Abstract
This study aimed to investigate whether lymphocyte-C-reactive protein ratio (LCR) upon admission can predict disease progression and intensive care unit (ICU) admission in adult patients with diabetic ketoacidosis (DKA). A single-center retrospective study was conducted, including adult DKA patients admitted to the First Affiliated Hospital of Harbin Medical University between March 2018 and March 2023. Multiple demographic and clinical data were collected from the medical records upon admission and during hospitalization. Subsequently, sequential organ failure assessment (SOFA) score and LCR were calculated based on relevant clinical parameters within 24 h of admission. These indicators were compared among different disease severity groups, and factors related to severe DKA, concurrent acute kidney injury (AKI), and ICU admission were further analyzed. Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity, specificity, area under the ROC curve (AUC), and cut-off value of LCR. A total of 271 adult DKA patients were enrolled and categorized into three groups: mild group (n = 42), moderate group (n = 64), and severe group (n = 165). Significant differences in demographic and clinical data were observed among these groups. Glasgow coma scale (GCS) score, LCR, pH, and bicarbonate (HCO3-) were identified as protective factors for severe DKA. Conversely, SOFA score, neutrophil count (NEUT), serum creatinine (SCr), and glucose (GLU) were risk factors for concurrent AKI. Concurrent AKI and SOFA score were risk factors for ICU admission, while pH was a protective factor. The areas under the ROC curve (AUC) of LCR to classify adult DKA patients into mild group, severe group, and ICU admission were 0.679, 0.718, and 0.621, respectively, with cut-off value of 212.80, 96.16, and 63.35, sensitivity of 54.8%, 76.4%, and 78.9%, and specificity of 76.0%, 62.4%, and 46.3%. LCR upon admission provides great potential to predict disease progression and ICU admission in adult patients with DKA.
Similar content being viewed by others
Introduction
Although the genesis of ketone bodies serves as a protective metabolic mechanism in times of nutrient deficiency, prolonged ketogenesis can give rise to a ketosis-prone state and potentially lead to ketoacidosis—a life-threatening metabolic crisis1. Diabetic ketoacidosis (DKA) remains one of the most frequently encountered and serious endocrine emergency, characterized primarily by the clinical features such as hyperglycemia, anion gap (AG) metabolic acidosis, ketosis, electrolyte imbalances, and dehydration2,3. In recent years, DKA has become a major health concern and thus attracted more and more attention due to its ever-increasing morbidity and mortality, and the substantial economic burden on the global healthcare system4,5,6. Adult DKA patients are categorized into different disease severity groups, including mild, moderate, and severe groups, based on the severity of metabolic acidosis and the presence of altered mental status, rather than absolute glucose (GLU) levels7. Regardless of disease severity, early recognition, risk stratification, prompt individualized interventions with varying intensities, and vigilant monitoring are the cornerstones of clinical management aimed at improving clinical outcomes in adult patients with DKA8,9. Effectively managing this hyperglycemic crisis necessitates a comprehensive and in-depth understanding of its pathogenesis and disease progression. Given the involvement of auto-immune destruction to insulin-producing pancreatic β cells and the activation of non-infectious pro-inflammatory response in the pathogenesis and disease progression of DKA, immuno-inflammatory crosstalk may point the way to address this clinical challenge10,11,12,13.
Lymphocyte-C-reactive protein ratio (LCR), calculated by multiplying lymphocyte count (LYMPH) by 10,000 and then dividing it by C-reactive protein (CRP), serves as an indicator of the strength of protective immune activation response and the severity of systemic inflammatory cascade reaction, and therefore reflects immuno-inflammatory crosstalk within the body under different disease conditions14. LCR, whether assessed upon admission or pre-operation, embodies the relative balance between immune status and inflammatory reaction of the patients at that specific moment, which holds far-reaching implications for disease progression and clinical outcomes15,16. As our understanding of LCR continues to evolve, its clinical utility has expanded beyond the initial application in malignant tumors to encompass many other fields, particularly in infectious diseases17,18,19,20,21,22. Previous research from our team has established that LCR upon admission can effectively predict disease progression in adult patients with coronavirus disease 2019 (COVID-19) and serve as a simple and objective auxiliary screening tool for admission to hospital and intensive care unit (ICU)14. However, there are currently no widely recognized clinical parameters or existing scoring systems for early identification of adult DKA patients at different risk of disease progression, nor have there been related clinical studies to explore the application potential of LCR in this patient population.
In response to this gap, our study represents the inaugural effort to investigate the predictive and prognostic potentials of LCR in adult patients with DKA, offering innovative insights with significant implications for clinical practice. Our findings will lay a solid foundation for the clinical application of this easy-to-obtain and cost-effective parameter to predict disease progression in adult patients with DKA. Early identification of adult DKA patients at different risk of disease progression will aid in tailoring individualized interventions of varying intensity, ultimately contributing to improved clinical outcomes, the rational allocation of limited ICU resources, and the effective alleviation of the burden on healthcare system, ICU and medical staff.
Methods
Study design
In this study, adult DKA patients who were admitted to the First Affiliated Hospital of Harbin Medical University from March 2018 to March 2023 were enrolled. Upon admission, demographic data, involving gender, age, height, weight, body mass index (BMI), history of hypertension, and clinical data, including white blood cell count (WBC), neutrophil count (NEUT), neutrophil proportion (NEUT%), LYMPH, lymphocyte percentage (LYM%), hemoglobin (HGB), platelet count (PLT), D-Dimer, CRP, procalcitonin (PCT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TBIL), blood urea nitrogen (BUN), serum creatinine (SCr), GLU, arterial blood gas analysis (ABGA) for serum potential hydrogen (pH), bicarbonate (HCO3−), actual base excess (ABE), and lactic acid (Lac), and glasgow coma scale (GCS) score, as well as disease severity, concurrent acute kidney injury (AKI), and intensive care unit (ICU) admission during hospitalization were collected and collated from the medical records of the enrolled patients. Subsequently, sequential organ failure assessment (SOFA) score and LCR were calculated based on the relevant clinical parameters within 24 h of admission. These indicators were compared among different disease severity groups, and the factors related to severe DKA, concurrent AKI, and ICU admission were further analyzed. Receiver operating characteristic (ROC) curve analysis was performed, and the corresponding sensitivity and specificity, area under the ROC curve (AUC), and cut-off value of LCR were calculated to explore the potential of LCR to predict disease progression and ICU admission in adult patients with DKA. The study protocol received approval from the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (IRB number: 2023IIT152). Written informed consent from the participants was not required for this study, in compliance with national legislation and institutional requirements. The study was conducted in adherence to the principles of the Helsinki Declaration.
Study population
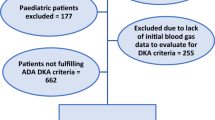
The inclusion criteria of this study were adult patients (aged 18 and above) with a confirmed diagnosis of DKA at the First Affiliated Hospital of Harbin Medical University between March 2018 and March 2023. The exclusion criteria encompassed co-existing infectious diseases, chronic organ failure, uncontrolled malignant tumors with multiple metastases, leukemia, acquired immunodeficiency syndrome (AIDS), immunotherapy or organ transplant within 6 months, auto-immune disorders, previous or current use of sodium-glucose cotransporter-2 (SGLT2) inhibitors, pregnant or breastfeeding women, and incomplete medical records (Fig. 1).
Diagnosis of hypertension
In accordance with the 2018 joint guideline from the European Society of Cardiology and Hypertension, hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg23.
Diagnosis and classification of DKA
In clinical practice, a comprehensive assessment of current and past medical history, clinical presentations, physical examinations, and laboratory parameters was typically necessary for the definitive diagnosis of DKA from clinical and laboratory point of view24,25. Adult DKA patients were categorized as mild, moderate, or severe groups based on the severity of metabolic acidosis (pH, HCO3−, and ketones) and the presence of altered mental status7. Among them, the diagnostic criteria for mild, moderate, and severe DKA were pH 7.25 ~ 7.29 and (or) HCO3− 15 ~ 17.9 mmol/L with AG > 10, pH 7.00 ~ 7.24 and (or) HCO3− 10 ~ 14.9 mmol/L with AG > 12, and DKA coma or pH < 7.00 and (or) HCO3− < 10 mmol/L with AG > 12 without DKA coma, respectively.
Calculation of LCR
Within 24 h of admission, LCR was calculated by multiplying LYMPH by 10,000 and then dividing it by CRP.
Data collection
Multiple demographic and clinical data were collected and collated from the medical records of the enrolled patients upon admission and during hospitalization by dedicated personnel within our research team. Other members of our research team had limited access to the personal information of the enrolled patients, only accessing what was necessary for this study.
Statistical analysis
Statistical analysis was conducted using SPSS 24.0 (SPSS, Inc., Chicago, IL, USA). Continuous data with a normal distribution were presented as mean ± standard deviation (SD), while those without a normal distribution were represented as median (P25, P75). Frequency was used to express counting data. One-way analysis of variance (ANOVA) was employed for inter-group comparisons of continuous data with a normal distribution. In case of a significant difference, least significant difference (LSD) method was further used for pairwise comparisons. Kruskal–Wallis rank-sum test was adopted for inter-group comparisons of continuous data without a normal distribution, and pairwise comparisons were performed in case of a significant difference. Chi-square (χ2) test was applied to inter-group comparisons of counting data, while Fisher’s exact probabilities test was adopted when the condition of χ2 test was not satisfied. Pairwise comparisons were conducted in case of a significant difference, and the significance was adjusted by Bonferroni correction. Variables with significance less than 0.1 in inter-group comparisons were screened by Wald backward method and then included in multiple logistic regression analysis. In case of a significant difference, the ROC curve was analyzed, and the corresponding sensitivity and specificity, AUC, and cut-off value of LCR were calculated. Statistical significance was indicated by a p-value < 0.05.
Results
Comparison of demographic and clinical data upon admission and during hospitalization among mild, moderate, and severe groups in adult patients with DKA
A total of 271 adult DKA patients, admitted to the First Affiliated Hospital of Harbin Medical University between March 2018 and March 2023, were enrolled in this study and subsequently categorized into three groups: mild group (n = 42), moderate group (n = 64), and severe group (n = 165). Significant differences in multiple demographic and clinical data upon admission and during hospitalization were observed among different disease severity groups, including age, NEUT%, LYMPH, LYMPH%, HGB, D-Dimer, CRP, PCT, ALB, BUN, SCr, GLU, pH, HCO3−, ABE, Lac, concurrent AKI, ICU admission, GCS score, SOFA score, and LCR (Table 1).
Analysis of the factors related to severe DKA
GCS score, LCR, pH, and HCO3− were identified as protective factors associated with severe DKA. With every unit change in GCS score, LCR, pH, and HCO3−, the possibility of severe DKA was 0.321, 0.995, 0.000016, and 0.664 times of the original values, respectively (Table 2).
Analysis of the factors related to concurrent AKI
SOFA score, NEUT, SCr, and GLU were considered as risk factors related to concurrent AKI. With every unit change in SOFA score, NEUT, SCr, and GLU, the possibility of concurrent AKI was 1.230, 1.053, 1.008, and 1.027 times of the original values, respectively (Table 3).
Analysis of the factors related to ICU admission
Concurrent AKI and SOFA score were determined as risk factors for ICU admission, while pH was recognized as a protective factor. The possibility of ICU admission in adult DKA patients with concurrent AKI was 11.658 times of that in those without concurrent AKI (Table 4). With every unit change in SOFA score, and pH, the possibility of ICU admission was 1.337, and 0.042 times of the original values, respectively (Table 4).
The ROC curve of LCR to classify adult DKA patients into mild group
Upon admission, there was a statistically significant difference in the comparison of LCR between mild group and moderate-severe combined group (p = 0.000) (Table 5). The AUC of LCR to classify adult DKA patients into mild group was 0.679 (Table 1 in Supplementary Material and Fig. 2). The identified cut-off value of LCR, along with the corresponding sensitivity and specificity, were 212.80, 54.8%, and 76.0%, respectively.
The ROC curve of LCR to classify adult DKA patients into severe group
Upon admission, there was a statistically significant difference in the comparison of LCR between mild-moderate combined group and severe group (p = 0.000) (Table 6). The AUC of LCR to classify adult DKA patients into severe group was 0.718 (Table 2 in Supplementary Material and Fig. 3). The identified cut-off value of LCR, along with the corresponding sensitivity and specificity, were determined as 96.16, 76.4%, and 62.4%, respectively.
The ROC curve of LCR to classify adult DKA patients with ICU admission
Upon admission, a significant difference was observed in the comparison of LCR between adult DKA patients with ICU admission and those without ICU admission (p = 0.001) (Table 7). The AUC of LCR to classify adult DKA patients with ICU admission was found to be 0.621 (Table 3 in Supplementary Material and Fig. 4). The cut-off value of LCR, as well as the corresponding sensitivity and specificity, were determined to be 63.35, 78.9%, and 46.3%, respectively.
Discussion
In both type I and type II diabetes mellitus (T1DM and T2DM) patients with insulin-dependence, DKA is a relatively common but largely preventable disorder that often presents as a new-onset clinical manifestation, even after hospitalization2,26,27. In clinical practice, poor compliance to prescribed treatments, erratic glycemic control, or inciting events such as infection or ischemia are the most common triggers and underlying precipitating factors of DKA28,29. The clinical signs and symptoms of new-onset DKA are generally atypical and similar-being unwell, including but not limited to polydipsia, polyphagia, polyuria, weight loss, fatigue, nausea, vomiting, dehydration, abdominal pain, rapid and shallow breathing, and lethargy, which can easily lead to delayed diagnosis and misdiagnosis in the context of the relative lack of awareness and vigilance among clinicians1. Relative or absolute insulin deficiency, stemming from reduced insulin production and elevated levels of counter-regulatory hormones, has been established to be a potential root cause of the occurrence and progression of DKA, which can further result in adverse metabolic consequences from increased lipolysis, glycogenolysis, gluconeogenesis, and muscle proteolysis, including hyperglycemia, urinary glucose excretion, osmotic diuresis, enhanced genesis and release of ketone bodies, and metabolic acidosis30,31. Additionally, insulin resistance (IR) and poor glucose utilization also play a role in this process32. At a deeper level, immunologic dysregulation, oxidative stress, activation of inflammatory mediators, and systemic inflammatory cascade reaction (also known as cytokine storm) have been identified with strong evidences as significant contributors to the occurrence and progression of DKA33,34,35.
Lymphocytes, originating from lymphoid organs, serve as the major effector cells in the body’s immune system, among which different cell subsets with unique developmental and regulatory pathways exhibit significant heterogeneity in cytokine expression, and thus play distinct roles in immune-mediated pathological processes36. A growing body of evidence indicates that CRP, a non-specific acute-phase reactant and an active modulator of innate immunity, has been demonstrated to be strongly correlated with the extent of systemic inflammatory cascade reaction and clinical outcomes in a range of inflammatory disorders, particularly infectious diseases, given its known biological effects of promoting inflammatory response and tissue damage37,38,39. At the genetic level, common biological pathways linking inflammation and metabolic traits are supported through the lipid and glucose metabolism genes annotated by many genetic loci related to CRP40. LCR, as the ratio of the above two clinical indicators and a novel immuno-inflammatory score, reflects the current state of microenvironment in the body at that time with a perspective towards immunity and inflammation, which aligns with the pathogenesis of DKA discussed in our study. Therefore, to the best of our knowledge, our study represents the first attempt to extend the clinical application of LCR from the widely-recognized field of oncology to DKA in order to assess its predictive and prognostic potentials and accordingly determine individualized therapeutic strategies with varying intensities.
From our results, there were significant differences in multiple demographic and clinical data upon admission and during hospitalization among different disease severity groups, indicating that disease severity of adult patients with DKA was determined by multiple factors. With every unit change in GCS score, LCR, pH, and HCO3−, the possibility of severe DKA was 0.321, 0.995, 0.000016, and 0.664 times of the original values, respectively, revealing that the above were all protective factors and every unit change in pH had the greatest impact. Similarly, with every unit change in SOFA score, NEUT, SCr, and GLU, the possibility of concurrent AKI was 1.230, 1.053, 1.008, and 1.027 times of the original values, respectively, suggesting that the above were all risk factors and every unit change in SOFA score showed the greatest impact. From the analysis of the factors related to ICU admission, every unit change in pH had the greatest impact on the possibility of ICU admission. In summary, all the above factors should be paid attention to and closely monitored during the clinical management of adult patients with DKA.
There is an urgent clinical need for early identification of adult DKA patients at different risk of disease progression and prompt initiation of tailored interventions with varying intensities, which will facilitate improved clinical outcomes, rationalize the allocation of limited ICU resources, and effectively alleviate the burden on the healthcare system, ICU and medical staff. However, to date, there are no simple and effective clinical parameters or combinations to predict disease progression and ICU admission in adult patients with DKA. Consequently, in this study, various cut-off values from the results were utilized to categorize LCR upon admission into three intervals: greater than 212.80, between 96.16 and 212.80, and less than 96.16, suggesting that adult patients with DKA in these different intervals would evolve into mild, moderate, and severe groups during hospitalization, respectively. In addition, the cut-off value of LCR to classify adult DKA patients with ICU admission was 63.35, revealing that the possibility of ICU admission was significantly increased when LCR was less than 63.35, with the corresponding sensitivity and specificity of 78.9% and 46.3%, respectively. Finally, the cut-off values of LCR to classify adult DKA patients into severe group and those with ICU admission were similar but not identical, indicating that disease severity was a significant but not sole determinant for ICU admission in adult patients with DKA.
Despite the positive findings, the limitations of this study should be taken into account. Firstly, the nature of single-center retrospective study with a relatively small sample size may restrict the reliability and generalizability of our conclusion. Therefore, our findings warrant further validation through future well-designed clinical trials with larger sample size. Secondly, the conclusion from this study is only applicable to adult patients with DKA and cannot be directly extrapolated to other patient populations. Thirdly, in this study, the AUC and corresponding sensitivity and specificity still have room for improvement in future studies. Fourthly, the predictive and prognostic potentials of dynamic changes in LCR were not investigated in this study, which may be the direction of further research. Lastly, the long-term effects of LCR on adult patients with DKA were not explored in this study.
Conclusion
In summary, LCR upon admission holds significant potential to predict disease progression and ICU admission in adult patients with DKA, which will facilitate early identification of adult DKA patients at different risk of disease progression and prompt initiation of individualized interventions with varying intensities, thereby improving clinical outcomes, rationalizing the allocation of limited ICU resources, and effectively relieving the burden on the healthcare system, ICU and medical staff. Our study represents the first attempt to investigate the predictive and prognostic potentials of LCR in adult patients with DKA and stratify risk accordingly to provide recommendations on optimal care. Consequently, our findings provide unique insights and necessitate further validation through future well-designed clinical trials with larger sample size.
Data availability
The authors are committed to providing the raw data that support the conclusions of this article without any reservation. If someone wants to request the data from this study, they should contact the corresponding author, Yang Gao.
References
Bashir, B., Fahmy, A. A., Raza, F. & Banerjee, M. Non-diabetic ketoacidosis: A case series and literature review. Postgrad. Med. J. 97, 667–671. https://doi.org/10.1136/postgradmedj-2020-138513 (2021).
Calimag, A., Chlebek, S., Lerma, E. V. & Chaiban, J. T. Diabetic ketoacidosis. Dis. Mon 69, 101418. https://doi.org/10.1016/j.disamonth.2022.101418 (2023).
Bonora, B. M., Avogaro, A. & Fadini, G. P. Euglycemic Ketoacidosis. Curr. Diab Rep. 20, 25. https://doi.org/10.1007/s11892-020-01307-x (2020).
Jensen, E. T. et al. Increase in Prevalence of Diabetic ketoacidosis at diagnosis among Youth with type 1 diabetes: The SEARCH for diabetes in Youth Study. Diabetes Care 44, 1573–1578. https://doi.org/10.2337/dc20-0389 (2021).
Gorchane, A. et al. Uncovering the alarming rise of diabetic ketoacidosis during COVID-19 pandemic: A pioneer African study and review of literature. Front. Endocrinol. (Lausanne) 14, 1234256. https://doi.org/10.3389/fendo.2023.1234256 (2023).
Ebrahimi, F., Kutz, A., Christ, E. R. & Szinnai, G. Lifetime risk and health-care burden of diabetic ketoacidosis: A population-based study. Front. Endocrinol. (Lausanne) 13, 940990. https://doi.org/10.3389/fendo.2022.940990 (2022).
Wu, X. Y. et al. Clinical profiles, outcomes and risk factors among type 2 diabetic inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: A hospital-based analysis over a 6-year period. BMC Endocr. Disord. 20, 182. https://doi.org/10.1186/s12902-020-00659-5 (2020).
Chow, E., Clement, S. & Garg, R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open. Diabetes Res. Care. 11, e003666. https://doi.org/10.1136/bmjdrc-2023-003666 (2023).
Eledrisi, M. S., Beshyah, S. A. & Malik, R. A. Management of diabetic ketoacidosis in special populations. Diabetes Res. Clin. Pract. 174, 108744. https://doi.org/10.1016/j.diabres.2021.108744 (2021).
Syed, F. Z. Type 1 diabetes Mellitus. Ann. Intern. Med. 175, ITC33–33ITC48. https://doi.org/10.7326/AITC202203150 (2022).
Mae, S., Kuriyama, A. & Tachibana, H. Diabetic Ketoacidosis as a delayed Immune-related event after discontinuation of Nivolumab. J. Emerg. Med. 60, 342–344. https://doi.org/10.1016/j.jemermed.2020.09.023 (2021).
Cheng, Y. et al. Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells. Open. Life Sci. 16, 1365–1376. https://doi.org/10.1515/biol-2021-0141 (2021).
Casillas, S., Pomerantz, A., Surani, S. & Varon, J. Role of vitamin C in diabetic ketoacidosis: Is it ready for prime time. World J. Diabetes. 9, 206–208. https://doi.org/10.4239/wjd.v9.i12.206 (2018).
Zhang, J. N. et al. Lymphocyte-C-reactive protein ratio can differentiate disease severity of COVID-19 patients and serve as an assistant screening tool for hospital and ICU admission. Front. Immunol. 13, 957407. https://doi.org/10.3389/fimmu.2022.957407 (2022).
Lu, L. H. et al. Lymphocyte-C-reactive protein ratio as a novel prognostic index in intrahepatic cholangiocarcinoma: A multicentre cohort study. Liver Int. 41, 378–387. https://doi.org/10.1111/liv.14567 (2021).
Ye, Y. et al. Prognostic role of preoperative lymphocyte/C-reactive protein associated with upper gastrointestinal cancer: A meta-analysis. Front. Oncol. 13, 1181649. https://doi.org/10.3389/fonc.2023.1181649 (2023).
Okugawa, Y. et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg. 272, 342–351. https://doi.org/10.1097/SLA.0000000000003239 (2020).
He, Z. et al. The modified lymphocyte C-reactive protein score is a promising indicator for predicting 3-year mortality in elderly patients with intertrochanteric fractures. BMC Geriatr. 23, 432. https://doi.org/10.1186/s12877-023-04065-z (2023).
Chen, B. et al. A promising new predictive factor for detecting bowel resection in childhood intussusception: The lymphocyte-C-reactive protein ratio. BMC Pediatr. 21, 577. https://doi.org/10.1186/s12887-021-03068-2 (2021).
Zhong, Y. et al. Risk factors to predict post-operative organ/space infection after appendectomy in the pediatric population: A retrospective case control analysis. Surg. Infect. (Larchmt) 24, 462–467. https://doi.org/10.1089/sur.2022.388 (2023).
Yildirim, M., Dasiran, F., Angin, Y. S. & Okan, I. Lymphocyte-C-reactive protein ratio: A putative predictive factor for intestinal ischemia in strangulated abdominal wall hernias. Hernia 25, 733-9. (2021). https://doi.org/10.1007/s10029-020-02174-x
Erdogan, A., Can, F. E. & Gönüllü, H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J. Med. Virol. 93, 5555–5559. https://doi.org/10.1002/jmv.27097 (2021).
Williams, B. et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Glaser, N. et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr. Diabetes 23, 835–856. https://doi.org/10.1111/pedi.13406 (2022).
Dhatariya, K. K., Glaser, N. S., Codner, E. & Umpierrez, G. E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 6, 40. https://doi.org/10.1038/s41572-020-0165-1 (2020).
Evans, K. Diabetic ketoacidosis: Update on management. Clin. Med. (Lond). 19, 396–398. https://doi.org/10.7861/clinmed.2019-0284 (2019).
Lapolla, A. et al. Diabetic ketoacidosis: A consensus statement of the Italian Association of Medical Diabetologists (AMD), Italian society of Diabetology (SID), Italian Society of Endocrinology and Pediatric Diabetoloy (SIEDP). Nutr. Metab. Cardiovasc. Dis. 30, 1633–1644. https://doi.org/10.1016/j.numecd.2020.06.006 (2020).
Atiase, Y. et al. Clinical characteristics and severity of diabetic ketoacidosis: A cross-sectional study from a tertiary hospital in Ghana. Trop. Med. Int. Health 28, 790–796. https://doi.org/10.1111/tmi.13919 (2023).
Alotaibi, R. et al. Diabetic ketoacidosis in Saudi Arabia: Factors precipitating initial admission and readmission. Ann. Saudi Med. 42, 119–126. https://doi.org/10.5144/0256-4947.2022.119 (2022).
Castellanos, L., Tuffaha, M., Koren, D. & Levitsky, L.L. Management of diabetic ketoacidosis in children and adolescents with type 1 diabetes Mellitus. Paediatr. Drugs 22, 357–367. https://doi.org/10.1007/s40272-020-00397-0 (2020).
Palermo, N. E., Sadhu, A. R. & McDonnell, M. E. Diabetic Ketoacidosis in COVID-19: Unique concerns and considerations. J. Clin. Endocrinol. Metab. 105, dgaa360. https://doi.org/10.1210/clinem/dgaa360 (2020).
Long, B., Lentz, S., Koyfman, A. & Gottlieb, M. Euglycemic diabetic ketoacidosis: Etiologies, evaluation, and management. Am. J. Emerg. Med. 44, 157–160. https://doi.org/10.1016/j.ajem.2021.02.015 (2021).
Hoffman, W. H., Whelan, S. A. & Lee, N. Tryptophan, kynurenine pathway, and diabetic ketoacidosis in type 1 diabetes. PLoS One 16, e0254116. https://doi.org/10.1371/journal.pone.0254116 (2021).
Nyunt, T., Mullol, J. & Snidvongs, K. Immune response to fungi in diabetic patients with invasive fungal rhinosinusitis. Asian Pac. J. Allergy Immunol. 38, 233–238. https://doi.org/10.12932/AP-080620-0874 (2020).
Eskandarani, R. M. & Sawan, S. Diabetic Ketoacidosis on hospitalization with COVID-19 in a previously nondiabetic patient: A review of pathophysiology. Clin. Med. Insights Endocrinol. Diabetes 13, 1179551420984125. https://doi.org/10.1177/1179551420984125 (2020).
Dong, C. Cytokine regulation and function in T cells. Annu Rev Immunol 39, 51–76. (2021). https://doi.org/10.1146/annurev-immunol-061020-053702
Potempa, L. A., Rajab, I. M., Olson, M. E. & Hart, P. C. C-Reactive protein and cancer: Interpreting the differential bioactivities of its pentameric and monomeric, modified isoforms. Front. Immunol. 12, 744129. https://doi.org/10.3389/fimmu.2021.744129 (2021).
Luan, Y. Y., Yin, C. H. & Yao, Y. M. Update advances on C-Reactive protein in COVID-19 and other viral infections. Front. Immunol. 12, 720363. https://doi.org/10.3389/fimmu.2021.720363 (2021).
Dix, C. et al. C-reactive protein, immunothrombosis and venous thromboembolism. Front. Immunol. 13, 1002652. https://doi.org/10.3389/fimmu.2022.1002652 (2022).
Koskeridis, F. et al. Pleiotropic genetic architecture and novel loci for C-reactive protein levels. Nat. Commun. 13, 6939. https://doi.org/10.1038/s41467-022-34688-6 (2022).
Acknowledgements
The authors express their gratitude to all those who provided invaluable assistance, advice, and support for this article.
Funding
This study was supported by the National Natural Science Foundation of China (No.82372172), the Key Research and Development Plan Project of Heilongjiang Province (No.GA23C007), the Heilongjiang Province Postdoctoral Start-up Fund (No.LBH-Q20037), the Special Fund for Clinical Research of Wu Jie-Ping Medical Foundation (No.320.6750.2022-02-16), the Research Project of Heilongjiang Provincial Health Commission (No.20231717010461), and the Scientific Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (No.2021M08).
Author information
Authors and Affiliations
Contributions
Yi-Jia Hu, Shu-Xiao Qiu, Jian-Nan Zhang, Qi-Qi Lai, Yan Gao, Kai Kang, and Yang Gao conducted the literature search, conceived and designed the study, performed statistical analysis, analyzed and discussed the results, and prepared, edited, and reviewed the manuscript. Yi-Lu Lin, Lin-Qiong Liu, Di Wu, Hui-Ying Liu, Huan Meng, Jia-Xi Xu, Jia-Ning Zhang, and Bo-Wen Liu contributed to the literature search, data acquisition and compilation, statistical analysis, analysis and discussion of results, and manuscript preparation. All authors read and approved the final article. Yi-Jia Hu, Shu-Xiao Qiu, Jian-Nan Zhang, and Qi-Qi Lai made equal contributions to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol received approval from the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (IRB number: 2023IIT152). The need for informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University, in compliance with national legislation and institutional requirements.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, YJ., Qiu, SX., Zhang, JN. et al. Lymphocyte-C-reactive protein ratio upon admission to predict disease progression and ICU admission in adult patients with diabetic ketoacidosis. Sci Rep 15, 3012 (2025). https://doi.org/10.1038/s41598-024-84054-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84054-3