Abstract
The increase of coal seam mining depth leads to the increase of ground temperature stress, which affects the fracture development and spontaneous combustion characteristics of coal samples. Taking anthracite as the research object, scanning electron microscopy, low-temperature N2 adsorption, temperature- programmed experiments and infrared spectroscopy tests were carried out to analyze the mechanism of the influence of pore structure and the number of oxygen-containing functional groups on the spontaneous combustion characteristics of coal samples from the physical and chemical perspectives. The results show that the connection between pores and fractures is enhanced and the scale of micro-fractures is also increased after the thermal and mechanical coupling. After treatment, the oxidation of the coal sample was enhanced, and the overall production rate of the three iconic gases increased. The thermal and mechanical coupling results in the increase of the content of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in coal. The thermal and mechanical coupling effects promote the occurrence and development of coal spontaneous combustion by changing the structure, temperature and stress state of coal and affecting the reaction process of coal and oxygen. The research results have laid a theoretical foundation for the prevention and control of multi-field coupling CSC.
Similar content being viewed by others
Introduction
In the process of coal mining, the coal body is affected by mining and suffers external stress. At the same time, the mining activity changes the ventilation condition of the coal body, thus affecting its heat dissipation1,2,3,4. In addition, the thermal stress generated during spontaneous combustion of coal further affects the pore structure and temperature distribution of coal, forming a heat-force coupling effect, which may have a significant impact on the spontaneous combustion characteristics of coal5,6,7.
A lot of research has been done on the evolution law of coal permeability under the action of thermal and mechanical coupling8,9,10. Liu et al.11 analyzed the interaction between temperature, deformation and matrix-fracture, and then built a characterization model of coal permeability under the coupling of thermal and mechanical forces, and verified the accuracy of the model. Cao12 studied the osmotic evolution of high temperature and high stress coal. In recent years, the thermal and mechanical coupling effect of coal deformation has attracted extensive attention from scholars. Yi et al.13 studied the deformation and permeability change characteristics of coal and rock permeability under the coupling of thermal and mechanical effects. Wang et al.14 studied the thermal deformation characteristics of long-flame coal under two different ground stress conditions, and the results show that the triaxial pressure has a significant influence on the thermal deformation of long-flame coal. The higher the pressure, the higher the temperature point at which thermal expansion deformation begins, and the smaller the coefficient of thermal expansion. Wang et al.15 obtained that the elastic modulus of coal samples decreased significantly with the increase of temperature, and Poisson’s ratio gradually increased. Confining pressure has little effect on Poisson’s ratio of coal samples, and Poisson’s ratio increases slightly with the increase of confining pressure. Feng et al.16 studied the deformation characteristics of anthracite coal under thermal coupling, and the results showed that thermal coupling and pyrolysis gas production were the key factors affecting coal deformation, especially in the high temperature stage, and pyrolysis gas production played a main role in deformation.
The above researches mainly focus on the permeability and deformation of coal under the action of thermal-mechanical coupling, while the researches on the spontaneous combustion characteristics of coal under the action of thermal-mechanical coupling are scarce. Taking anthracite as the research object, scanning electron microscopy, low-temperature N2 adsorption, temperature programmed experiments and infrared spectroscopy tests were carried out to analyze the mechanism of the influence of pore structure and the number of oxygen-containing functional groups on the spontaneous combustion characteristics of coal samples from the physical and chemical perspectives. By analyzing the physical and chemical properties of coal and combining the theory of multi-field coupling, this study provides a more comprehensive theoretical basis for coal spontaneous combustion prevention and control.
Experimental process
Preparation of coal sample
The test coal sample was anthracite. The large lump coal retrieved from the fresh exposed area of the mining face was divided into coal samples with a particle size of 0.83 ~ 1 mm after crushing and screening, and the coal sample was placed in an oven at 50 °C for 12 h for use. Table 1 shows the industrial analysis of coal samples.
The thermal coupling test equipment is a servo-controlled multi-functional high temperature triaxial rock testing machine, which is mainly used to simulate deep high temperature and high pressure rock mass environment, and can carry out thermal-force coupling rock mechanics experiments. The testing machine is composed of a system control platform, a high-temperature triaxial stress chamber and a heating system. In the course of the experiment, the rock sample is heated by heating system, and the rock sample is pressured by triaxial loading system, so as to realize the coupling effect of heat. During the triaxial test, the stress control was carried out at 1 MPa/min cyclic loading to the coal sample, and the test confining pressures were 1.0, 2.0 and 4.0 MPa, respectively. In the seepage test, the axial loading rate is 0.0015 mm/s, confining pressure is 1.0, 2.0 and 4.0 MPa, respectively, and the temperature is raised to 50 °C and 100 °C to prepare the thermodynamically coupled coal sample.
SEM test
Axia ChemiSEM scanning electron microscope was used to test the coal samples under different pretreatment conditions, and the pore distribution characteristics under different conditions were obtained. Scanning electron microscope (SEM) is based on electron beam as light source, through the three-stage electromagnetic lens to do raster scanning on the surface of the material, and obtain the scanning electron image of the material. First, the surface attachments were blown off with the ear wash ball, and then the sample was fixed on the sample table with conductive adhesive17,18,19. After the hatch door was closed and the parameters were adjusted, the electron microscope image with magnification of 10,000× was obtained.
Low-temperature N2 adsorption experiment
The pore structure of coal samples under different pretreatment conditions was measured by ASAP2020 physical adsorption instrument. The low-temperature N2 adsorption experiment is an experimental method based on the equivalent substitution method and tested at the liquid nitrogen saturation temperature of 77 K. The pore structure parameters of coal are obtained by analyzing the N2 isothermal adsorption-desorption curve, and the changes of coal pore structure are quantitatively characterized. First, make sure the equipment is running properly; Then, complete the coal sample weighing, loading, degassing and other pretreatment work; Finally, the pre-treated sample tube is placed in the analysis station for testing20,21,22.
Programmed temperature experiment
The programmed temperature experiment was carried out under the condition of 30 ml/min air flow. Weigh 10 g of the sample into the sample container to ensure that the sample is evenly distributed, and place the sample container into the heating area of the instrument. When the coal sample temperature reaches 40 °C, the temperature control box is set to 1 °C/min. This means that at the beginning of the experiment, the coal sample is first heated to 40 °C and then heated at a rate of 1 °C per minute. During the heating process, the sample response, such as chromatographic peaks, is continuously monitored. In the experiment, when the temperature of the coal sample reaches 40 °C, the oxygen concentration and the indicator gas at the outlet of the coal sample tank are measured by gas chromatograph every 20 °C, and the data are recorded. At the same time, start the data acquisition system to collect temperature parameters. When the temperature reaches the preset final temperature of 200 °C, it is kept for a period of time according to the experiment to ensure that the sample is fully reacted or reaches an equilibrium state.
FTIR test
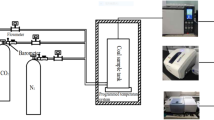
The functional groups of the coal samples were determined by Fourier transform infrared absorption spectrometer Nicolet 6700. During the test, the coal sample was mixed with potassium bromide at the ratio of 1∶100 and fully ground, and the grinding was pressed into a sheet on the tablet press and scanned for detection. The scanning range of the instrument is between 4000 and 400 cm−1, and the resolution is 4 cm−123,24,25. The experimental flow chart is shown in Fig. 1.
Results and discussion
Microscopic surface features
Scanning electron microscopy (SEM) is used to observe the surface microstructure of coal under thermal and mechanical coupling, and the pore type and size can be directly observed. The influence of thermal-mechanical coupling on the surface microstructure of coal is discussed. Figure 2 shows the SEM images of raw coal before and after the thermal-mechanical coupling, with a magnification of 10,000 times.
In SEM images at the same temperature, with the increase of pressure, the observed pores in coal gradually increase and the types are diverse. These include the pores formed by “gas production” and “gas accumulation”, the dissolved pores formed by the dissolution of minerals, and the pores formed by the stress caused by the difference in the hardness of minerals and organic matter. At 50 °C, the shape and size of the pores vary, mainly with approximately elliptical pores, accompanied by a small number of irregularly shaped pores. When entering 100 °C, a small number of elongated cracks can be observed, and the surface morphology is more irregular.
When coal is oxidized, the irregularity of its surface morphology increases significantly. The micropores are further expanded into mesopores, and a large number of micropores are interconnected to form microcracks, so a large number of slits and fissures can be observed. In the process of oxidation, organic matter and inorganic matter on the surface of coal combine with oxygen, and a series of complex physical and chemical changes occur under the action of heat-force coupling. These changes include the pyrolysis of easily broken side chains and bridge bonds, the removal and spillover of water and free alkanes, N2, CO and other compounds in the coal, and depolymerization and polycondensation reactions. These reactions cause the surface structure of coal to collapse and loosen, which in turn produces more microcracks, and the microporous structure is destroyed and transformed into mesoporous structure. The connectivity between pores and fractures is enhanced, and the scale of microfractures also increases significantly. This means that the thermo-mechanical coupling promotes the development of micro-scale pores and fractures in coal.
Pore structure characteristics
The isothermal adsorption curves of each coal sample under the action of thermo-mechanical coupling are shown in Fig. 3. It can be seen that the curve conforms to the isothermal adsorption type IV or V in the IUPAC standard, indicating that the corresponding test material is microporous or mesoporous material26,27,28. The protrusion in the high specific pressure area of the figure is due to pore condensation, and the adsorption and desorption amount of coal sample in the high specific pressure area is higher than that of raw coal under the action of thermal and mechanical coupling. The overall adsorption capacity of 7 coal samples: 4 MPa (100 °C) coal sample > 2 MPa (100 °C) coal sample > 1 MPa (100 °C) coal sample > 4 MPa (50 °C) coal sample > 2 MPa (50 °C) coal sample > 1 MPa (50 °C) coal sample > Raw coal, it can be seen that the thermal and mechanical coupling treatment of coal sample changes the pore structure of raw coal, resulting in changes in adsorption capacity. It can be seen that raw coal in goaf will increase the degree of spontaneous combustion after thermal-mechanical coupling.
As shown in Fig. 4, compared with the raw coal sample, the specific surface area and average pore diameter of the coal sample show a trend of increasing gradually with the increase of pressure. The influence of thermal and mechanical coupling on the pore structure of coal is mainly reflected in “parallel pores” and “reaming pores”. With the increase of pressure, pores in coal are connected with each other, so the specific surface area and average pore diameter in coal show an increasing trend. Therefore, the thermal-force coupling increases the coal-oxygen contact area, and the coal oxidation spontaneous combustion is enhanced, that is, the spontaneous combustion tendency of coal under the thermal-force coupling is higher than that of raw coal.
Gas product analysis
CO concentration
The variation curve of CO with temperature is shown in Fig. 5. It can be seen that at 70 ~ 150 °C, the CO concentration in the early stage of coal fire gradually begins to rise, at this time, the CO production is less, and the first to measure CO gas is raw coal. At this time, the coal sample is in the stage of oxygen gain and slow oxidation. Other pre-oxidized coal samples are heated again and oxidized, and the initial temperature of CO production increases, because the functional groups that are easy to participate in oxidation have participated in the reaction during the pre-oxidation. On the whole, with the increase of temperature, the coal sample is reoxidized gradually, and the CO gas produced has the same change trend as the raw coal. The CO concentration of pre-oxidized coal samples is higher than that of raw coal. This is because the coal active groups of the pre-oxidized coal sample increase, and the pore structure changes, it is easier to contact with oxygen. With the accumulation of heat in the coal body, the coal-oxygen reaction intensifies, and the spontaneous combustion tendency of the pre-oxidized coal sample increases.
Alkyl olefin gas analysis
CH4 and C2H4 are high-temperature decomposition products and pyrolysis products of coal redox reaction, and their gas concentration changes are shown in Fig. 6. It can be seen that the pre-oxidized coal sample and raw coal have similar gas generation rules. As the temperature of the coal sample increases, alkene gases such as CH4 and C2H4 are detected successively. The temperature of CH4 production is 80 ~ 200 °C, and the temperature of C2H4 production is 160 ~ 200 °C. The temperature point of CH4 and C2H4 gas in the pre-oxidized coal sample is measured to move backward. This indicates that the transfer and consumption of CH3 groups in coal is caused by pre-oxidation at low temperature. At the same time, it can also be seen from the data in Fig. 6 that this kind of coal sample is in the latent stage of combustion at 0 ~ 80 °C, the initial reaction of internal chemical reaction begins at 80 ~ 200 °C, and the oxidation reaction of coal begins to accelerate after 200 °C. After the thermal and mechanical coupling, the measured time of coal sample gas is advanced, and the measured gas content is also increasing. It can be seen that the proximity standard of gas judgment should be adjusted during the secondary mining of coal seam.
Content of chemical active groups in coal
The infrared spectral curve of each coal sample under the action of heat-force coupling is shown in Fig. 7. As can be seen from Fig. 7, The FTIR curves of all coal samples have similar distribution trends. According to the absorption peak distribution characteristics of the curves, the infrared spectrum curves of coal samples can be divided into four stages. The wave number ranges from 700 to 900 cm−1 band of aromatic hydrocarbons, 1000 to 1800 cm−1 band of oxygen-containing functional groups, 2800 to 3000 cm−1 band of aliphatic hydrocarbons and 3000 to 3600 cm−1 band of hydroxyl groups29,30,31. The main absorption peaks are shown in Table 2.
In order to explore the influence of thermal-mechanical coupling on the content of functional groups on the coal surface, the FTIR curves of each coal sample were fitted by Peakfit, a peak-splitting fitting software. The absorption peak areas of the main functional groups of each coal sample were obtained, as shown in Fig. 8.
It can be seen from the infrared spectrum test results that the thermo-mechanical coupling promotes the pyrolysis and volatilization of organic substances in coal, resulting in changes in the content of active functional groups in coal. Compared with raw coal, the contents of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in 1 MPa and 4 MPa samples are 0.72 and 1.21, 0.38 and 4.60, 0.25 and 1.49, respectively, as the temperature rises to 50 °C. At 100 °C, the contents of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in 1 MPa and 4 MPa samples were 1.51 and 1.93, 5.55 and 10.20, 0.05 and 1.27, respectively. The content of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in coal increases. The increase of oxygen-containing functional groups will increase the oxidation activity of coal and promote the occurrence of coal oxidation reaction. Aliphatic hydrocarbon can react with oxygen to produce hydroxyl group during coal oxidation, and the hydroxyl group has strong activity and is easily consumed by oxidation.
Mechanism
The effect of stress will change the pore structure of the coal, including the size, shape and connectivity of the pores, thus affecting the contact area between coal and oxygen and the diffusion rate of oxygen in the coal. This will affect the oxidation reaction rate and heat release process of coal. When coal is subjected to external pressure or internal stress, friction heat or deformation heat may be generated under the action of heat-force coupling, which will accelerate the temperature rise process of coal and promote the occurrence of spontaneous combustion of coal32,33.
There is a coupling effect between temperature and stress, high temperature will increase the thermal stress of coal, and the stress concentration area may become a high temperature point, and this mutual promoting effect will accelerate the process of coal spontaneous combustion34,35.
In summary, the thermo-mechanical coupling can promote the occurrence and development of spontaneous combustion of coal by changing the structure, temperature and stress state of coal and affecting the reaction process of coal and oxygen. Under laboratory conditions, the oxidation reaction rate, heat release and spontaneous combustion tendency of coal were observed by simulating the effect of different stress and temperature conditions on coal. These experimental results show that the thermal and mechanical coupling effects significantly affect the spontaneous combustion characteristics of coal.
Conclusions
In this manuscript, scanning electron microscopy, low-temperature N2 adsorption, temperature programmed experiment and infrared spectroscopy tests were carried out to analyze the mechanism of the influence of pore structure and the number of oxygen-containing functional groups on the spontaneous combustion characteristics of coal samples from the physical and chemical angles. The main conclusions are as follows:
-
(1)
After the thermal and mechanical coupling of raw coal, the connectivity between pores and fractures is enhanced, and the scale of micro-fractures is also significantly increased. With the increase of temperature and stress, the new cracks in the coal body further expand and form a more complex fracture network. The air seepage channel expands and the diffusion of oxygen in the fracture network is strengthened, thus aggravating the risk of coal spontaneous combustion in the underground.
-
(2)
From the analysis of product changes, after the thermo-mechanical coupling, the measured signature gases CO, CH4 and C2H4 lead to the advance of gas production temperature with the increase of pressure, indicating that the oxidation of the treated coal sample is enhanced. As the overall production rate of the three signature gases increases, it is easier to accumulate combustible gases in the reaction and then spontaneous combustion occurs.
-
(3)
Compared with raw coal, the contents of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in 1 MPa and 4 MPa samples are 0.72 and 1.21, 0.38 and 4.60, 0.25 and 1.49, respectively, as the temperature rises to 50 °C. At 100 °C, the contents of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in 1 MPa and 4 MPa samples were 1.51 and 1.93, 5.55 and 10.20, 0.05 and 1.27, respectively. The content of aromatic hydrocarbon, oxygen-containing functional group and aliphatic hydrocarbon in coal increases.
-
(4)
The thermo-mechanical coupling jointly promotes the occurrence and development of spontaneous combustion of coal by changing the structure, temperature and stress state of coal and affecting the reaction process of coal and oxygen.
Data availability
Correspondence and requests for materials should be addressed to J. Wu.
References
Miao, G. et al. Insight into the formation mechanism of higher-molecular-weight gases during the spontaneous combustion of coal. Fuel 373, 132383 (2024).
Li, Y. et al. Study on the thermal reaction characteristics and kinetics of coal and coal gangue coexisting spontaneous combustion. Energy 288, 129781 (2024).
Yu, S. L. & Liu, X. Study on multi-indicator quantitative risk evaluation methods for different periods of coal spontaneous combustion in coal mines. Combust. Sci. Technol. 196 (11), 1628–1641 (2024).
Du, Q. et al. Preparation of complex physicochemical synergistic inhibitors and study on inhibition of spontaneous combustion of coal. Int. J. Coal Prep. Util. 1–24 (2024).
Feng, W. et al. Multiphysics multicoupled modeling of rock fragmentation under high-voltage electrical pulse. Int. J. Geomech. 24(9), 04024176 (2024).
Yu, J. et al. An improved model for discrete element method simulation of spatial gradient distributions of freeze-thaw-induced damage to sandstone. Comput. Geotech. 171, 106412 (2024).
Shangguan, J. et al. Experimental study on dynamic deformation and breaking mechanism of high-temperature hard rock cutting by abrasive water jet. Int. J. Rock Mech. Min. Sci. 180, 105797 (2024).
Li, S. et al. Research on the welding process of Q355ND thick plate with narrow gap double wire submerged arc welding. In Journal of Physics: Conference Series Vol. 2785 012012 (IOP Publishing, 2024).
Zheng, C. et al. Measurement of uniaxial compression mechanical properties of thermally dried coal samples by energy and fragmentation characteristic analyses. Measurement 207, 112363 (2023).
Tong, H. et al. A True Triaxial Creep Damage Constitutive Model of Rock Considering Thermal-Force Coupling. Available at SSRN 4534675.
Liu, X. Under the action of thermal-mechanical coupling seepage law of evolution of coal is study. J. Energy Environ. Prot. 46–48(6), 45–51. (2024).
Cao, N. Study on coal permeability and its influence on gas extraction characteristics under thermodynamic coupling (Coal Science Research Institute, 2023).
Yi, X. et al. Thermal–mechanical coupling coal and rock under the action of seepage characteristics study. J. Coal Mine Saf. (04), 56–61 (2022).
Wang, X. & Feng, Z. Experimental study on thermal deformation of long flame coal under thermodynamic coupling. Coal Eng. 53(06), 135–139 (2014).
Wang, G., Zhao, X. & Wang, G. Thermal coupling coal sample mechanical behavior under the influence of the experimental study. J. China Coal 4, 90–93 (2016).
Feng, Z. et al. Experimental study on deformation characteristics of anthracite coal under thermodynamic coupling. Chin. J. Rock Mech. Eng. 29(08), 1624–1630 (2010).
Zou, G. et al. Two-dimensional SEM image-based analysis of coal porosity and its pore structure. Int. J. Coal Sci. Technol. 7, 350–361 (2020).
Li, H. et al. Spectroscopic (FTIR, 1H NMR) and SEM investigation of physicochemical structure changes of coal subjected to microwave-assisted oxidant stimulation. Fuel 317, 123473 (2022).
Wu, D. et al. Fine characterization of the macromolecular structure of huainan coal using XRD, FTIR, 13 C-CP/MAS NMR, SEM, and AFM techniques. Molecules 25(11), 2661 (2020).
Wazir, A. H. et al. Preparation and characterization of activated carbon from coal by chemical activation with KOH. Int. J. Coal Prep. Util. 42(5), 1477–1488 (2022).
Xu, S. et al. Quantitative analysis of pore structure and its impact on methane adsorption capacity of coal. Nat. Resour. Res. 30, 605–620 (2021).
Zhao, S. et al. Experimental analysis of the effect of temperature on coal pore structure transformation. Fuel 305, 121613 (2021).
Bin, L. et al. FTIR and XRD microscopic characterisation of coal samples with different degrees of metamorphism. J. Mol. Struct. 1309, 138270 (2024).
Ghosh, A. K. & Bandopadhyay, A. K. Formation of thermogenic gases with coalification: FTIR and DFT examination of vitrinite rich coals. Int. J. Coal Geol. 219, 103379 (2020).
Zhao, L. et al. Molecular structure characterization of lignite treated with ionic liquid via FTIR and XRD spectroscopy. Fuel 272, 117705 (2020).
Zhang, Y. et al. Study on properties of coal-sludge-slurry prepared by sludge from coal chemical industry. Powder Technol. 366, 552–559 (2020).
Wang, D. et al. Evolution of pore structure and fractal characteristics of coal char during coal gasification. J. Energy Inst. 93(5), 1999–2005 (2020).
Liu, D. et al. Pore structure characterization and its significance for gas adsorption in coals: A comprehensive review. Unconv. Resour. 2, 139–157 (2022).
Liu, J. et al. Pyrolysis mechanisms of coal extracts based on TG-FTIR and ReaxFF MD study. Fuel Process. Technol. 227, 107124 (2022).
Zhou, G. et al. Experimental study and analysis on physicochemical properties of coal treated with clean fracturing fluid for coal seam water injection. J. Ind. Eng. Chem. 108, 356–365 (2022).
Fatima, N. et al. Extraction and chemical characterization of humic acid from nitric acid treated lignite and bituminous coal samples. Sustainability 13(16), 8969 (2021).
Lin, B., Li, Q. & Zhou, Y. Research progress on multi-field evolution of combined thermal dynamic disasters of gas and coal spontaneous combustion in goaf. J. Coal 46(6), 1715–1726 (2021).
Zhu, C. et al. Study on CO distribution in spontaneous combustion of coal in goaf under multiple heat sources. Mine Eng. 12(1), 92–102 (2024).
Nie, X. et al. Research on thermodynamic coupling stamping considering deformation heat and friction heat effect. China Mech. Eng. 31(16), 2005–2015 (2020).
Liu, Z. et al. Temperature rise characteristics of spontaneous combustion oxidation of coal with different particle sizes under thermo-stress coupling. Autom. Ind. Min. 47(9), 91–95 (2021).
Acknowledgements
The authors greatly acknowledge the financial support from the National Natural Science Foundation of China(51774170). The authors would like to thank all the reviewers who participated in the review.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 51774170].
Author information
Authors and Affiliations
Contributions
J.W. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, J., Li, Z., Huang, S. et al. Study on spontaneous combustion characteristics of coal under thermo mechanical coupling. Sci Rep 14, 31914 (2024). https://doi.org/10.1038/s41598-024-83448-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83448-7