid content was linearly positively correlated with the initial sugar concentration (y = 0.0115x − 3.4698, p < 0.05, R2 = 0.9936) but negatively correlated with the initial sugar concentration in the early stage. Finally, the maximal acetic acid content was linearly negatively correlated with the maximal viable yeast quantity (y = − 0.8193x + 3.46, p < 0.05, R2 = 0.9684), consistent with what was reported by Bely et al.26, which implied that increasing the consumption of dissolved oxygen for greater yeast growth results in less acetic acid production by oxidation. Both the acetic acid production per gram of sugar consumed and the acetic acid production per gram of YAN consumed were linearly positively correlated with the initial sugar concentration (y = 0.0003x − 0.1, p < 0.05, R2 = 0.9225). The acetic acid production per gram of sugar consumed was linearly positively correlated with the glycerol production per gram of sugar consumed (y = 0.3562x − 0.0142, p < 0.05, R2 = 0.9935), which is consistent with earlier findings8. The final acetic acid contents of 2.14 and 2.85 g/L at initial sugar concentrations of 500 and 550 g/L, respectively, exceeded the international standard of 0.8–1.5 g/L for ice wine products9. However, the greater amount of residual sugar and glycerol maintained the organoleptic balance of the ice wine, at least for fermentation with 500 g/L initial sugar.
Effect of the initial sugar concentration on the content of higher alcohols and esters during ice wine fermentation
The contents of n-butanol and n-hexanol decreased throughout fermentation at initial sugar contents of 370 and 450 g/L and increased to a certain extent at initial sugar contents of 500 and 550 g/L. The contents of other higher alcohols, except n-butanol and n-hexanol, initially increased but then stabilized or decreased somewhat, and their increase was negatively related to the initial sugar concentration. The final contents of 2-phenyl ethanol, n-butanol, and n-hexanol were positively related to the initial sugar concentration (Fig. 5A-F).
Higher alcohol contents during ice wine fermentation at different initial sugar concentrations. (A) Isobutanol, (B) isopentanol, (C) n-propanol, (D) n-hexanol, (E) 2-phenylethanol, (F) butanol; ■, initial sugar concentration of 370 g/L; ●, initial sugar concentration of 450 g/L; ▲, initial sugar concentration of 500 g/L; ▼, initial sugar concentration of 550 g/L.
The contents of isopentanol, n-propanol, and isobutanol in all the groups were greater than those of the other higher alcohols, accounting for 41–51%, 26–31%, and 18–21% of the total higher alcohols, respectively (Fig. 5A‒C). The three higher alcohols were produced faster at the early stage, concomitantly with ethanol. There were no significant differences in the contents of isobutanol, isopentanol, or n-propanol among the groups with initial sugar contents of 450, 500, and 550 g/L (p > 0.05). At the initial sugar concentration of 370 g/L, the contents of isobutanol and n-propanol were significantly lower, whereas the isopentanol content was significantly greater than that of the other groups (p < 0.05).
These results indicated that n-butanol and n-hexanol in the final ice wine were derived mainly from the grapes and could be converted into other products during fermentation. In contrast, n-propanol, isobutanol, isopentanol, and 2-phenylethanol were produced mainly by fermentation, and only 2-phenylethanol was produced during the late fermentation stage. The conversion of n-butanol and n-hexanol in the ice wine juice was greatly inhibited, whereas the production of 2-phenylethanol was significantly improved by increasing the initial sugar concentration (500 and 550 g/L). High initial sugar concentrations (≥ 450 g/L) increased isobutanol production and inhibited isopentanol production.
During the entire process of ice wine fermentation, the content of esters decreased with increasing initial sugar concentration (Fig. 6A-F). The contents of ethyl octanoate (Fig. 6B), diethyl succinate (Fig. 6D), and isoamyl acetate (Fig. 6E) increased in the early stage but decreased in the late stage, whereas the contents of the other esters increased more slowly at initial sugar concentrations of 370 and 450 g/L. The concentrations of almost all the esters were lower at initial sugar concentrations of 500 and 550 g/L than at lower initial sugar concentrations, among which the contents of isoamyl acetate, diethyl succinate, ethyl hexanoate, and ethyl octanoate slightly changed during fermentation. Compared with those of the other esters, the contents of ethyl butyrate (Fig. 6A) and ethyl acetate increased significantly after 17 days (Fig. 6F). Significant differences were observed in the final contents of ethyl hexanoate (Fig. 6C), diethyl succinate, and isoamyl acetate in all the groups. The differences in the contents of ethyl butyrate between the groups with lower and higher sugar concentrations were statistically significant (p < 0.05). No ethyl lactate or ethyl phenylacetate was detected.
Ester contents during ice wine fermentation at different initial sugar concentrations. (A) ethyl butyrate, (B) ethyl octanoate, (C) ethyl hexanoate, (D) diethyl succinate, (E) isoamyl acetate, (F) ethyl acetate; ■, initial sugar concentration of 370 g/L; ●, initial sugar concentration of 450 g/L; ▲, initial sugar concentration of 500 g/L; ▼, initial sugar concentration of 550 g/L.
The results indicated that the production of isoamyl acetate, diethyl succinate, ethyl hexanoate and ethyl octanoate was strongly inhibited by higher initial sugar concentrations (500 and 550 g/L; Fig. 6). The ethyl hexanoate, diethyl succinate, and ethyl octanoate in the final ice wine were mainly introduced by the grapes as raw materials, although they were also produced to a certain extent during processing and in the early stage of fermentation. In contrast, fermentation mainly produced isoamyl acetate, ethyl butyrate, and ethyl acetate. The ethyl acetate content accounted for more than 90% of all esters, which was related to the high contents of ethanol and acetic acid. Isoamyl acetate production depends on the production of isopentanol and acetic acid and is especially limited by isopentanol29.
The higher yield of ethanol in the present study than in previous studies can be explained by the excess YAN at lower initial sugar concentrations, the lower fructose-to-glucose ratio at higher initial sugar concentrations, and the different compositions of the utilized grape juices. The specific reasons merit further detailed investigation. The results indicated that ethanol was produced in advance of glycerol, acetic acid, and acetaldehyde; the lower production of glycerol, acetic acid, and acetaldehyde resulted from a higher ethanol yield. After the amount of consumed sugar or YAN was normalized, the production of ethanol, acetaldehyde, acetic acid, and glycerol was linearly positively correlated with the initial sugar concentration (p < 0.05). However, the maximal yeast biomass was negatively correlated with the initial sugar concentration (y = -0.0002x + 0.1789, p > 0.05, R2 = 0.9815). It can be deduced that sufficient and appropriate nitrogen and supplementation with preferable sugars contribute to biomass production and maintain cell activity for a long time at high sugar concentrations, thereby promoting ethanol production and preventing sluggish fermentation.
The final ice wines fermented for 50 days under different initial sugar concentrations presented significant differences in the contents of residual sugar, YAN, ethanol, acetic acid, acetaldehyde, 2-phenylethanol, n-propanol, n-butanol, n-hexanol, ethyl butyrate, ethyl hexanoate, isoamyl acetate, and ethyl octanoate (p < 0.05). The final ice wine produced with 370 g/L initial sugar had an acrid taste due to its high ethanol content and low residual sugar content, whereas the wine produced with 550 g/L initial sugar was obviously dark, cloying, and unbalanced in taste owing to an increased content of residual sugar and residual YAN. The initial sugar concentration affected the production of glycerol, isobutanol, and ethyl acetate, as well as the conversion of diethyl succinate. Nevertheless, it did not affect the final contents of the ice wine.
Conclusions
The current research indicates that when the sugar concentration in ice grape juice is 500 and 550 g/L, yeast growth and reproduction are inhibited by high osmotic pressure, which leads to slow cell growth, slow fermentation initiation, low consumption of assimilable nitrogen, high residual sugar, and lower production of ester compounds. Additionally, elevated sugar concentrations induce aberrant yeast metabolism, leading to increased acetic acid production, with levels reaching 2.14 and 2.85 g/L. The groups with sugar concentrations of 370 and 450 g/L demonstrated comparable yeast growth and reproduction, as well as similar higher alcohol and ester contents following fermentation, with no notable differences. Nevertheless, the group with a sugar concentration of 370 g/L presented elevated levels of glycerol and ethanol, accompanied by a diminished acetic acid content. The production of ice wine via fermentation at various sugar concentrations has demonstrated that as the concentration of sugar increases, the color of the wine becomes more intense. The high sugar concentration groups (500 and 550 g/L) presented pronounced sweetness; however, the flavor profile was imbalanced, and the aftertaste was deficient. In contrast, the low sugar concentration groups (370 and 450 g/L) produced ice wine that was clear, pleasant tasting, and well balanced, and had a superior aftertaste. Therefore, the initial sugar concentration for ice wine fermentation should be controlled below 500 g/L, which is beneficial for initiating yeast fermentation and improving the ice wine quality.
Data availability
The authors declare that all data supporting the fndings of this study are available within the paper. Moreover, the datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Erasmus, D., Vandermerwe, G. & Vanvuuren, H. Genome-wide expression analyses: metabolic adaptation of to high sugar stress. FEMS Yeast Res. 3, 375–399. https://doi.org/10.1016/s1567-1356(02)00203-9 (2003).
Kontkanen, D. et al. Effect of yeast inoculation rate, acclimatization, and nutrient addition on icewine fermentation. Am. J. Enol. Viticult. 55 (4), 363–370 (2004).
Ma, Y., Xu, Y. & Tang, K. Aroma of icewine: A review on how environmental, viticultural, and oenological factors affect the aroma of icewine. J. Agric. Food Chem. 69, 6943–6957. https://doi.org/10.1021/acs.jafc.1c01958 (2021).
Boss, P. K., Böttcher, C. & Davies, C. Various influences of harvest date and fruit sugar content on different wine flavor and aroma compounds. Am. J. Enol. Viticult. 65, 341–353. https://doi.org/10.5344/ajev.2014.13137 (2014).
Lutskova, V. & Martirosyan, I. Influence of harvest date and grape variety on sensory attributes and aroma compounds in experimental icewines of Ukraine. Fermentation 7 https://doi.org/10.3390/fermentation7010007 (2021).
Hong, M. et al. Impact of mixed non-saccharomyces yeast during fermentation on volatile aroma compounds of Vidal blanc icewine. Lwt 145, 111342. https://doi.org/10.1016/j.lwt.2021.111342 (2021).
Childs, B. C., Bohlscheid, J. C. & Edwards, C. G. Impact of available nitrogen and sugar concentration in musts on alcoholic fermentation and subsequent wine spoilage by Brettanomyces bruxellensis. Food Microbiol. 46, 604–609. https://doi.org/10.1016/j.fm.2014.10.006 (2015).
Pigeau, G. M. et al. Concentration effect of Riesling icewine juice on yeast performance and wine acidity. J. Appl. Microbiol. 103, 1691–1698. https://doi.org/10.1111/j.1365-2672.2007.03397.x (2007).
Grumezescu, A. M. & Holban, A. M. Advances in Biotechnology for Food Industry. Vol. 14180-181 (Academic, 2018).
Pei Guangren, L. et al. Influencing factors of high content volatile acid in icewine. Food Ferment. Ind. 40 (03), 58–62. https://doi.org/10.13995/j.cnki.11-1802/ts.2014.03.022 (2014).
Malacrinò, P. et al. The vinification of partially dried grapes: A comparative fermentation study of Saccharomyces cerevisiae strains under high sugar stress. Lett. Appl. Microbiol. 40, 466–472. https://doi.org/10.1111/j.1472-765X.2005.01713.x (2005).
Remize, F. et al. Glycerol overproduction by engineered saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 65 (1), 143–149 (1999).
Bisson, L. F. & Butzke, C. E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Viticult. 51 (2), 168–177 (2000).
Heit, C. et al. Osmoadaptation of wine yeast (Saccharomyces cerevisiae) during icewine fermentation leads to high levels of acetic acid. J. Appl. Microbiol. 124, 1506–1520. https://doi.org/10.1111/jam.13733 (2018).
Lleixa, J. et al. Saccharomyces and non-saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 7 https://doi.org/10.3389/fmicb.2016.01959 (2016).
Yang, F., Heit, C. & Inglis, D. L. Cytosolic redox status of wine yeast (Saccharomyces cerevisiae) under hyperosmotic stress during icewine fermentation. Fermentation 3 https://doi.org/10.3390/fermentation3040061 (2017).
Liu, S. Q. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 35 (1), 49–61 (2000).
Cojocaru, G. A. A. A. O. Optimization of the alcoholic fermentation by correlating the initial sugar concentration with the inoculum size of yeasts and assimilable nitrogen requirements. Sci. Pap. 171–180 (2014).
Sami, M., Ikeda, M. & Yabuuchi, S. Evaluation of the alkaline methylene blue staining method for yeast activity determination. J. Ferment. Bioeng. 78 (3), 212–216 (1994).
Negrulescu, A. & Mincea, P. V. Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 23, 2176–2182 (2012).
Baroň, M. Yeast assimilable nitrogen in South Moravian grape musts and its effect on acetic acid production during fermentation. Czech J. Food Sci. 29, 603–609. https://doi.org/10.17221/259/2010-cjfs (2011).
Yeganeh-Zare, S., Farhadi, K. & Amiri, S. Rapid detection of apple juice concentrate adulteration with date concentrate, fructose and glucose syrup using HPLC-RID incorporated with chemometric tools. Food Chem. 370 https://doi.org/10.1016/j.foodchem.2021.131015 (2022).
Ma, Y. et al. Characterization of the key aroma compounds in Chinese Vidal icewine by gas chromatography–olfactometry, quantitative measurements, aroma recombination, and omission tests. J. Agric. Food Chem. 65, 394–401. https://doi.org/10.1021/acs.jafc.6b04509 (2017).
Perrot, L. & Charpentier, C. M. Yeast adapted to wine: Nitrogen compounds released during induced autolysis in a model wine. J. Ind. Microbiol. Biotechnol. 29 (3), 134–139 (2002).
Martínez-Moreno, R. et al. Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 12, 477–485. https://doi.org/10.1111/j.1567-1364.2012.00802.x (2012).
Bely, M., Rinaldi, A. & Dubourdieu, D. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 96, 507–512. https://doi.org/10.1016/s1389-1723(04)70141-3 (2003).
Orlić, S. & Huić-Babić, A. L. F. N. A comparative study of the wine fermentation performance of Saccharomyces paradoxus under different nitrogen concentrations and glucose/fructose ratios. J. Appl. Microbiol. 108 (1), 73–80 (2010).
Jing, W. et al. Syntax of referencing. In Advances in Biotechnology for Food Industry (ed. Jing, W.). 267–300 (Academic Press, 2018).
Fermentativa, I. & Vinho, D. The impact of nitrogen on yeast fermentation and wine quality. Ciênc. Téc Vitiv. 26 (1), 17–32 (2011).
Acknowledgements
This study was supported by the Key Laboratory for Resource Plants Protection and Utilization of Yili Valley in Xinjiang (2024HGZD04) and Yili Prefecture Science and Technology Bureau under Grant (YZ2023A05). The authors would like to thank all those who helped with this research.
Author information
Authors and Affiliations
Contributions
Y.Z. and Z.J. designed the study; Y.Z., C.C., T.Z., and X.F. performed experiments; Y.Z. and C.C. analyzed the data; Y.Z., C.C., and Z.J. wrote the manuscript; all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Chang, C.H., Fan, X.H. et al. Effect of the initial glucose concentration on the performance of ice wine fermentation of Vidal grape juice. Sci Rep 14, 31341 (2024). https://doi.org/10.1038/s41598-024-82721-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82721-z