Abstract
Malaria is a significant public health challenge in Gabon, with high prevalence rates in rural and semi-urban areas. This study investigated Plasmodium infection prevalence among outpatients at a medical laboratory in Franceville, Gabon, in 2020. Data from 500 patients were analyzed, revealing an overall infection rate of 33.2% and the presence of four Plasmodium species: P. falciparum, P. malariae, P. ovale, and possibly P. vivax for the first time in Gabon. Co-infections were common, with P. falciparum and P. ovale spp. being the most prevalent at 23.5%. Asymptomatic infections accounted for 81.3% of cases, while symptomatic infections were 18.7%. P. falciparum was associated with symptomatic cases, while non-falciparum species were linked to asymptomatic infections. The findings suggest Franceville has perennial malaria transmission, highlighting the role of Plasmodium species diversity in disease severity and clinical presentation, including the first report of P. vivax infection in the Gabonese population.
Similar content being viewed by others
Introduction
Malaria is a significant public health threat in sub-Saharan Africa1. It is an infectious disease caused by protozoan parasites belonging to Plasmodium genus and transmitted by female mosquitoes of the genus Anopheles2. According to a World Health Organization (WHO) report (2022), this region (sub-Saharan Africa) witnessed approximately 233 million malaria cases, resulting in 580,000 deaths, with 80% of fatalities occurring in children under five years old3. This alarming situation serves to highlight the pressing need to intensify prevention, diagnosis and treatment efforts to reduce malaria burden among the most vulnerable populations. Malaria is caused by a variety of parasites species affecting its incidence4. Currently, five malaria species affect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale spp. (Plasmodium ovale-curtesi and Plasmodium ovale-wallekiri), Plasmodium malariae and Plasmodium knowlesi5. Among these, P. falciparum is main responsible for severe infection and fatalities if left untreated6.
In addition to a single infection by a plasmodial species, individuals can also experience mixed infections, where multiple plasmodial species coexist within the same individual7. The interactions between these mixed infections can significantly influence course and outcome of the disease8. However, there is conflicting evidence on the severity of mixed-species Plasmodium infections compared with single-species infections9,10. Some studies suggest that mixed infections may be associated with more severe disease, while others suggest that the severity depends on which species is dominant11,12. In mixed infections, different Plasmodium species can interact each other’s and could affect parasitemia as well as clinical features of the patient13. For instance, P. falciparum may suppress P. vivax parasitemia through interspecies inhibition, while a P. vivax super-infection over an existing P. falciparum infection can lead to increase P. falciparum parasitemia and severe malaria13.
Since 2015, the WHO has implemented a global technical strategy to reduce the incidence of malaria and related mortality by at least 90% by 2030, with primarily focus on combating P. falciparum14. However, research has shown that other Plasmodium species can also cause significant morbidity11,12. For instance, P. malariae has been linked to severe complications such as severe anaemia and nephrotic syndrome, which can lead to progressive renal failure in adolescents and young adults15,16. Similarly, infection with P. ovale spp. has been associated with severe forms of malaria17. Additionally, P. vivax, which is resurfacing in Black Africa, can cause persistent severe anaemia by disrupting the formation of mature red blood cells and leading to premature death of reticulocytes18. All these observations emphasise the importance of considering other species of Plasmodium in malaria control, prevention and treatment efforts.
Malaria is a significant public health concern in Gabon, with year-round transmission facilitated by the equatorial climate that provides optimal breeding conditions for mosquitoes19. The prevalence of Plasmodium infection ranges from 53 to 79% in rural areas, 21.2% to 36.1% in semi-urban and urban areas, with variations across different regions, including Lastoursville, Fougamou, Koula-Moutou, and Franceville20,21. Furthermore, the disruption of routine health services due to the COVID-19 pandemic in 2020 led to an increase in malaria cases and deaths in Gabon, underscoring the challenges faced during the pandemic20. Of the three Plasmodium species (P. falciparum, P. malariae, and P. ovale spp) responsible for malaria in Gabon19, P. falciparum is the most prevalent, accounting for 94–99% of cases, followed by P. malariae (0.5–5%) and P. ovale spp. (0.5–2.4%)19. However, the lower incidence of malaria attributed to P. malariae and P. ovale spp. can be linked to challenges in laboratory infrastructure or competition between P. falciparum and this non-falciparum species. First, conventional microscopy techniques, commonly utilized for identification, have limitations in sensitivity22,23. Secondly, rapid diagnostic tests (RDTs) used are highly specific for detecting P. falciparum24,25, resulting in under-diagnosis of non-falciparum malaria, which could impede an accurate assessment of their contribution to malaria incidence24,25,26. Consequently, these limitations emphasise the necessity for the development of improved diagnostic techniques with enhanced sensitivity, and specificity in order to increase the accuracy of identification of the less common Plasmodium species.
Franceville is a city of Gabon, the capital of Haut-Ogooué province, and the country’s third largest city in terms of population. The region is characterised by meso-endemic malaria, with a prevalence of between 26 and 33%, as indicated by the most recent studies20,21. The region experiences seasonal rainfall of between 1000 and 2000 mm and average annual temperatures of between 15 and 29 °C21. The region’s principal rivers facilitate the dispersal of Anopheles species, which are responsible for the transmission of malaria. Eliminating malaria in this region requires the implementation of a comprehensive surveillance programme, which should be designed to control all malaria species. Thus, the objective of this study was to monitor Plasmodium infection in patients consulting at the CIRMF’s Medical Laboratory (LAM) in 2020 to characterize the diversity of Plasmodium species circulating in the study area (Franceville, South-East of Gabon) and to assess its association to some biological parameters associated with infection.
Results
Socio-demographic and haematological characteristics of patients.
In 2020, the medical laboratory of the CIRMF received 500 patients for malaria diagnosis. Of these patients, 52.8% (264/500) were females and 47.2% (236/500) males, resulting in a sex ratio (male/female) of 0.9. The median age of the entire group was 28 years [IQR: 10–40]. A total of 166 patients were diagnosed with malaria parasites, with a median age of 28 years [IQR: 10–40]. The age group most affected was the 5–15 year-olds, accounting for 42.6% (40/166) of cases, however, this discrepancy was not statistically significant when compared with other age groups (P = 0.0893). In malaria-infected patients, the clinical and biological data revealed a median temperature of 36 °C [IQR: 36–40] and a mean haemoglobin level of 13 g/dl [IQR: 11–15]. The median parasitaemia (× 103 μl) was 258 [IQR: 197–321], while the median erythrocyte (× 106 μl) was 4.5 [IQR: 4–4.5]. Table 1 presents the sociodemographic and clinical characteristics of the malaria and non-malaria parasites infected patients.
Malaria prevalence and plasmodial species by molecular detection
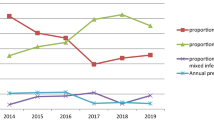
The study findings indicated that the overall prevalence of malaria was 33.2% (166/500), with three notable peaks observed. The first peak occurred in January, with a prevalence rate of 42.6%, followed by a second peak in June, with a prevalence rate of 42.4%, and a third peak was observed in October (32.5%). This study identified four plasmodial species: P. falciparum, P. malariae, P. ovale spp. and P. vivax (Figs. 1A and Supplementary Fig. S1). The overall prevalence by species showed that P. falciparum was the most prevalent species with 89.1% (148/166), followed by P. ovale spp. with 38.5% (64/166) [(ovale curtisi, 24.7% (41/166) and ovale wallikeri, 13.8% (23/166)], P. malariae with 30.1% (50/166) and P. vivax with 3% (5/166). The five P. vivax cases reported here were identified in blood material from three native Gabonese subjects and two Asians from India, all employees of the same mining company. In terms of symptomatology, the data demonstrate that the proportion of symptomatic carriers was markedly higher than that of asymptomatic carriers (96.9%, [31/32] vs 28.8% [135/468], P < 0.001). The study revealed that both women and children exhibited high levels of parasitemia. Furthermore, a significant correlation was observed between high parasitemia and the presence of P. falciparum either as a mono-infection or in combination with other species (Fig. 2A,B).
The Venn diagram depicts the interrelationships between the various types of parasites that are responsible for malaria (Fig. 1B). Mono-infections accounted for 49% of plasmodial infections, with P. falciparum accounting for 44% (73/166, [CI95%, 36.3–51.9]), followed by P. malariae with the remaining 3.6% (6/166, [CI95%, 1.5–8]) were caused by P. ovale spp., while 0.6% (1/166, [CI95%, 0.03–3.8]) were attributed to P. ovale spp. No monoinfection with P. vivax was found. The most prevalent co-infections were those between P. falciparum and P. ovale spp., accounting for 23.5% (39/166, [CI95%, 17.4–30.8]). The second most common co-infection was between P. falciparum and P. malariae, which accounted for 10.9% (18/166, [CI95%, 6.7–16.8]) of cases. P. vivax was found in co-infection with P. falciparum in only 1.8% of cases (3/166, [IC95%, 0.5–5.6]), and in triple infection with P. falciparum, P. malariae and P. vivax in only 1.2% of cases (2/166, [IC95%, 0.2–4.7]).
Furthermore, the data indicated a considerable prevalence of submicroscopic infections throughout the year (Fig. 3). The proportion of submicroscopic infections in the population was 39.1% (65/166, [95% CI 31.8–47]) (Table 2). In terms of the performance of microscopy and RDT versus PCR in detecting Plasmodium spp. parasites, the data indicated sensitivities of 60.8% (95% CI 52.9–68.2) and 59.03% (95% CI 51.1–66.5), while their specificities were 100% (95% CI 98.6–100) and 99.4% (95% CI 97.6–99.9), respectively (Table 2). Furthermore, the level of agreement between the RDT and microscopy was evaluated, and it was found to be excellent, with a kappa value 0.97 (95% CI 0.94–1) (Table 3).
Correlation between malaria parasite species on haematological parameters in patients
A principal component analysis (PCA) was performed on the patients’ various biological markers. The PCA retained the first two principal components (PC1 and PC2, Fig. 4A) using the broken-bar model test. These two principal components accounted for 37.8% of the total variance. PC1 (21.2%) can be interpreted as a stability of blood components, characterised by positive associations with haemoglobin (Hb) (r = 0.89, p < 0.0001), haematocrit (Hte) (r = 0.89, p < 0.0001), red blood cells (RBC) (r = 0.52, p < 0.0001), mean corpuscular volume (MCV) (r = 0.65, p < 0.0001), and mean corpuscular haemoglobin concentration (MCHC) (r = 0.5, p < 0.0001) and lymphocytes (r = 0.09, p = 0.038) (Fig. 4A and Table S1).
Characterisation of malaria infection as a function of haematological and biochemical variables, colored according to the cosine squared value (measuring the quality of the variables on the PCA graph). (A) Canonical weight of each variable on the principal components; the arrow size indicates the importance of the parameter. PCA diagrams showing correlations between haemato-biochemical characteristics and (B) Presence/absence of plasmodial infection. (C) Different clinical manifestations of Plasmodium infection and (D) the different plasmodial species involved.
PC2 (16.8%) demonstrated a correlation with variables associated with an inflammatory response of the immune system against Plasmodium (Fig. 4B), including CRP (r = 0.65, p < 0.0001), temperature (r = 0.51, p < 0.0001), and parasitaemia. The correlation coefficient was 0.51 (p < 0.0001), with platelets (r = 0.51, p < 0.0001), neutrophils (r = 0.14, p = 0.0012), monocytes (r = 0.16, p = 0.004. Furthermore, the correlation between eosinophils (r = 0.10, p = 0.0229), basophils (r = 0.35, p < 0.0001) and platelets (r = − 0.52, p < 0.0001) was also observed (Table S2). The results of this investigation indicate that symptomatic infections are most closely associated with the presence of a single P. falciparum infection. Conversely, asymptomatic infections are associated with either co-infection with P. falciparum or solely with other plasmodial species (Fig. 4C,D).
Discussion
The objective of this study was to provide a comprehensive overview of the prevalence of malaria and the diversity of Plasmodium species circulating among outpatients visiting Medical Laboratory of CIRMF in Franceville, an urban area in south-east Gabon, over the period from January to December 2020.
The prevalence of malaria found in this study (33.2%) is consistent with the results of a previous study carried out in the same region in 202020. These results underscore the need to reassess and adapt control strategies to the current epidemiological context in order to hope to achieve the WHO’s goals of eliminating malaria by 2030. The data indicate that malaria transmission persists throughout the year in the study area, with peaks of prevalence observed mainly during the rainy season. This trend is consistent with recent observations made in Franceville20,21, likely due to increased Anopheles mosquitoes populations during the rainy season, facilitated by expanded larval habitats27,28, which elevates risk of Plasmodium-infected mosquitoes bites.
With regard to the impact of malaria according to age group, the results indicate that the age group of 5 to 15 presented the highest prevalence, in accordance with findings from previous studies conducted in Gabon and in several other countries in sub-Saharan Africa21,29. This trend may be attributed to the intensification of control strategies, which are more pronounced in children under five and delayed immunity acquisition in children over the age of five, in addition to increased exposure in the latter group30,31. It is therefore evident that a balanced approach to control strategies addressing both age groups is essential for more effective infection management.
The diversity of malaria parasites can have a significant impact on the management of the disease. In the present study, four species were identified, which align with findings from previous studies32,33, with the exception of P. vivax, all the species identified have already been reported in Gabon. Of the species identified, P. falciparum was the most prevalent. This predominance, comparable to that observed in previous studies in Gabon21,34, could be attributed to a several factors. Firstly, the high transmission rate of P. falciparum plays a crucial role. Indeed, P. falciparum is known for its high transmission capacity and its adaptation to various environmental conditions, which has resulted in it becoming the most widespread malaria parasite in the region35,36. This predominance is also due to the specificity of the Anopheles gambiae vector, which is the main malaria vector in Central Africa. This vector appears to have a strong preference for transmitting P. falciparum, which contributes to its high prevalence in the region37,38.
In comparison to previous studies, our findings indicate a higher prevalence of P. ovale spp. and P. malariae than previously reported19,32,39, which underlines the need to include them in malaria eradication strategies. This difference can be explained to the using of molecular tools, which are more sensitive than conventional methods, such as microscopy and RDTs used in previous research40,41. The challenge in detecting these parasites, which are typically associated with less severity and lower parasite densities, particularly with microscopy, underscores the importance of molecular tools22 to objectively assess their impact on malaria prevalence.
In addition, the prevalence of submicroscopic infections, which accounted for 39.1% of cases in our study, represents a significant challenge to malaria elimination efforts. These data indicate the need for more sensitive diagnostic tools to minimize these infections, as they can act as reservoirs, perpetuating transmission cycles and hampering eradication efforts7. In this sense, results obtained in this study indicate that the diagnostic tests for Plasmodium spp. parasites exhibited low sensitivity but high specificity. This is in stark contrast to the findings of previous evaluations conducted in Libreville and Lambaréné42,43. This discrepancy may be attributed to the fact that the studies in question exclusively included patients presenting with fever, and employed microscopy as the gold standard for diagnosis. In contrast, our study incorporated both febrile and apyretic individuals and utilised PCR, which is known for its superior sensitivity compared to microscopy. Given these differences, it is essential to consider the performance of rapid diagnostic tests (RDTs) across diverse populations, regardless of fever status. Addressing this issue is crucial for achieving the goal of eradicating malaria by 2030. Concerning co-infections, the most prevalent was that of P. falciparum-P. ovale spp, with a prevalence of 23.5%. This was followed by that of P. falciparum and P. malariae, with a prevalence of 10.9%. It is therefore essential to gain an understanding of the interactions between these species in order to assess their impact on the host’s health.
Finally, with regard to malaria parasite diversity, the present study is the first in our knowledge to report the presence of P. vivax in the Gabonese population. The parasite was identified in individuals of Asian and Gabonese. The last has never left Gabon but live in a rural area near Franceville. All of them are employed in a local company who attended routine health check-ups. These individuals were co-infected with P. falciparum and in triple infection with P. falciparum and P. malariae. Our findings are consistent with previous reports of P. vivax circulation in the sub-region, including in the Democratic Republic of Congo44, Cameroon45, Equatorial Guinea and Angola46. Gabon is the fifth Central African country to document the presence of P. vivax in Duffy-negative populations. This study provides evidence that Plasmodium vivax is present in Gabon, as is its chimpanzee- and gorilla-infecting Plasmodium vivax-like homologue, which has been demonstrated to infect humans47. Recent years have witnessed the emergence of P. vivax malaria in sub-Saharan African populations, prompting questions about the mechanisms facilitating its invasion18. Historically, the absence of the Duffy chemokine receptor antigen (DARC) on the surface of erythrocytes in these populations suggested that natural protection against P. vivax48,49. Nevertheless, recent research indicates that P. vivax merozoites can invade a subset of Duffy-negative erythroblasts transiently expressing DARC at erythropoiesis sites50. Furthermore, individuals who are Duffy-positive may act as reservoirs for P. vivax, facilitating the infection of Duffy-negative hepatocytes and selecting new strains capable of invading Duffy-negative erythrocytes independently of DARC51. The failure to take prompt and decisive action to contain the spread of P. vivax in Central Africa could impede the elimination of malaria in the region, where P. falciparum is already a significant concern. One potential solution would be the implementation of prophylactic measures for expatriates originating from high-risk areas such as Asia.
The findings of this study indicated a significantly higher prevalence of asymptomatic infections compared to symptomatic ones. This difference may be attributed to the composition of the studied population, which was predominantly composed of older children and adults. These findings are corroborated with those of a previous study conducted in the Amazon region52, which also identified a positive correlation between age and asymptomatic malaria. In malaria-endemic regions, age plays a pivotal role in the protection against clinical malaria. Young children are more susceptible to infection, whereas adults and older children tend to carry asymptomatic infections, having developed immunity after multiple parasite exposures53,54. The majority of symptomatic infections were associated with P. falciparum alone, whereas asymptomatic infections arose from co-infections of P. falciparum with other species or from infections by other plasmodial species alone. Furthermore, individuals infected solely with P. falciparum exhibited significantly higher parasitemia compared to those with mixed infections involving P. falciparum and other plasmodial species. Similar observations have been reported in Tanzania55 and Nigeria56. The data indicate that P. malariae and P. ovale spp. may confer a protective effect, reducing the virulence of P. falciparum and impeding its progression to symptomatic or severe malaria. For example, studies have demonstrated that P. malariae infection can result in a 50% reduction in P. falciparum parasitemia through intra-host competition57. Furthermore, the absence of co-infection in symptomatic patients has been linked to the proliferation of specific P. falciparum clones, which results in the exclusion of other species within the infection and leads to clinical symptoms in the host58. Furthermore, the absence of co-infection in symptomatic patients has been linked to an acute innate immune response in the host, which eliminates slower-growing species59.
Conclusion
This work highlights the need for further epidemiological investigations on the dynamics of malaria infection in the country. Indeed, it reports for the first time the presence of Plasmodium vivax in the Gabonese population, raising important questions about the invasion mechanisms of this parasite in sub-Saharan African populations, traditionally considered to be naturally protected due to the absence of the Duffy chemokine receptor antigen. The discrepancy in prevalence between molecular tools and microscopy/TDR diagnosis involves the necessity for the development of more sensitive tools if malaria is to be eradicated, particularly in regions where the disease persists as a significant public health concern.
Materials and methods
Study area and population
This is a prospective, descriptive and analytical study conducted at the medical laboratory of the Centre de Recherches Médicales Interdisciplinaires (CIRMF) in Franceville, the urban capital of the province of Haut-Ogooué in south-eastern Gabon (Fig. 5). From January to December 2020, patients of all ages and sexes were enrolled for malaria diagnosis. Sociodemographic, biological and clinical parameters of the patients, including age, sex, bed net use, haematological and biochemical parameters, weight, temperature and parasitaemia, and species diversity, were recorded and reported using a Microsoft Excel 2016 (Microsoft Corporation, United States of America).
Localization of study site. The map of Gabon was created using QGIS software version 2.2.0 (https://qgis.org/fr/site/) and enhanced using Adobe Illustrator CS6 version 16.0.0 (http://www.adobe.com/de/products/illustrator.html). The brown area represents the province where the study was conducted, while the building indicates the geographical position of the CIRMF research center.
Case definition
In the context of this study, any individual who presented to the medical analysis laboratory for a general check-up was classified as asymptomatic. Conversely, a patient was considered symptomatic if they sought laboratory diagnosis for malaria, thereby indicating a potential malaria infection. Asymptomatic malaria infection was defined as a positive test result for Plasmodium spp., no fever (temperature ≤ 37.5 °C) at the time of sampling and no history of fever in the previous 72 h. A case of malaria was defined as symptomatic if P. falciparum spp. was detected in the patient by rapid diagnostic test (RDT) or microscopy and was accompanied by fever (axillary temperature ≥ 37.5 °C) or a history of fever (in the previous 72 h) with at least one of the following clinical signs: headache, chills, weakness, joint pain, vomiting or diarrhoea. Submicroscopic infection was defined as infection that was negative by mRDT and positive by polymerase chain reaction (PCR).
Collection of blood samples
A total of 5 ml of blood was collected from each patient in EDTA tubes for thick and thin blood smears and RDTs at the CIRMF medical laboratory. The remaining blood was collected in 1.5 ml microtubes, transported to the Health Ecology Research Laboratory and stored at − 20 °C for molecular diagnosis of malaria, where these samples were stored at − 20 °C for less than two weeks prior to DNA extraction. All samples from each study participant were uniquely coded and stored until use.
Malaria diagnosis by microscopy and RDT
The CIRMF medical laboratory uses a malaria screening algorithm that combines a rapid diagnostic test (RDT) and microscopy, irrespective of the RDT result. The MERISCREEN Malaria Pf/Pan Ag rapid diagnostic test (Meril Diagnostics Pvt. Ltd, India) was used following the manufacturer’s instructions. The parasite load was quantified on a May-Grünwald-Giemsa (MGG) stained blood smear and examined microscopically using the Lambaréné method60. Experienced technicians read the slides at least twice, and any discrepancies were resolved by a third reader to ensure the reliability of the results. Slides were considered negative only after examination of one hundred fields at 1000× magnification, with a detection limit of approximately two parasites per microliter in accordance with WHO recommendations. Haemoglobin levels were quantified using an automated analyser (HORIBA Medical, France).
Molecular analyses for Plasmodium spp. detection
Total DNA was extracted from 200 µl of each blood sample using the DNeasy Blood & Tissue kit (Qiagen) and used for the detection of parasites of the genus Plasmodium spp. according to a previously described protocol61. Refer to Fig. 6 and supplementary data for detailed molecular tools used for parasite characterization.
Data management and analysis
The database for this study was constructed and cleaned using Microsoft Excel 2016 software (Microsoft Corporation, USA). All statistical analyses were carried out using the R 4.0.2 software (https://cran.r-project.org). Simple proportions were calculated for qualitative variables and means, standard deviations (SD) and medians with interquartile ranges (IQR) were calculated for quantitative variables. Categorical variables were compared using either the chi-square test or Fisher’s exact test, depending on whether or not the number of observations was less than five. A 95% confidence interval (CI) was employed, with a statistical significance level of α = 5%. All graphs were generated using the Ggplot2 package functions62. To categorize the distinct plasmodial infection profiles according to the immuno-haematological variables, a principal component analysis (PCA) was conducted on the quantitative variables using the ade463 and FactoMineR packages64. PCA also permitted the integration of the significant principal components selected using the Broken Stick model as independent variables65, thus reducing the risk of multi-colinearity between the immuno-haematological variables. The subsequent step involved the examination of the variation in parasitaemia in relation to a number of sociodemographic parameters, the type of P. falciparum infection (mono-infection and co-infection), and the newly identified variables resulting from the principal component analysis (PCA). This was achieved by constructing a binomial generalised linear model utilising the lme4 package66. The response variable was the parasitaemia of each patient, while the explanatory variables were the various socio-demographic and immuno-haematological parameters of each patient. The results of the model were extracted and illustrated using the plot model function in the sjPlot package67.
Efficacy of microscopy and rapid diagnostic tests (RDTs) for malaria diagnosis
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of microscopy and RDT were compared using PCR as the gold standard for malaria parasite detection. The concordance between RDT and microscopy for malaria diagnosis was evaluated using the Cohen’s Kappa coefficient (https://www.graphpad.com/quickcalcs/kappa1/) at the confidence interval 95% (95% CI).
References
Papaioannou, I., Utzinger, J. & Vounatsou, P. Malaria-anemia comorbidity prevalence as a measure of malaria-related deaths in sub-Saharan Africa. Sci. Rep. 9(1), 11323 (2019).
Soma, D. D. et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: A pre-intervention study. PLoS ONE 15(8), e0236920 (2020).
World Health Organization. World malaria report 2023. World malaria report 2023 (2023).
Milner, D. A. Malaria pathogenesis. Cold Spring Harb. Perspect. Med. 8(1), a025569 (2018).
Oriero, E. C., Van Geertruyden, J.-P., Nwakanma, D. C., D’Alessandro, U. & Jacobs, J. Novel techniques and future directions in molecular diagnosis of malaria in resource-limited settings. Expert Rev. Mol. Diagn. 15(11), 1419–1426 (2015).
Imboumy-Limoukou, R. K. et al. Severe malaria in Gabon: epidemiological, clinical and laboratory features in Amissa Bongo Hospital of Franceville. Malar. J. 22(1), 1–8 (2023).
Kojom Foko, L. P. et al. Non-falciparum species and submicroscopic infections in three epidemiological malaria facets in Cameroon. BMC Infect. Dis. 22(1), 1–13 (2022).
May, J. et al. Impact of subpatent multi-species and multi-clonal plasmodial infections on anaemia in children from Nigeria. Trans. R. Soc. Trop. Med. Hyg. 94(4), 399–403 (2000).
Tajebe, A., Magoma, G., Aemero, M. & Kimani, F. Detection of mixed infection level of Plasmodium falciparum and Plasmodium vivax by SYBR Green I-based real-time PCR in North Gondar, north-west Ethiopia. Malar. J. 13, 1–8 (2014).
Leonard, C. M. et al. Missed Plasmodium falciparum and Plasmodium vivax mixed infections in Ethiopia threaten malaria elimination. Am. J. Trop. Med. Hyg. 106(2), 667 (2022).
Kotepui, M., Kotepui, K. U., Milanez, G. D. & Masangkay, F. R. Prevalence and proportion of Plasmodium spp. triple mixed infections compared with double mixed infections: a systematic review and meta-analysis. Malar. J. 19, 1–22 (2020).
Kotepui, M., Kotepui, K. U., De Jesus, M. G. & Masangkay, F. R. Plasmodium spp. mixed infection leading to severe malaria: a systematic review and meta-analysis. Sci. Rep. 10(1), 11068 (2020).
Mayxay, M., Pukrittayakamee, S., Newton, P. N. & White, N. J. Mixed-species malaria infections in humans. Trends Parasitol. 20(5), 233–240 (2004).
World Health Organization. Global Technical Strategy for Malaria 2016–2030 (World Health Organization, 2015).
Eiam-Ong, S. Malarial nephropathy. Paper presented at: Seminars in nephrology (2003).
Langford, S. et al. Plasmodium malariae infection associated with a high burden of anemia: a hospital-based surveillance study. PLoS Negl. Trop. Dis. 9(12), e0004195 (2015).
Singh, R. et al. First report of detection and molecular confirmation of Plasmodium ovale from severe malaria cases in central India. Trop. Med. Int. Health 18(11), 1416–1420 (2013).
Wilairatana, P., Masangkay, F. R., Kotepui, K. U., De Jesus, M. G. & Kotepui, M. Prevalence and risk of Plasmodium vivax infection among Duffy-negative individuals: a systematic review and meta-analysis. Sci. Rep. 12(1), 3998 (2022).
Maghendji-Nzondo, S., Nzoughe, H. & Lemamy, G. J. et al. Prevalence of malaria, prevention measures, and main clinical features in febrile children admitted to the Franceville Regional Hospital, Gabon. Parasite 23 (2016).
Lendongo-Wombo, J.-B., Oyegue-Liabagui, S.-L., Biteghe-Bi-Essone, J.-C., Ngoungou, E. B. & Lekana-Douki, J.-B. Epidémiology of malaria from 2019 to 2021 in the southeastern city of Franceville, Gabon. BMC Public Health 22(1), 2313 (2022).
Boundenga, L., Bignoumba, M., Dibakou, S.-E. et al. Decrease on malaria clinical cases from 2017 to 2019 in Franceville, Southeast Gabon, Central Africa. J. Public Health Afr. 14(3) (2023).
Lalremruata, A. et al. Species and genotype diversity of Plasmodium in malaria patients from Gabon analysed by next generation sequencing. Malar. J. 16(1), 1–11 (2017).
Opoku Afriyie, S. et al. Accuracy of diagnosis among clinical malaria patients: comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar. J. 22(1), 76 (2023).
Lupaka, M., Degefa, T., Eba, K., Zeynudin, A. & Yewhalaw, D. Diagnostic performance of ultrasensitive rapid diagnostic test for the detection of Plasmodium falciparum infections in asymptomatic individuals in Kisangani, Northeast Democratic Republic of Congo. Malar. J. 22(1), 354 (2023).
Lin, K. et al. Evaluation of an innovative point-of-care rapid diagnostic test for the identification of imported malaria parasites in China. Trop. Med. Infect. Dis. 8(6), 296 (2023).
Ongonda, J. K., Ayieko, C. & Miheso, S. Meta-analytic review on the impact of factors that affect performance of malaria rapid diagnostic test in Africa. J. Health Sci. Med. 7(2), 236–243 (2024).
Coetzee, M. et al. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619(3), 246–274 (2013).
Boussougou-Sambe, S. T. et al. Assessment of malaria transmission intensity and insecticide resistance mechanisms in three rural areas of the Moyen Ogooué Province of Gabon. Parasit. Vectors 15(1), 1–13 (2022).
Drakeley, C. et al. Longitudinal estimation of Plasmodium falciparum prevalence in relation to malaria prevention measures in six sub-Saharan African countries. Malar. J. 16, 1–15 (2017).
Snow, R. W. et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349(9066), 1650–1654 (1997).
Bejon, P. et al. Analysis of immunity to febrile malaria in children that distinguishes immunity from lack of exposure. Infect. Immun. 77(5), 1917–1923 (2009).
Delicat-Loembet, L. et al. No evidence for ape Plasmodium infections in humans in Gabon. PLoS One 10(6), e0126933 (2015).
Groger, M. et al. Prospective clinical trial assessing species-specific efficacy of artemether-lumefantrine for the treatment of Plasmodium malariae, Plasmodium ovale, and mixed Plasmodium malaria in Gabon. Antimicrob. Agents Chemother. 62(3), e01758-17. https://doi.org/10.1128/aac.01758-01717 (2018).
Manego, R. Z. et al. Demography, maternal health and the epidemiology of malaria and other major infectious diseases in the rural department Tsamba-Magotsi, Ngounie Province, in central African Gabon. BMC Public Health. 17(1), 1–7 (2017).
Rono, M. K. et al. Adaptation of Plasmodium falciparum to its transmission environment. Nat. Ecol. Evol. 2(2), 377–387 (2018).
Rougeron, V. et al. A population genetic perspective on the origin, spread and adaptation of the human malaria agents Plasmodium falciparum and Plasmodium vivax. FEMS Microbiol. Rev. 46(1), fuab047 (2022).
Mbama Ntabi, J. D. et al. Prevalence of non-Plasmodium falciparum species in southern districts of Brazzaville in The Republic of the Congo. Parasites Vectors 15(1), 209 (2022).
Mbama Ntabi, J. D. et al. Contribution of Anopheles gambiae sensu lato mosquitoes to malaria transmission during the dry season in Djoumouna and Ntoula villages in the Republic of the Congo. Parasites Vectors 17(1), 104 (2024).
Mawili-Mboumba, D. P., Akotet, M. K. B., Ngoungou, E. B. & Kombila, M. Evaluation of rapid diagnostic tests for malaria case management in Gabon. Diagn. Microbiol. Infect. Dis. 66(2), 162–168 (2010).
Mens, P. et al. Is molecular biology the best alternative for diagnosis of malaria to microscopy? A comparison between microscopy, antigen detection and molecular tests in rural Kenya and urban Tanzania. Trop. Med. Int. Health 12(2), 238–244 (2007).
Fontecha, G. A. et al. Comparison of molecular tests for the diagnosis of malaria in Honduras. Malar. J. 11, 1–5 (2012).
Ditombi, B. C. M. et al. Comparative performance of four malaria rapid diagnostic tests, Vikia malaria pf/Pan, Meriline-Meriscreen pf/Pv/Pan, right sign malaria pf/Pan, and right sign malaria pf, among febrile patients in Gabon. Revista da Sociedade Brasileira de Medicina Tropical 53, e20190274 (2020).
Alabi, A. et al. Performance evaluation of a combination Plasmodium dual-antigen CRP rapid diagnostic test in Lambaréné, Gabon. Infection https://doi.org/10.1007/s15010-024-02366-y (2024).
Brazeau, N. F. et al. The epidemiology of Plasmodium vivax among adults in the Democratic Republic of the Congo. Nat. Commun. 12(1), 4169 (2021).
Dongho, G. B. D. et al. Plasmodium vivax infections detected in a large number of febrile Duffy-negative Africans in Dschang, Cameroon. Am. J. Trop. Med. Hyg. 104(3), 987 (2021).
Mendes, C. et al. Duffy negative antigen is no longer a barrier to Plasmodium vivax–molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl. Trop. Dis. 5(6), e1192 (2011).
Prugnolle, F. et al. Diversity, host switching and evolution of Plasmodium vivax infecting African great apes. Proc. Natl. Acad. Sci. 110(20), 8123–8128 (2013).
Young, M. D., Eyles, D. E., Burgess, R. W. & Jeffery, G. M. Experimental testing of the immunity of Negroes to Plasmodium vivax. J. Parasitol. 41(3), 315–318 (1955).
Miller, L. H., Mason, S. J., Clyde, D. F. & McGinniss, M. H. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 295(6), 302–304 (1976).
Bouyssou, I. et al. Unveiling P. vivax invasion pathways in Duffy-negative individuals. Cell Host Microbe 31(12), 2080-2092.e2085 (2023).
Ménard, D. et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc. Natl. Acad. Sci. 107(13), 5967–5971 (2010).
Alves, F. P. et al. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am. J. Trop. Med. Hyg. 66(6), 641–648 (2002).
Jenkins, R. et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar. J. 14, 1–6 (2015).
Dzeing-Ella, A. et al. Severe falciparum malaria in Gabonese children: clinical and laboratory features. Malar. J. 4(1), 1–8 (2005).
Yman, V. et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl. Trop. Dis. 13(5), e0007414 (2019).
Abdulraheem, M. A. et al. High prevalence of Plasmodium malariae and Plasmodium ovale in co-infections with Plasmodium falciparum in asymptomatic malaria parasite carriers in southwestern Nigeria. Int. J. Parasitol. 52(1), 23–33 (2022).
Mason, D. P., McKenzie, F. E. & Bossert, W. H. The blood-stage dynamics of mixed Plasmodium malariae–Plasmodium falciparum infections. J. Theor. Biol. 198(4), 549–566 (1999).
Tang, J., Templeton, T. J., Cao, J. & Culleton, R. The consequences of mixed-species malaria parasite co-infections in mice and mosquitoes for disease severity, parasite fitness, and transmission success. Front. Immunol. 10, 3072 (2020).
Molineaux, L., Storey, J., Cohen, J. E. & Thomas, A. A longitudinal study of human malaria in the West African Savanna in the absence of control measures: relationships between different Plasmodium species, in particular P. falciparum and P. malariae. Am. J. Trop. Med. Hyg. 29(5), 725–737 (1980).
Planche, T. et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am. J. Trop. Med. Hyg. 65(5), 599–602 (2001).
Prugnolle, F. et al. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc. Natl. Acad. Sci. 107(4), 1458–1463 (2010).
Wickham, H., Chang, W. & Wickham, M. H. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2(1), 1–189 (2016).
Dray, S. & Siberchicot, M. A. Package ‘ade4’ (Université de Lyon, 2017).
Husson, F., Josse, J., Le, S., Mazet, J. & Husson, M. F. Package ‘factominer’. An R Package 96(96), 698 (2016).
Peres-Neto, P. R., Jackson, D. A. & Somers, K. M. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput. Stat. Data Anal. 49(4), 974–997 (2005).
Bates, D. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 1, 1 (2016).
Lüdecke MD. Package ‘sjPlot’ (2023).
Acknowledgements
Thanks to patients who accepted the study participation.
Funding
CIRMF is supported by Gabonese government via PID/PIH by Total Energies Gabon. CIRMF is a member of CANTAM funded by EDCTP [1045 CANTAM2 EDCTP ReNet2015].
Author information
Authors and Affiliations
Contributions
Conceptualization: Larson Boundenga, Formal analysis: Neil Michel Longo-Pendy, Clark Mbou-Boutambe, Yann Vital Sima-Biyang, Michelle Bignoumba, Clauve Jauvert Moukagni-Mussadji, Dorothé Marielle Wora, Fabrice Kassa-Kassa, Richard Onanga, Funding acquisition: Larson Boundenga and Jean Bernard Lekana-Douki Investigation: Neil Michel Longo-Pendy, Clark Mbou-Boutambe, Yann Vital Sima-Biyang, Larson Boundenga, Michelle Bignoumba, Clauve Jauvert Moukagni-Mussadji, Dorothé Marielle Wora, Methodology: Neil Michel Longo-Pendy, Clark Mbou-Boutambe, Yann Vital Sima-Biyang, Michelle Bignoumba, Clauve Jauvert Moukagni-Mussadji, Dorothé Marielle Wora, Fabrice Kassa-Kassa, Project administration and Supervision: Larson Boundenga. Validation: Larson Boundenga, Jean Bernard Lekana-Douki Writing original draft: Yann Vital Sima-Biyang, Neil Michel Longo-Pendy, Larson Boundenga, Draft editing: Larson Boundenga, Cyrille Bisseye Francine Ntoumi, Ayola Akim Adegnika, Jean-Bernard Lekana-Douki.
Data availability
All data (DNA sequences) generated during this study are included in this published article [and its supplementary information files] are available in Genbank (file number SUB14713119).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was done according to the Declaration of Helsinki. It was approved by the National Research Ethics Committee of Gabon (Approval No. 001/PR/SG/CNER/2018). Prior to participation, written informed consent was obtained from adult participants or their legal guardians for minor children (< 18 years). The data collection process was conducted anonymously as part of the routine analyses performed in the Medical Analysis Laboratory at CIRMF.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Boundenga, L., Sima-Biyang, Y.V., Longo-Pendy, N.M. et al. Epidemiology and diversity of Plasmodium species in Franceville and their implications for malaria control. Sci Rep 14, 31977 (2024). https://doi.org/10.1038/s41598-024-83487-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83487-0