Abstract
Postpartum depression (PPD) affects up to 20% of new mothers and has adverse consequences for the well-being of both mother and child. Exposure to stress during pregnancy as well as dysregulation in the mesolimbic dopamine (DA) reward system and its upstream modulator oxytocin (OT) have been independently linked to PPD. However, no studies have directly examined DA or OT signaling in the postpartum brain after gestational stress. Here we employed a chronic variable stress procedure during pregnancy and evaluated behavioral measures of mood and reward along with assessments of DA and OT signaling in postpartum rats. Our results show that gestational stress induced postpartum depressive-like and anxiety-like behavior in addition to producing reward-related deficits including anhedonia, impaired maternal care, and reduced maternal motivation. Consistent with a hypodopaminergic state, histological analysis revealed reduced expression of tyrosine hydroxylase in the NAc shell and core as well as reduced expression of the dopamine transporter and dopamine D2 receptor in the NAc shell of postpartum females exposed to gestational stress. A reduction in accumbal DA content as determined by liquid chromatography-mass spectrometry was also observed in gestationally-stressed dams. Lastly, we assessed mRNA expression of OT and OT receptors (OTR) and found that gestational stress increased OT expression in the hypothalamus but reduced OTR expression in the postpartum ventral tegmental area (VTA), a target of hypothalamic OT neurons. In the VTA, a reduction in OT-immunoreactive fibers following gestational stress was also seen. Taken together, these data demonstrate that the DA and OT systems within the postpartum reward circuit are sensitive to gestational stress and suggest that mood and maternal disruptions in PPD may arise from dysfunctional oxytocinergic regulation of the dopaminergic reward system.
Similar content being viewed by others
Introduction
An estimated 20% of new mothers experience postpartum depression (PPD) making it the most common non-obstetric complication of childbirth1. PPD is detrimental for maternal well-being and is the leading cause of maternal mortality resulting from suicide2,3. Among the core symptoms of PPD are depressed mood and anhedonia which can negatively impact mother-infant interactions and maternal bonding4,5. Consequently, children of mothers with PPD are at heightened risk for impaired cognitive, emotional, and social development as well as later life psychopathology6,7,8. Despite the high incidence of PPD, and the detrimental consequences for both mother and child, the underlying neurobiological mechanisms of PPD remain poorly understood.
Animal models that incorporate known risk factors for PPD provide a valuable translational tool to address its neurobiological underpinnings9,10,11. Several meta-analyses and systematic reviews have identified chronic stress during pregnancy as a major risk factor for PPD12,13,14 with greater numbers of life stressors being associated higher incidence of PPD15. Analogous to PPD, pregnant rodents exposed to chronic stress exhibit increased depressive-like behaviors during the postpartum period along with deficits in maternal behavior16,17,18,19,20,21,22,23,24,25 that too can have adverse effects on offspring development26,27. Thus, gestational stress can be used to mechanistically study impaired mood and maternal functioning seen in depressed mothers.
A prime candidate to mediate the mood and maternal behavioral disruptions caused by gestational stress is the mesolimbic reward system28,29. This system, which consists predominantly of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc), plays a well-established role in processing rewards and motivated behavior30, including maternal behavior31,32. Both clinical studies as well as preclinical work in rodents have implicated mesolimbic DA dysregulation in depression/depression-related behaviors33,34,35 and although few have focused on PPD specifically, the available evidence suggests aberrant mesolimbic DA system function is also relevant to maternal depression29,36,37,38,39. We have previously shown that in rats, gestational stress induces structural changes within the postpartum NAc19,40 but the extent to which gestational stress impacts the DA system is otherwise largely unknown. Here we aimed to fill this gap by examining behavioral measures of mood and reward along with assessments of DA signaling in the NAc of postpartum females exposed to chronic variable stress (CVS) during pregnancy.
An important modulator of the mesolimbic DA system is the neuropeptide oxytocin (OT)41. OT producing neurons in the paraventricular nucleus (PVN) and medial preoptic area (mPOA) of the hypothalamus project to DA neurons in the VTA which express oxytocin receptors (OTR)42,43. Within the VTA, OT has been shown to enhance the activity of DA neurons and to stimulate DA release44,45,46,47,48. Studies in rodents48,49,50 and humans51,52 have shown that interactions between the OT and DA systems regulate maternal care, that if disrupted can derail mothering48,52. OT itself has also been implicated in depressive behavior including PPD53,54,55. Thus, in addition to DA, we investigated the effects of gestational stress on measures of OT signaling in the postpartum hypothalamus and VTA56,57,58.
Results
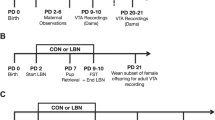
The experimental timeline and cohorts are depicted in Fig. 1.
Experimental timeline. Pregnant rats were exposed to a chronic variable stress procedure from gestation day 7 (GD7)-GD20. No stress controls were handled daily. The day of birth (DOB) was designated as postpartum day 0 (PD0). Behavioral testing and brain collection occurred from PD2-PD10. In Expt. 1, rats (No Stress = 10; Stress = 9) underwent testing for maternal behavior and brains were collected on PD7 for qPCR gene expression analysis of oxytocin (OT) and oxytocin receptor (OTR) in the hypothalamus and ventral tegmental area (VTA). In Expt. 2, rats (No Stress = 6; Stress = 5) were tested on the conditioned place preference (CPP) paradigm. In Expt. 3, rats (No Stress = 9; Stress = 9) underwent testing on the sucrose preference test (SPT) and brains were collected on PD8 for immunohistochemistry (IHC) and densiometric analysis of dopamine (DA) markers (DAT, dopamine transporter; D1R; dopamine 1 receptor; D2R, dopamine 2 receptor; TH, tyrosine hydroxylase) in the NAc. Maternal and litter characteristics were measured in the SPT dams. In Expt. 4, rats (No Stress = 12; Stress = 10) were tested on the elevated plus maze (EPM) and forced swim test (FST) and brains were collected on PD10 for IHC and densiometric analysis of additional DA markers in the NAc (pTH, phosphorylated tyrosine hydroxylase; VMAT, vesicular monoamine transporter) as well as counts of TH + cells in the VTA. OT IHC was also done to assess the effects of gestational stress on OT + cells in the hypothalamus and OT fibers in the VTA. In Expt. 5, dams (No Stress = 6; Stress = 6), the NAc was dissected on PD6 for Liquid Chromatography-Mass Tandem Spectrometry (LC–MS/MS) to measure DA content and the dopamine metabolite, DOPAC. Figure created in https://BioRender.com.
Gestational stress effects on maternal and litter characteristics
Exposure to CVS during pregnancy reduced gestational weight gain as compared to unstressed controls (t16 = 3.28, p = 0.005). During the postpartum period, there was no effect of gestational stress on weight gain (t16 = 0.29, p > 0.05). There was also no detectable difference in litter size (Mann Whitney U = 29.5, p > 0.05) or litter weight on PD1 (Mann Whitney U = 27, p > 0.05) and no effect on litter weight gain (t16 = 1.35, p > 0.05). Data are shown in Table 1.
Gestational stress induces a PPD-like behavioral phenotype
Exposure to gestational stress impaired maternal behavior. In a pup retrieval test, stressed dams were slower to retrieve all pups to the nest (i.e., longer latencies) as compared to unstressed controls (t14.90 = 2.50, p = 0.03; Fig. 2A). Observations of home-cage maternal behavior further showed that in stressed dams, there was a reduction in the percent time spent hovering over pups (t17 = 2.91, p = 0.009; Fig. 2B) and a reduction in pup licking and grooming (Mann Whitney U = 9, p = 0.002; Fig. 2C). Instead of pup-directed behaviors, stressed dams engaged more in self-grooming (t17 = 2.39, p = 0.03; Fig. 2D). There was no significant effect of gestational stress on time spent nesting (t11.14 = 1.77, p > 0.05; data not shown).
Gestational stress disrupts maternal care and maternal motivation. (A) In a pup retrieval test, dams exposed to gestational stress exhibited longer latencies to retrieve pups to the nest as compared to unstressed controls. In home cage observations of maternal care, gestationally stressed dams spent (B) less time hovering over their pups and (C) less time licking and grooming their pups but instead engaged in (D) more self-grooming. (E) During habituation for the conditioned place preference test, there was no effect of gestational stress on shuttling. (F) Following conditioning, unstressed dams spent more time in the pup-associated chamber generating a positive preference ratio. In contrast, gestational stress reduced time in the pup-associated chamber following conditioning generating a negative preference ratio. *p < 0.05; ** p < 0.01.
The effects of gestational stress on maternal care might arise via disruptions in maternal motivation which was assessed using a two-chamber conditioned place preference paradigm. During habituation, shuttling between the chambers was similar in the stressed and unstressed dams indicating no effect of gestational stress on locomotor activity (t9 = 0.66, p > 0.05; Fig. 2E). Following conditioning, there was a significant effect of gestational stress on preference for the pup-associated chamber (t9 = 3.25, p = 0.01; Fig. 2F). Specifically, control dams showed a positive preference ratio suggesting a preference for the pup-associated chamber. In contrast, stressed dams showed a negative preference ratio suggesting a preference for the non-pup chamber and a shift in the motivational value of pups following gestational stress.
To validate the effects of chronic variable gestational stress on PPD-relevant emotional behaviors, dams were tested on the forced swim test (FST), sucrose preference test (SPT) and elevated plus maze (EPM). In the FST, gestational stress increased immobility (t20 = 2.17, p = 0.04; Fig. 3A) and decreased climbing (t20 = 3.98, p = 0.0007; Fig. 3B), indicative of passive stress coping and a depressive-like phenotype. Anhedonia, as measured with SPT, was also evident in gestationally stressed dams who exhibited reduced sucrose preference compared with control dams (t9.147 = 2.63, p = 0.03; Fig. 3C). Baseline water consumption was unchanged between groups (t16 = 2.02, p > 0.05; data not shown). Dams exposed to gestational stress also displayed increased threat appraisal/anxiety-like behavior in the EPM as evidenced by a reduction in time spent in open arms of the EPM (Mann Whitney U = 27.5, p = 0.03; Fig. 3D) and fewer open arm entries (Mann Whitney U = 20, p = 0.006; Fig. 3E). There was no difference in closed arm entries indicating no effect of gestational stress on locomotor behavior (t20 = 0.67, p > 0.05; Fig. 3F).
Gestational stress induces PPD-relevant emotional behaviors. Compared to unstressed controls, dams that experienced stress during pregnancy displayed (A) increased immobility and (B) decreased climbing behavior in the forced swim test. (C) Preference for a 2% sucrose solution was also reduced in gestationally stressed dams as compared to unstressed controls. In the elevated plus maze, gestational stress (D) reduced the percentage of time spent in the open arms and (E) reduced open arms entries without affecting closed arms entries (F). *p < 0.05; **p < 0.01; ***p < 0.005.
Gestational stress induces a hypodopaminergic state in postpartum dams
We next evaluated the effects of gestational stress on DA signaling in reward regions of the postpartum brain. Using immunohistochemistry, we found that gestational stress reduced percent area staining of several dopaminergic signaling markers in the NAc shell (Fig. 4A). Specifically, percent area staining of tyrosine hydroxylase (TH; t16 = 4.33, p = 0.0005; Fig. 4B), phosphorylated tyrosine hydroxylase (pTH; t18 = 2.35, p = 0.03; Fig. 4C), dopamine transporter (DAT; t16 = 3.28, p = 0.005; Fig. 4D), and dopamine D2 receptor (D2R: t15 = 3.69, p = 0.002; Fig. 4G) was reduced in gestationally stressed dams. In contrast, there was no effect of gestational stress on percent area staining for vesicular monoamine transporter (VMAT; Mann Whitney U = 34, p > 0.05; Fig. 4E) or dopamine D1 receptor (D1R; t16 = 0.09, p > 0.05; Fig. 4F) in the NAc shell. In the NAc core (Supplemental Fig. 2A-D), gestational stress reduced percent area staining of TH (t15 = 2.47, p = 0.03) but did not alter percent area of DAT (t16 = 1.48, p > 0.05), D1R (t16 = 0.72, p > 0.05) or D2R staining (Mann Whitney U = 18, p > 0.05). Lastly, there was no difference between unstressed and stressed dams in the number of TH + somas in the VTA after gestational stress (t17 = 0.25, p > 0.05; Supplemental Fig. 3).
Gestational stress alters markers of dopaminergic signaling in the NAc shell. (A) In the nucleus accumbens (NAc) shell, markers associated with dopaminergic signaling were examined via immunohistochemistry on PD8 or PD10. Compared to unstressed controls, dams exposed to gestational stress showed a reduction in percent area staining for several dopaminergic markers including (B) tyrosine hydroxylase, TH, (C) phosphorylated TH, pTH and (D) the dopamine transporter, DAT. (E) There was no effect of gestational stress on vesicular monoamine transporter, VMAT. Percent area staining for dopamine receptors (F) D1R and (G) D2R in the NAc shell was also examined and only the D2R was reduced by gestational stress. Representative images of each marker analyzed are shown in (A). *p < 0.05; **p < 0.005; ***p ≤ 0.0005.
Using Liquid Chromatography-Mass Tandem Spectrometry (LC–MS/MS) we also assessed DA content and turnover in the NAc of stressed and unstressed dams (Fig. 5A). We found gestational stress reduced accumbal DA (t10 = 1.95, p = 0.04; Fig. 5B) without affecting accumbal DOPAC (Mann Whitney U = 9, p > 0.05; Fig. 5C) resulting in an increase in the DOPAC/DA turnover ratio (t10 = 2.65, p = 0.02; Fig. 5D).
Gestational stress alters accumbal dopamine content. (A) Dopamine (DA) content, the DA metabolite DOPAC, and turnover rate in the nucleus accumbens (NAc) was assessed via Liquid Chromatography-Mass Tandem Spectrometry on PD6. (B) Gestationally stressed mothers had significantly lower DA as compared to unstressed controls. (C) DOPAC did not differ between unstressed dams and those exposed to gestational stress. (D) The DOPAC/DA ratio, a measure of DA turnover, was increased in NAc of dams exposed to gestational stress. *p < 0.05.
Gestational stress differentially impacts the OT system of the postpartum hypothalamus and VTA
As OT is an important modulator of the DA system, we analyzed the impact of gestational stress on OT signaling. Gene expression analysis of the hypothalamus (Fig. 6A) showed increased expression of OT mRNA in dams that underwent gestational stress (t10.83 = 2.12, p = 0.03; Fig. 6B). No effect of gestational stress was noted on hypothalamic expression of OTR (t9.499 = 0.89, p > 0.05; Fig. 6C). In the VTA (Fig. 6D), gestational stress did not alter OT expression (Mann Whitney U = 30, p > 0.05, Fig. 6E) but significantly reduced OTR expression (t17 = 1.76, p = 0.05; Fig. 6F). Using immunohistochemistry, the effects of gestational stress on hypothalamic OT + cells and OT fibers in the VTA were also examined. These analyses (Supplemental Fig. 4) showed no significant differences between unstressed and gestationally stressed dams in the numbers of OT + cells in either the mPOA (t20 = 0.65, p > 0.05) or PVN (t11.78 = 1.79, p > 0.05). However, there was an effect of gestational stress on OT fibers within the VTA (Fig. 6G) such that percent area staining for OT was lower in gestationally stressed dams in comparison with controls (t7.802 = 5.09, p = 0.001, Fig. 6H).
Gestational stress differentially altered oxytocin and oxytocin receptor expression in the hypothalamus and VTA as well as oxytocin fibers in the VTA. Gene expression analysis was conducted in the hypothalamus and ventral tegmental area (VTA) on PD7. (A) In the hypothalamus, gestationally stressed mothers had higher expression of oxytocin (OT), whereas no differences between stress and control dams were noted in oxytocin receptor (OTR) expression (B, C). (D) In the VTA, gestational stress didn’t alter OT expression, but reduced OTR expression (E, F). (G, H) Immunohistochemical analysis of the VTA indicated that gestationally stressed mothers also had lower density of OT fibers in the VTA in comparison with unstressed dams on PD10. Representative images of OT fibers in the VTA are shown. *p ≤ 0.05, ***p ≤ 0.001.
Discussion
Here we show that exposure to CVS during pregnancy leads to postpartum emotional dysregulation, reduced maternal motivation, and impaired maternal care. These stress-induced behavioral changes were associated with disrupted DA and OT signaling in areas of the mesolimbic reward system. Overall, these data highlight the potential for oxytocinergic regulation of the dopamine system to act as a mediator for the pathological effects of stress on behavior in the postpartum period.
Gestational stress paradigms are often selected for not influencing maternal weight gain and litter size, so as to limit confounding factors59. Here we used CVS applied from GD7-20 and found it had little impact on somatic measures in mothers or offspring. Aside from a reduction in weight gain during pregnancy, stressed and unstressed groups did not differ in gestational length suggesting that our maternal stress model did not interfere with mechanisms important for parturition. Moreover, litter size and litter weight at birth was similar for stressed and unstressed dams and thus gestational stress did not affect fetal resorptions or placental nutrient transfer capacity essential for fetal growth. Finally, postpartum maternal and litter weight gain were similar in the stressed and unstressed dams. Overall, these data indicate that chronic variable gestational stress limits normal pregnancy weight gain without compromising overall litter size or offspring viability.
Maternal behavior is sensitive to gestational stress including CVS 17,18,21,24,40,60 which we show impaired pup retrieval, time spent with pups on the nest, and pup licking/grooming. Instead, stressed dams engaged in more self-grooming which is an anxiety-like response61 that could interfere with the ability to adequately display maternal care62,63. Another way stress-induced deficits in maternal care might arise is through disrupted maternal motivation. Pup retrieval is considered to be a motivated maternal behavior64,65 and thus the longer retrieval latencies in stressed dams support this possibility. To further assess the effects of gestational stress on maternal motivation we used CPP. Previous work has shown that early postpartum dams develop a strong preference to their pups in the CPP paradigm over a pup-size plastic piece of tubing or even cocaine indicating that pups have high incentive value28,66,67. Here, using CPP we show that postpartum females exposed to stress in pregnancy exhibit impaired maternal motivation and failure to develop a preference for the pup-associated chamber. In fact, gestationally stressed dams seemed to exhibit a pup aversion as they showed a negative preference ratio in the CPP test. Adequate display of maternal behavior requires not only an increase in reward and approach behavior towards pups but also a suppression in pup aversion68. Since stressed dams displayed pup-aversion like behavior in the CPP, gestational stress might not only interfere with the neurobiological processes that mediate approach but also those that mediate suppression of pup aversion.
Our behavioral analysis additionally showed that gestational stress induces anhedonia-like behavior during the postpartum period as demonstrated by a reduction in sucrose preference. These results align with other studies using variable stress paradigms during pregnancy which also found reductions in postpartum sucrose preference21,22,27. However, the ability of gestational stress to induce postpartum anhedonia may depend on the timing, duration, and type of stressor as one week of late gestation sub-chronic stress in the form of daily forced swimming was insufficient to induce postpartum anhedonia69. Variable chronic mild stress has also been shown to decrease sucrose preference in nulliparous female rats70,71 but these effects are seen following three or more weeks of stress. In contrast, gestationally stressed mothers in this study demonstrated a reduction in sucrose preference following two weeks of variable stress and almost one week of recovery. These differences between our results and those in nulliparous females may suggest that pregnancy is a time of heightened stress susceptibility in females.
Coinciding with previous research utilizing other gestational stress paradigms16,18,19,20,21,56 our CVS procedure increased immobility and decreased climbing in the FST, suggesting a passive coping response which has been previously interpreted as depressive-like behavior72. In dams exposed to chronic variable gestational stress, we also observed reduced open arm exploration in the EPM which could reflect an increase in postpartum threat appraisal or anxiety-like behavior. However, it’s worth mentioning that the effects of pregnancy stress on behavior in the EPM are variable across studies with some finding reduced open arm exploration in stressed dams17,56,73 and others finding no effect of gestational stress20,22. These discrepancies are likely due to the specifics of the stress paradigm or testing procedure employed. Nonetheless, the emergence of both depressive-like and anxiety-like behavior following exposure to CVS during pregnancy in the present study aligns with epidemiological evidence showing high comorbidity of anxiety and depression in PPD 74, and together with the reward-related deficits, further support the strength of this translational model.
In rodents, maternal motivation and maternal care are impaired following pharmaceutical interventions and electrolytic lesions to different components of the reward circuit32,75,76,77. The neurobiological mechanisms that contribute to depressive-like behaviors also involve alterations in the DA system30,33,78. As such, the maternal and depressive phenotype following gestational stress as observed here and in other studies could be due to disruptions in the functional integrity of the mesolimbic DA system. Indeed, our immunohistochemical analysis of the NAc revealed significant reductions in the density of several dopaminergic markers including TH, pTH, and DAT. Further, gestational stress reduced D2R expression in the NAc without altering D1R expression. Decreased D2R aligns with the critical role for this DA receptor subtype in maternal motivation75,79,80 and when combined with the reduction in TH and DAT suggest that gestational stress induced a hypodopaminergic state. To further explore this possibility, we examined DA content in the NAc using LC–MS/MS and found a significant decrease in gestationally stressed dams. Moreover, we found that the DA metabolite DOPAC was unaffected by gestational stress leading to an increase in the DOPAC/DA ratio. The DOPAC/DA ratio is a measure of DA turnover, an increase in which may reflect a compensatory response driven by decreases in DA. Increased DA turnover could also suggest that gestational stress increases DA catabolism. As DA catabolism is catalyzed by monoamine oxidase (MAO) and Catechol-O-methyltransferase (COMT), increased catabolism could result from greater expression/activity of these enzymes. This possibility is supported by a human PET imaging study showing increased MAO-A VT, an index of MAO-A density, within several brain regions including the striatum during PPD as well as data associating the development of peripartum depressive symptoms with gene polymorphisms of MAO-A and COMT81,82. Overall, the present findings align with previous work linking aberrations in accumbal DA signaling with impairments in maternal care and maternal motivation83,84,85,86 as well as depressive-like behavior78. Thus, it is likely that the PPD-like behavioral phenotype seen following gestational stress is mediated, at least in part, by attenuated DA signaling with NAc.
It is important to note that the effects of gestational stress on markers of DA signaling were largely anatomically specific. Aside from a reduction in TH in both NAc regions following gestational stress, expression of DAT and D2R was only impacted by gestational stress in the NAc shell. These findings are consistent with data showing greater sensitivity of the NAc shell to stress87,88. Further, they expand upon our previous work which found an effect of gestational stress on structural plasticity of medium spiny neurons in the NAc that was restricted to the shell region19,40. Considering that DA is a positive regulator of striatal structural plasticity and dendritic spine density89, it is possible that lower levels of accumbal DA following gestational stress lead to reductions in spine density.
OT is an important modulator of the DA system41,46,90. Here we show that gestational stress had differential effects on measures of OT signaling in the hypothalamus and VTA. In the hypothalamus, gestational stress increased postpartum expression of OT without altering the numbers of hypothalamic OT + cells or hypothalamic expression of OTR. In contrast, gestational stress did not alter OT mRNA expression in the VTA but did attenuate the expression of OTR mRNA. Notably, genetic studies implicate hypermethylation of the OTR gene in PPD91. Hypermethylation is typically associated with suppression of gene transcription which eventually leads to reduced mRNA and thus reduced OTR expression observed in the current study coincides with the genetic findings. Within the VTA, gestational stress also reduced OT fiber density which has been used as a proxy for OT release92. Reduced OTR coupled with reduced OT fiber density suggests that OT signaling within the VTA is compromised by gestational stress exposure such that despite greater hypothalamic OT expression, there may be less availability of OT in the VTA and reduced sensitivity to the OT that is present. Since OT signaling in the VTA is important for the regulation of accumbal DA release and the regulation of maternal care31,48, these data may provide a mechanistic explanation for how gestational stress may lower DA release to ultimately disrupt mother-pups interactions.
In summary, our data recapitulate the link between disrupted maternal care and increased depression-like behaviors in rodent models of PPD. This study also extends current knowledge regarding stress-induced changes in the maternal brain by demonstrating that gestational stress compromises DA and OT signaling in the reward system and points to a mechanism by which stress disrupts maternal mood, motivation, and mothering to drive maladaptive behavioral states in new mothers with PPD.
Methods
Animals
Timed pregnant Sprague–Dawley rats (Taconic; Albany, NY) arrived at our facility on gestational day (GD) 4 and were individually housed with unlimited access to food and water, except as described below. Colony conditions were maintained at 23◦ ± 1 ◦C with a 12:12 light: dark cycle (lights on at 6 AM). The day of pup delivery was designated as postpartum day (PD)0 and on PD1, litters were culled to 8–10 pups (4–5 males, 4–5 females). To minimize the possibility that any observed differences between stressed and unstressed mothers were indirectly due to the effects of gestational stress on the pups, litters were combined at the time of culling and randomly assigned to a postpartum female. All procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines93.
Gestational stress protocol
Pregnant rats were randomly assigned to stress or no stress groups. Stressed rats underwent a CVS protocol from GD7-GD20. CVS was performed during the day, unless specified otherwise and consisted of one or two of the following stressors per day: restraint in ventilated clear Plexiglas cylinders (21 cm long, 6 cm internal diameter) for either 45 min or 2 h, overcrowding during the day for 12 h, overnight food deprivation (12 h), wet bedding either during the day or the night (12 h), cage change stress (pregnant female transferred into the home cage of an unfamiliar, sexually experienced resident male) or electric foot shock (0.5 mA, 1 ms shock/per min for 10 min). On days of 12 h long stressors (overcrowding or wet bedding), stressed rats were subjected to only that stress. On days when 12 h long overnight stressors were delivered (food deprivation or wet bedding), one of the short duration stressors (restraint, cage change, electric foot shock) was delivered on the preceding and proceeding days. When two short duration stressors were employed, they were separated by at least 4 h. All stressed animals followed the same schedule of stressors. Unstressed controls were handled daily for 5 min.
Behavioral testing
Behavioral tests were conducted from PD2-PD9 as outlined in Fig. 1. Except for SPT, all behavior was digitally recorded and analyzed blind using BEST analysis software (Education Consulting Inc.; Hobe Sound, FL).
Maternal behavior. Maternal behavior (No stress, n = 10; Stress, n = 9) was evaluated twice daily on PD2, PD4 and PD6. One session took place during lights on (7–10 AM), and the other session took place during lights off (7–10 PM) under red light illumination. Each session began with a 30 min acclimation to the testing room, followed by a 30 min home cage observation. Litters were then removed from the home cage for 15 min and nests disturbed. A 15 min pup retrieval test was then performed by reintroducing all pups into the home cage in a scattered manner. Home cage tests were analyzed for pup licking/grooming, hovering over pups, nest building, and self-grooming. Percent time engaged in each behavior was calculated. For the pup retrieval test, latency to retrieve and group all pups into the nest was measured.We report the behaviors averaged across postpartum days and times.
CPP. The CPP apparatus consisted of two chambers (27.5 cm × 27.5 cm × 20.5 cm), one white and one black, separated by a removable divider. On PD2, dams (No stress, n = 6; Stress, n = 5) were habituated to the CPP apparatus for 30 min without the divider. Percent time spent in each chamber was determined to assess pre-conditioning preferences and the number of crossings between the two chambers (shuttling) was determined to assess locomotion. On PD3-PD6, dams underwent conditioning for 2 h/day in an alternating sequence. Before conditioning, there was no effect of gestational stress on time spent in the white versus black chamber, although all rats preferred the black chamber (F1,18 = 5.76, p < 0.05; Supplemental Fig. 1). Because of this preference, three pup-sized plastic tubes were conditioned to the black chamber and three pups were conditioned to the white chamber. Throughout conditioning, an opaque Plexiglas divider separated the chambers. Testing took place on PD7. The divider was removed and rats allowed to freely explore the apparatus in the absence of pups or plastic tubing for 30 min. A preference ratio was calculated as time spent in the pup-associated white chamber during testing on PD7/time spent in the same chamber during habituation. All sessions took place in the AM under red light illumination. The apparatus was cleaned with soapy water between each rat.
SPT. The SPT was performed from PD2-7 (No stress, n = 9; Stress, n = 9). On PD3, lixits were removed and two plastic water bottles were placed in the cage. Each day from PD3-PD5, rats were deprived of water for 3 h from 3-6 PM. Access to water was then restored and intake over the next hour assessed by subtracting water bottle weight at the end of the assessment period from water bottle weight at beginning of the assessment period. On PD6 and PD7, water bottles were removed at 3 PM for 3 h after which one bottle filled with water and one with 2% sucrose was placed back into the cage for 1 h in a counterbalanced manner across days, and intake measured by weighing bottles. Sucrose preference was calculated as the percentage of sucrose solution ingested/total amount of sucrose and water consumed. Maternal (body weight measured daily from GD7-GD21 and from PD2-PD7) and litter (litter size and litter weight on PD1 prior to culling, daily litter weights from PD2-7) characteristics were measured in the SPT animals.
EPM. The EPM consisted of a plus-shaped elevated platform (height: 50 cm) with four arms (width: 10 cm, length: 50 cm), two of which were enclosed by walls 50 cm high. On PD7, dams (No stress, n = 12; Stress, n = 10) were acclimated to the testing room for 10 min. Dams were then placed in the 10 cm × 10 cm center zone of the EPM allowed to explore the maze for 5 min. Number of entries into the open arms and the percent time spent in the open arms [(time in open arms/time in open and closed arms) × 100] were used as measures anxiety-like behavior. Closed arm entries were used to assess locomotion. EPM testing was done between 7-10 PM under red lighting. The EPM was cleaned with soapy water between each rat.
FST. 48 h following the EPM (PD9), rats were subjected to the FST to assess behavioral despair/passive stress coping. The FST was performed on two consecutive days between 10 AM-12 PM under ambient lighting (550 lx). Plexiglas cylinders (diameter, 30.5 cm; height, 49 cm) were filled to a depth of 30 cm with 25 ± 0.5 °C water. On day 1, rats were individually placed into the cylinders for 15 min, towel-dried, and returned to their home cage. 24 h later, rats were returned to the cylinders for 5 min. Percent time immobile (floating in the water only making movements necessary to maintain the head above water) and percent time climbing the walls of the cylinder was measured on day 2. Water was replaced between each rat.
Tissue collection
All tissue was collected 24 h after behavioral testing (Fig. 1) except tissue for LC–MS/MS which was collected from a separate cohort of PD6 (No stress, n = 6; Stress, n = 6) dams that did not undergo behavioral testing. Rats used for immunostaining were deeply anesthetized with Euthasol (Virbac; Fort Worth, TX) and transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed, postfixed in 4% paraformaldehyde overnight, and then transferred to 0.1 M PBS. A 1:12 series of 40 µm thick coronal sections was obtained using a Vibratome (Leica, Model VTS1000S; Deer Park, IL) and kept at 4 °C until immunostaining. Rats used for qPCR were deeply anesthetized with Isoflurane and then rapidly decapitated. Brains were removed, flash-frozen in 2-methylbutane over dry ice, and stored at -80 °C. Using a 1000 µm tissue biopsy punch, the hypothalamus (0.8 mm and 1.6 mm posterior to Bregma; Paxinos & Watson, 1998), VTA (5.2 mm and 6.4 mm posterior to Bregma; Paxinos & Watson, 1998), and NAc (1.7 mm and 1 mm anterior to Bregma; Paxinos and Watson, 1998) were obtained from coronal brain slices 1 mm in thickness made using a rat brain matrix. Punches were homogenized in a Tris-Triton lysis buffer and samples kept at -80 °C. Rats used for LC–MS/MS were deeply anesthetized with Euthasol and rapidly decapitated. Brains were quickly removed and the NAc dissected and placed in pre-weighed 2 mL screw cap tubes which were flash-frozen in liquid nitrogen. Punches were homogenized in Tris-Triton lysis buffer and samples kept at -80 °C.
Dopamine signaling
Immunohistochemistry for dopaminergic markers. Tissue from animals that underwent SPT was used to analyze the density of TH, DAT, D1R, and D2R separately in the NAc shell and NAc core. For densitometry analysis of VMAT and pTH in the NAc shell as well as the analysis of TH + cells in the VTA, tissue from animals that underwent the EPM and FST was used.
Free floating sections were rinsed in 0.1 M PBS and quenched of native peroxidase activity in 0.3% H2O2 in 50% methanol for 1 h at RT. Next, sections were blocked in 5% BSA in 0.1 M PBS for 1 h at RT and incubated with one of the following primary antibodies: rabbit anti-D1R (1:50; Cat#sc-14001, Santa Cruz Biotechnology), goat anti-dopamine D2R (1:100; Cat#sc-7522, Santa Cruz Biotechnology), rabbit anti-TH (1:1000; Cat#AB152, Millipore), rabbit anti-DAT (1:200; Cat#AB1591P, Millipore), rabbit anti-VMAT(1:100; Cat#PA5-22,864, ThermoFisher), and rabbit anti- pTH (1:500; Cat#AB5935, Millipore) overnight at 4 °C. Next, sections were rinsed in 0.1 M PBS and incubated with a corresponding biotinylated secondary antibody (1:500; Vector Laboratories) for 2 h at RT. Sections were then rinsed, incubated with avidin–biotin complex (Vector Laboratories), rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2. Sections were mounted onto Fisher Superfrost Plus slides (Fisher Scientific), dried, and coverslipped with Permount. Negative control sections, in which the primary antibody was omitted, were included.
Microscopic analysis. Brightfield images were obtained of the NAc (1.7 mm and 1 mm anterior to Bregma; Paxinos and Watson, 1998) or the VTA (-5.2 mm and -6.3 posterior to Bregma; Paxinos and Watson, 1998) at 20X using a Nikon 90i microscope (Nikon Instruments; Melville, NY). Three to six images across three to six brain sections were captured. For the NAc shell and core, images were analyzed for the density of TH, D1R, D2R, and DAT staining. pTH and VMAT2 staining density were also analyzed in the NAc shell. Densiometric analyses were performed using NIS Elements software (Nikon Instruments). For each image, a threshold for positive labeling was determined against a region of background staining within a region of interest (ROI). Following thresholding, percentage area of positive labeling was determined for each ROI as follows: area in positive threshold/total area × 100. For each marker and brain region, percentages were averaged across images to obtain a mean density of percent area staining for each animal. For the VTA, images were analyzed for TH + somas. For this analysis, a ROI was defined within an image and the number of TH + somas counted. TH + cell counts were divided by the area of the ROI then averaged for each animal and data expressed as the number of immunoreactive cells per mm2. All analyses were performed blind. Tissue from one no stress dam for the pTH NAc shell analysis and three no stress dams for the TH VTA analysis were compromised during staining and excluded from analyses.
LC–MS/MS. Brain samples were lyophilized for 48 h at -83 °C. Dry weights were recorded and a 5 mm tungsten bead was added to each tube standing on ice. The extracts were disrupted using a bead beater for 2 min at a frequency of 30 Hz. The grounded samples were put back on ice, centrifuged (17,000 g) for 30 s at 4 °C, and placed on ice. 300 µL of boiling ultrapure water was added to each sample, vortexed and incubated in a boiling water bath for 10 min. After incubation, the extracts were cooled down on ice for 5 min, and 300 µL of an acetonitrile (LC–MS grade, Fisher Scientific) solution containing 0.2% formic acid (LC–MS grade, Fisher Scientific) was added to each tube. The samples were spun (17,000 g) for 10 min at 4 °C, their upper phase was collected and transferred to a 1.5 mL microcentrifuge tube. The extracts were then placed in a speed vacuum to remove acetonitrile and formic acid prior to lyophilization at -83 °C overnight. Dried extracts were re-suspended in 100 µL of a 0.1% formic acid aqueous solution, vortexed, centrifuged (17,000 g) for 5 min at 20 °C and transferred to LC–MS/MS glass vials. Analysis was carried out using an Ultra High-Pressure Liquid Chromatography 1290 (Agilent Technologies, Inc; Santa Clara, CA) coupled to a hybrid Triple Quadrupole/Ion trap mass spectrometer QTRAP 5500 (AB Sciex; Framingham, MA).
Standards (Sigma) were diluted to a concentration of 0.05 µM/L for DA and 1 µM/L for DOPAC. Metabolites were injected individually and directly into the mass spectrometer apparatus. This step (direct infusion) optimizes four major parameters of a specific metabolite: (1) the declustering potential, (2) the entrance potential, (3) the collision energy potential, and (4) the collision cell exit potential. Compound parameters are shown in Supplemental Table 1.
The extracts were placed in an autosampler kept at 10 ºC. 20 µL of sample was injected onto the column and the liquid chromatography analysis carried out at 50 ºC. DA and DOPAC were separated using a Waters C18 BEH column (2.1 × 100 mm; 1.7 µm) with a Vanguard HSS T3 pre-column (2.1 × 5 mm; 1.8 µm). The gradient used to separate the neurotransmitters consisted of acetonitrile plus 0.1% formic acid (solvent A) and 0.1% formic acid in water (solvent B). The total LC–MS/MS run was 10 min with a of 500 µl/min flow rate. The gradient went as follows: A = 0–1 min 0%, 1–1.2 min 20%, 1.2–4 min 20%, 4–4.1 min 90%, 4.1–6 min 90%, 6–6.1 min 0%, and 6.1–10 min 0%.
The mass spectra were acquired using turbo spray ionization in positive (5000 V) and negative (-4500 V) modes for DA and DOPAC, respectively. DA and DOPAC were detected in the same LC–MS/MS run using multiple reaction monitoring by switching back and forth from negative to positive mode with a settling time of 50 ms. Source parameters are shown in Supplemental Table 2. The temperature of the source was fixed at 550o C. The mass spectrometer was set to have a dwell time of 50 ms. LC–MS/MS data were acquired and processed using Analyst 1.6.1 software.
Oxytocin signaling
OT immunostaining. Tissue from animals that underwent the FST and EPM was used for OT immunostaining. Free floating sections were rinsed in 0.1 M PBS and quenched of native peroxidase activity in 1% H2O2 in 50% methanol for 1 h at RT. Next, sections were blocked in 3% NHS in 0.1 M PBS for 1 h at RT and then incubated with mouse anti-OT (1:8000; Cat#MAB5296, Millipore) overnight at 4 °C. Next, sections were rinsed in 0.1 M PBS and incubated with a biotinylated anti-mouse secondary antibody (1:500, Vector Laboratories) for 2 h at RT. Sections were then rinsed, incubated with avidin–biotin complex (Vector Laboratories), rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2. Sections were mounted onto Fisher Superfrost Plus slides, dried, and coverslipped with Permount. For OT immunofluorescence, free floating sections were rinsed in 0.1 M PBS and blocked in 3% NHS in 0.1 M PBS for 1 h at RT. Sections were then incubated with mouse anti-OT (1:4000; Cat #MAB5296, Millipore) overnight at 4 °C. Next, sections were rinsed in 0.1 M PBS and incubated with DyLight549 secondary anti-mouse antibody (1:200; Cat.#DI-2549, Vector Laboratories) for 2 h at RT. Sections were mounted onto Fisher Superfrost Plus slides and coverslipped with DABCO.
Microscopic analysis. For OT fiber density analysis, three-six brightfield images of the VTA (-5.2 mm and -6.3 posterior to Bregma; Paxinos and Watson, 1998) were obtained at 20X with a Nikon 90i microscope across three to six brain sections. Percent area OT staining was performed as described above for the DA markers. For analysis of OT + cells, two to four 20X z-stacked images of the mPOA (-0.3 mm posterior to Bregma; Paxinos and Watson, 1998) and PVN (-1.3 mm posterior to Bregma; Paxinos and Watson, 1998) were acquired across two to four sections using a confocal microscope (Nikon C1 confocal microscope; Nikon Instruments, Melville, NY). An ROI was defined within an image and the number of OT + somas counted. OT + cell counts were divided by the area of the ROI then averaged for each animal and data expressed as the number of immunoreactive cells per mm2. All analyses were performed blind. For the VTA analysis, tissue from four no stress dams were compromised during staining and excluded from the analyses.
q-PCR. Tissue from dams that underwent maternal behavior testing was used for qPCR. Tissue was homogenized in Trizol (Life Technologies, Carlsbad, CA). Total RNA was extracted with a NucleoSpin RNA II kit (Macherey–Nagel; Düren, Germany). A cDNA library was reverse-transcribed from RNA using the iScript reverse transcription Supermix for RT-qPCR (Bio-Rad; Hercules, CA) and diluted with RNase-free water, yielding a 5 µg/µl working dilution. Primers were designed with PrimerQuest software (Integrated DNA Technologies; Coralville, IA). Primer pairs included oxytocin (accession number: NM_012996.3, forward sequence: ATCTCGGACTGAACACCAAC, reverse sequence: GGCAGTTCTGGATGTAGCA), oxytocin receptor (accession number: NM_012871.2, forward sequence: AGACGAGTGTCAGCAAGAAG, reverse sequence: ATGCTGAAGATGGCTGAGAG) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, accession number: NM_017008, forward sequence: TGACTCTACCCACGGCAAGTTCAA, reverse sequence: ACGACATACTCAGCACCAGCATCA). Primer pairs and SsoAdvanced SYBR Green Supermix (BioRad) were prepared in a master mix according to manufacturer directions. PCR amplification reactions each included 1 µl diluted cDNA and 19 µl of master mix. Each tissue sample was run in triplicate reactions for each primer pair. Two negative controls included reaction of sample without reverse transcription and reaction without a cDNA template. PCR reactions were run on a CFX96, C1000 Thermal Cycler (Bio-Rad). Amplification conditions were: 95 °C for 30 s and 40 cycles of PCR (denaturation: 95 °C for 5 s, annealing/extension: 60 °C for 30 s), followed by melting curves to verify the absence of primer dimers. The cycle at which expression levels crossed threshold (Ct) was normalized to the Ct of the reference gene GAPDH, producing ∆Ct with arbitrary units of total gene expression, and gene expression in MBS was expressed as fold-change from control.
Statistical analyses
Group data are reported as the mean ± SEM. ROUT (Q = 1%) was used to identify outliers. Normality was determined via the Shapiro–Wilk test. Normally distributed data were analyzed using two-tailed unpaired t-tests, with Welch’s correction applied when group variances were unequal. For non-normal data, variances were first analyzed with Levene’s test. If the homogeneity of variance assumption was met, non-normal data were analyzed using the Mann-Whitney test. If variances were unequal, non-normal data were log transformed and analyzed with a two-tailed unpaired t-test with Welch’s correction. Analyses for which Welch’s correction was applied are indicated in the results by non-integer values for the degrees of freedom. Except for Levene’s test, all analyses were conducted using GraphPad Prism software version 10.1.0 (GraphPad; La Jolla, CA) with significance set at p < 0.05.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author [BL].
References
O’Hara, M. W. & Wisner, K. L. Perinatal mental illness: definition, description and aetiology. Best Pract. Res. Clin. Obstet. Gynaecol. 28(1), 3–12. https://doi.org/10.1016/j.bpobgyn.2013.09.002 (2014).
Chesney, E., Goodwin, G. M. & Fazel, S. 4102288; risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry. 13(2), 153–160. https://doi.org/10.1002/wps.20128 (2014).
Slomian, J., Honvo, G., Emonts, P., Reginster, J. & Bruyere, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health (Lond). 15, 1745506519844044. https://doi.org/10.1177/1745506519844044 (2019).
Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behav. Dev. 33(1), 1–6. https://doi.org/10.1016/j.infbeh.2009.10.005 (2010).
Lovejoy, M. C., Graczyk, P. A., O’Hare, E. & Neuman, G. Maternal depression and parenting behavior: A meta-analytic review. Clin. Psychol. Rev. 20(5), 561–592. https://doi.org/10.1016/S0272-7358(98)00100-7 (2000).
Grace, S. L., Evindar, A. & Stewart, D. E. The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Arch. Womens Ment. Health. 6(4), 263–274. https://doi.org/10.1007/s00737-003-0024-6 (2003).
Letourneau, N. L., Tramonte, L. & Willms, J. D. Maternal depression, family functioning and children’s longitudinal development. J. Pediatr. Nurs. 28(3), 223–234. https://doi.org/10.1016/j.pedn.2012.07.014 (2013).
Verbeek, T. et al. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J. Affect. Disord. 136(3), 948–954. https://doi.org/10.1016/j.jad.2011.08.035 (2012).
Perani, C. V. & Slattery, D. A. Using animal models to study post-partum psychiatric disorders. Br. J. Pharmacol. 171(20), 4539–4555. https://doi.org/10.1111/bph.12640 (2014).
Brummelte, S. & Galea, L. A. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm. Behav. 77, 153–166. https://doi.org/10.1016/j.yhbeh.2015.08.008 (2016).
Li, M. & Chou, S. Y. Modeling postpartum depression in rats: theoretic and methodological issues. Dongwuxue Yanjiu. 37(4), 229–236. https://doi.org/10.13918/j.issn.2095-8137.2016.4.229 (2016).
Beck, C. T. Predictors of postpartum depression: An update. Nurs. Res. 50(5), 275–285. https://doi.org/10.1097/00006199-200109000-00004 (2001).
Robertson, E., Grace, S., Wallington, T. & Stewart, D. E. Antenatal risk factors for postpartum depression: A synthesis of recent literature. Gen. Hosp. Psychiatry. 26(4), 289–295. https://doi.org/10.1016/j.genhosppsych.2004.02.006 (2004).
Biaggi, A., Conroy, S., Pawlby, S. & Pariante, C. M. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 191, 62–77. https://doi.org/10.1016/j.jad.2015.11.014 (2016).
Lawrence, B. et al. The impact of psychosocial stress on maternal health outcomes: A multi-state PRAMS 8 (2016-2018) analysis. J. Health Dispar. Res. Pract. 15(2), 7 (2022).
Smith, J. W., Seckl, J. R., Evans, A. T., Costall, B. & Smythe, J. W. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology. 29(2), 227–244. https://doi.org/10.1016/s0306-4530(03)00025-8 (2004).
Baker, S. et al. Effects of gestational stress: 1. evaluation of maternal and juvenile offspring behavior. Brain Res. 1213, 98–110. https://doi.org/10.1016/j.brainres.2008.03.035 (2008).
O’Mahony, S. M. et al. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 13(2), 82–88 (2006).
Haim, A., Albin-Brooks, C., Sherer, M., Mills, E. & Leuner, B. The effects of gestational stress and selective serotonin reuptake inhibitor antidepressant treatment on structural plasticity in the postpartum brain–A translational model for postpartum depression. Horm. Behav. 77, 124–131. https://doi.org/10.1016/j.yhbeh.2015.05.005 (2016).
Leuner, B., Fredericks, P. J., Nealer, C. & Albin-Brooks, C. Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PLoS One. 9(3), e89912. https://doi.org/10.1371/journal.pone.0089912 (2014).
Zoubovsky, S. P. et al. Chronic psychosocial stress during pregnancy affects maternal behavior and neuroendocrine function and modulates hypothalamic CRH and nuclear steroid receptor expression. Transl. Psychiatr. 10, 1. https://doi.org/10.1038/s41398-020-0704-2 (2020).
Gorman-Sandler, E. et al. Gestational stress decreases postpartum mitochondrial respiration in the prefrontal cortex of female rats. bioRxiv. https://doi.org/10.1016/j.ynstr.2023.100563 (2022).
Patin, V. et al. Effects of prenatal stress on maternal behavior in the rat. Brain Res. Dev. Brain Res. 139(1), 1–8. https://doi.org/10.1016/S0165-3806(02)00491-1 (2002).
Bourke, C. H. et al. Prenatal exposure to escitalopram and/or stress in rats. Psychopharmacology. 228(2), 231–241. https://doi.org/10.1007/s00213-013-3030-z (2013).
Liu, J. et al. The BDNF-FoxO1 axis in the medial prefrontal cortex modulates depressive-like behaviors induced by chronic unpredictable stress in postpartum female mice. Mol. Brain. 13(1), 1–91. https://doi.org/10.1186/s13041-020-00631-3 (2020).
Champagne, F. A. & Meaney, M. J. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatr. 59(12), 1227–1235. https://doi.org/10.1016/j.biopsych.2005.10.016 (2006).
Fatima, M., Srivastav, S., Ahmad, M. H. & Mondal, A. C. Effects of chronic unpredictable mild stress induced prenatal stress on neurodevelopment of neonates: Role of GSK-3β. Sci. Rep. 9(1), 1305. https://doi.org/10.1038/s41598-018-38085-2 (2019).
Rincon-Cortes, M. & Grace, A. A. Adaptations in reward-related behaviors and mesolimbic dopamine function during motherhood and the postpartum period. Front Neuroendocrinol. 57, 100839. https://doi.org/10.1016/j.yfrne.2020.100839 (2020).
Post, C. & Leuner, B. The maternal reward system in postpartum depression. Arch. Womens Ment. Health. 22(3), 417–429. https://doi.org/10.1007/s00737-018-0926-y (2019).
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14(9), 609–625. https://doi.org/10.1038/nrn3381 (2013).
Numan, M. & Stolzenberg, D. S. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front. Neuroendocrinol. 30(1), 46–64. https://doi.org/10.1016/j.yfrne.2008.10.002 (2009).
Grieb, Z. A., Vitale, E. M., Morrell, J. I., Lonstein, J. S. & Pereira, M. Decreased mesolimbic dopaminergic signaling underlies the waning of maternal caregiving across the postpartum period in rats. Psychopharmacol. (Berl). 237(4), 1107–1119. https://doi.org/10.1007/s00213-019-05441-7 (2020).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 493(7433), 537–541. https://doi.org/10.1038/nature11740 (2013).
Admon, R. & Pizzagalli, D. A. Dysfunctional reward processing in depression. Curr. Opin. Psychol. 4, 114–118. https://doi.org/10.1016/j.copsyc.2014.12.011 (2015).
Kaufling, J. Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression. Cell Tissue Res. 377(1), 59–71. https://doi.org/10.1007/s00441-019-03007-9 (2019).
Rincón-Cortés, M. & Grace, A. A. Dopamine downregulation in novel rodent models useful for the study of postpartum depression. Front. Behav. Neurosci. 16, 1065558. https://doi.org/10.3389/fnbeh.2022.1065558 (2022).
Moses-Kolko, E. et al. 3454468; rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol. Psychiatry. 70(4), 395–399. https://doi.org/10.1016/j.biopsych.2011.02.021 (2011).
Laurent, H. K. & Ablow, J. C. 3277361; A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Soc. Cognit. Affect. Neurosci. 7(2), 125–134. https://doi.org/10.1093/scan/nsq091 (2012).
Nephew, B. C., Murgatroyd, C., Pittet, F. & Febo, M. Brain reward pathway dysfunction in maternal depression and addiction: A present and future transgenerational risk. J. Reward Defic. Syndr. 1(3), 105–116. https://doi.org/10.17756/jrds.2015-017 (2015).
Haim, A., Sherer, M. & Leuner, B. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur. J. Neurosci. 40(12), 3766–3773. https://doi.org/10.1111/ejn.12752 (2014).
Grieb, Z. A. & Lonstein, J. S. Oxytocin interactions with central dopamine and serotonin systems regulate different components of motherhood. Phil. Trans. R. Soc. B. 2022(377), 20210062. https://doi.org/10.1098/rstb.2021.0062 (1858).
Sofroniew, M. V. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog. Brain Res. 60, 101–114. https://doi.org/10.1016/S0079-6123(08)64378-2 (1983).
Peris, J. et al. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 525(5), 1094–1108. https://doi.org/10.1002/cne.24116 (2017).
Song, Z., Borland, J. M., Larkin, T. E., O’Malley, M. & Albers, H. E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 74, 164–172. https://doi.org/10.1016/j.psyneuen.2016.09.001 (2016).
Melis, M. R. et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 26(4), 1026–1035. https://doi.org/10.1111/j.1460-9568.2007.05721.x (2007).
Xiao, L., Priest, M. F., Nasenbeny, J., Lu, T. & Kozorovitskiy, Y. Biased oxytocinergic modulation of midbrain dopamine systems. Neuron 95(2), 368-384.e5. https://doi.org/10.1016/j.neuron.2017.06.003 (2017).
Hung, L. W. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357(6358), 1406–1411. https://doi.org/10.1126/science.aan4994 (2017).
Shahrokh, D. K., Zhang, T. Y., Diorio, J., Gratton, A. & Meaney, M. J. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 151(5), 2276–2286. https://doi.org/10.1210/en.2009-1271 (2010).
Febo, M. & Ferris, C. F. Oxytocin and vasopressin modulation of the neural correlates of motivation and emotion: Results from functional MRI studies in awake rats. Brain Res. 1580, 8–21. https://doi.org/10.1016/j.brainres.2014.01.019 (2014).
Stolzenberg, D. S. & Numan, M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev. 35(3), 826–847. https://doi.org/10.1016/j.neubiorev.2010.10.003 (2011).
Atzil, S. et al. Dopamine in the medial amygdala network mediates human bonding. Proc. Natl. Acad. Sci. U S A. 114(9), 2361–2366. https://doi.org/10.1073/pnas.1612233114 (2017).
Strathearn, L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. J. Neuroendocrinol. 23(11), 1054–1065. https://doi.org/10.1111/j.1365-2826.2011.02228.x (2011).
Kim, S. et al. Oxytocin and postpartum depression: Delivering on what’s known and what’s not. Brain Res. 1580, 219–232. https://doi.org/10.1016/j.brainres.2013.11.009 (2014).
Thul, T. A., Corwin, E. J., Carlson, N. S., Brennan, P. A. & Young, L. J. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology 120, 104793. https://doi.org/10.1016/j.psyneuen.2020.104793 (2020).
McQuaid, R. J., McInnis, O. A., Abizaid, A. & Anisman, H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 45, 305–322. https://doi.org/10.1016/j.neubiorev.2014.07.005 (2014).
Hillerer, K. M., Reber, S. O., Neumann, I. D. & Slattery, D. A. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology 152(10), 3930–3940. https://doi.org/10.1210/en.2011-1091 (2011).
Murgatroyd, C. A. & Nephew, B. C. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38(2), 219–228. https://doi.org/10.1016/j.psyneuen.2012.05.020 (2013).
Wang, T. et al. Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav. Brain Res. 336, 236–243. https://doi.org/10.1016/j.bbr.2017.09.012 (2018).
Antonson, A. M. et al. Unique maternal immune and functional microbial profiles during prenatal stress. Sci. Rep. 10(1), 20288–x. https://doi.org/10.1038/s41598-020-77265-x (2020).
Gorman-Sandler, E. et al. Gestational stress decreases postpartum mitochondrial respiration in the prefrontal cortex of female rats. Neurobiol. Stress. 26, 100563. https://doi.org/10.1016/j.ynstr.2023.100563 (2023).
Kalueff, A. V. et al. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 17(1), 45. https://doi.org/10.1038/nrn.2015.8 (2016).
Curley, J. P., Jensen, C. L., Franks, B. & Champagne, F. A. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 61(3), 454–461. https://doi.org/10.1016/j.yhbeh.2012.01.013 (2012).
Lonstein, J. S. Regulation of anxiety during the postpartum period. Front. Neuroendocrinol. 28(2–3), 115–141. https://doi.org/10.1016/j.yfrne.2007.05.002 (2007).
Li, M. & Fleming, A. S. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav. Brain Res. 145(1–2), 99–111. https://doi.org/10.1016/s0166-4328(03)00135-9 (2003).
Numan, M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 49(1), 12–21. https://doi.org/10.1002/dev.20198 (2007).
Mattson, B. J., Williams, S., Rosenblatt, J. S. & Morrell, J. I. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behav. Neurosci. 115(3), 683–694. https://doi.org/10.1037/0735-7044.115.3.683 (2001).
Wansaw, M. P., Pereira, M. & Morrell, J. I. 2574572; characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Horm. Behav. 54(2), 294–301. https://doi.org/10.1016/j.yhbeh.2008.03.005 (2008).
Numan, M. & Insel, T. The neurobiology of parental behavior (Springer-Verlag, 2003).
Posillico, C. K. & Schwarz, J. M. 4688102; an investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behav. Brain Res. 298, 218–228. https://doi.org/10.1016/j.bbr.2015.11.011 (2016).
Dalla, C. et al. Chronic mild stress impact: Are females more vulnerable?. Neuroscience 135(3), 703–714. https://doi.org/10.1016/j.neuroscience.2005.06.068 (2005).
Baker, S. L., Kentner, A. C., Konkle, A. T. M., Santa-Maria Barbagallo, L. & Bielajew, C. Behavioral and physiological effects of chronic mild stress in female rats. Physiol. Behav. 87(2), 314–322. https://doi.org/10.1016/j.physbeh.2005.10.019 (2006).
Molendijk, M. L. & de Kloet, E. R. Coping with the forced swim stressor: Current state-of-the-art. Behav. Brain Res. 364, 1–10. https://doi.org/10.1016/j.bbr.2019.02.005 (2019).
Darnaudery, M., Dutriez, I., Viltart, O., Morley-Fletcher, S. & Maccari, S. Stress during gestation induces lasting effects on emotional reactivity of the dam rat. Behav. Brain Res. 153(1), 211–216. https://doi.org/10.1016/j.bbr.2003.12.001 (2004).
Farr, S. L., Dietz, P. M., O’Hara, M. W., Burley, K. & Ko, J. Y. Postpartum anxiety and comorbid depression in a population-based sample of women. J. Women’s Health 23(2), 12–128. https://doi.org/10.1089/jwh.2013.4438 (2014).
Pereira, M. & Morrell, J. I. Functional mapping of the neural circuitry of rat maternal motivation: Effects of site-specific transient neural inactivation. J. Neuroendocrinol. 23(11), 1020–1035. https://doi.org/10.1111/j.1365-2826.2011.02200.x (2011).
Seip, K. M. & Morrell, J. I. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav. Neurosci. 123(6), 1325–1338. https://doi.org/10.1037/a0017666 (2009).
Pereira, M. & Morrell, J. I. The medial preoptic area is necessary for motivated choice of pup- over cocaine-associated environments by early postpartum rats. Neuroscience. 167(2), 216–231. https://doi.org/10.1016/j.neuroscience.2010.02.015 (2010).
Fox, M. E. & Lobo, M. K. The molecular and cellular mechanisms of depression: A focus on reward circuitry. Mol. Psychiatry. 24(12), 1798–1815. https://doi.org/10.1038/s41380-019-0415-3 (2019).
Zhao, C. & Li, M. The receptor mechanisms underlying the disruptive effects of haloperidol and clozapine on rat maternal behavior: A double dissociation between dopamine D(2) and 5-HT(2A/2C) receptors. Pharmacol. Biochem. Behav. 93(4), 433–442. https://doi.org/10.1016/j.pbb.2009.06.005 (2009).
Pereira, M. & Ferreira, A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav. Brain Res. 175(1), 139–148. https://doi.org/10.1016/j.bbr.2006.08.013 (2006).
Doornbos, B. et al. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog. Neuro-psychopharmacol. Boil. Psychiatry. 33(7), 1250–1254. https://doi.org/10.1016/j.pnpbp.2009.07.013 (2009).
Sacher, J. et al. Relationship of monoamine oxidase-A distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology (New York, N.Y.) 40(2), 429–435. https://doi.org/10.1038/npp.2014.190 (2015).
Rincón-Cortés, M. & Grace, A. A. Postpartum scarcity-adversity disrupts maternal behavior and induces a hypodopaminergic state in the rat dam and adult female offspring. Neuropsychopharmacology (New York, N.Y.) 47(2), 488–496. https://doi.org/10.1038/s41386-021-01210-3 (2022).
Winokur, S. B., Lopes, K. L., Moparthi, Y. & Pereira, M. Depression-related disturbances in rat maternal behaviour are associated with altered monoamine levels within mesocorticolimbic structures. J. Neuroendocrinol. https://doi.org/10.1111/jne.12766 (2019).
Lavi-Avnon, Y. et al. The reward system and maternal behavior in an animal model of depression: A microdialysis study. Psychopharmacology (Berl ). 196(2), 281–291. https://doi.org/10.1007/s00213-007-0961-2 (2008).
Champagne, F. A. et al. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 24(17), 4113–4123. https://doi.org/10.1523/JNEUROSCI.5322-03.2004 (2004).
Campioni, M. R., Xu, M. & McGehee, D. S. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol. 101(6), 3192–3198. https://doi.org/10.1152/jn.91111.2008 (2009).
Kalivas, P. W. & Duffy, P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 675(1–2), 325–328. https://doi.org/10.1016/0006-8993(95)00013-g (1995).
Meredith, G. E., Ypma, P. & Zahm, D. S. Effects of dopamine depletion on the morphology of medium spiny neurons in the shell and core of the rat nucleus accumbens. J. Neurosci. 15(5), 3808–3820 (1995).
Love, T. M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 119, 49–60. https://doi.org/10.1016/j.pbb.2013.06.011 (2014).
Bell, A. F. et al. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front. Genet. 6, 243. https://doi.org/10.3389/fgene.2015.00243 (2015).
Smith, C. J. W. et al. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: Focus on the social decision-making network. Brain. Struct. Funct. 222(2), 981–1006. https://doi.org/10.1007/s00429-016-1260-7 (2017).
Kilkenny, C., Browne, W. J., Cuthi, I., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 41(1), 27–31. https://doi.org/10.1111/j.1939-165X.2012.00418.x (2012).
Acknowledgements
We thank The Ohio State University Targeted Metabolomics Laboratory for access to their LC-MS/MS equipment funded by the Translational Plant Sciences Targeted Investment in Excellence (TIE) and for performing LC-MS/MS analysis. We also thank undergraduate research assistants Harry Hoffman, Dominic Julian, Emily Mills, Connor Nealer, Morgan Sherer, and Brandon Springer.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development R21-HD083791.
Author information
Authors and Affiliations
Contributions
B.L. and A.H. designed experiments. A.H. and C.A.B. performed the experiments including the stress procedure, behavioral testing, immunohistochemistry, microscopy and Western Blot. H.B performed qPCR. B.L. performed the statistical analyses. A.H. wrote the initial draft of the manuscript. B.L. revised the manuscript. M.B prepared figures, helped with statistical analyses and participated in manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Haim, A., Albin-Brooks, C., Brothers, H. et al. Gestational stress disrupts dopamine and oxytocin signaling in the postpartum reward system of rats: implications for mood, motivation and mothering. Sci Rep 15, 1450 (2025). https://doi.org/10.1038/s41598-024-84043-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84043-6