Abstract
This study investigates a nanoparticle-based doxycycline (DOX) delivery system targeting cervical cancer cells via the CD44 receptor. Molecular docking revealed a strong binding affinity between hyaluronic acid (HA) and CD44 (binding energy: -7.2 kJ/mol). Characterization of the HA-Chitosan nanoparticles showed a particle size of 284.6 nm, a zeta potential of 16.9 mV, and a polydispersity index of 0.314, with SEM confirming smooth surface morphology. The encapsulation efficiency of DOX-loaded nanoparticles was 89.32%, exhibiting a sustained release profile, with 67.45% released over 72 h in acidic conditions (pH 5.5). Cytotoxicity assays demonstrated a significant reduction in HeLa cell viability to 22% at 72 h, compared to 67% in normal HEK cells. Stability tests confirmed the maintenance of nanoparticle integrity and a consistent drug release profile over three months. Cell migration was reduced by 45%, and RT-PCR analysis revealed a 53% downregulation of TNF-α expression, suggesting effective targeting of inflammatory pathways. These results underscore the potential of HA-Chitosan-based DOX nanoparticles in improving cervical cancer treatment through enhanced targeted delivery and inhibition of tumor-promoting mechanisms.
Similar content being viewed by others
Introduction
Cervical cancer (CCa), a prevalent form of cancer among women worldwide, ranks as the third most common. Its impact is particularly devastating in Pakistan, where it claims the lives of over 7000 women annually1. Presently, around 85% of deaths are reported to occur in lower and middle socioeconomic countries. This makes it the principal root of disaese-related fatalities among females in the developing world. Mostly CCa is either squamous cell carcinoma or adenocarcinoma2. The primary treatment approaches including chemotherapy and radiotherapy are used as therapeutic modalities for metastatic and recurrent CCa. A three-drug regimen consisting of carboplatin, paclitaxel, and bevacizumab has emerged as a new standard of care. However, despite their improved outcomes, bevacizumab may not be the most cost-effective therapy.
Additionally, this regimen has a toxicity profile that limits its application in older women or in those with comorbidities such as hypertension or renal disease3. While these conventional options have shown promise in improving the prognosis of CCa, but sometime their severe adverse outcomes are challenging for patients to tolerate. In recent years, nanotechnology has significantly advanced, discovering unique physical, chemical, and biological properties of nanoscale materials. Nano-oncology focuses on utilizing nanobiotechnology in cancer treatment. The drug delivery systems have shown immense potential in enhancing drug bioavailability solubility, altering drug distribution, combating drug resistance, and reducing nonspecific toxicity. Most importantly, it can minimize the side effects of chemotherapy and increase their survival time by improving sufferer’s quality of life4.
Doxycycline (DOX), a tetracycline antibiotic, was repurposed as an anticancer drug. It inhibits the synthesis of bacterial protein after it binds to the active aminoacyl-tRNAs at the A-site of the 30 S subunit of bacterial ribosomes. As a result, DOX can unintentionally inhibit mitochondrial biogenesis, leading to its repurposing to be used therapeutically in oncology for targeting cancer cells. DOX offers excellent oral bioavailability and sustained action with a long half-life of 18–22 h. Despite its effectiveness as an antibiotic, DOX has some drawbacks. Its limitations include instability in the biological environment, rapid clearance, and metabolism. Additionally, large concentrations of DOX can potentially be toxic for healthy tissues, and the issue of tetracycline resistance remains a global challenge5.
Nanotechnology offers promising solutions for efficiently delivering antibiotics to infection sites while enabling better control over dosage regimens and reducing toxicity associated with conventional therapy6. When therapeutic agents are encapsulated in nanoparticles, several advantages arise compared to using the naked drug. Firstly, nanoparticles provide a larger drug mass to the target cells, enhancing the therapeutic efficacy. Additionally, they can aid in overcoming drug resistance by impeding the cell’s capacity to expel the drug molecules. Additionally, nanoparticles can be targeted to the specific disease site through localized delivery agents, achieved either by passive or active targeting. Lastly, nanoformulations can minimize conventional methods’ immunogenicity and related adverse effects (Buyuk et al.., 2020).
Polymeric biocompatible nanoparticles have become a promising avenue for drug delivery. These nanoparticles offer the advantage of being easily customizable through surface modification by incorporating specific targeting moieties on their surfaces (Gagliardi et al.., 2021). Chitosan, a linear cationic polymer, is an excellent candidate for such applications (Nayak et al.., 2022). Its flexible structure is attributed to the presence of amine groups (+NH2) that are charged, it allows for various physical modifications. Hyaluronic acid (HA) is a mucopolysaccharide, a hydrophilic, non-sulfated and negatively charged naturally occurring compound. HA’s molecular weight varies depending on its chain length and weight in Daltons, determining its functions and other properties. The ionic gelation process relies on electrostatic forces between oppositely charged groups involving an anionic crosslinker, such as tripolyphosphate polyanion (TPP)7.
Surface functionalization of nano-drug delivery systems (nDDS) with Hyaluronic acid (HA) results in longer blood circulation time, improved compatibility with the body, and the ability to specifically target CD44 receptors on cancer cells. CD44 is observed to be excessively produced in different types of solid tumors. It performs vital functions in the growth and development of cells, the differentiation of cells, the migration of cells, angiogenesis, and the displaying of signaling molecules such as cytokines, chemokines, and growth factors (Zhong et al., 2016). The interaction between CD44 and HA demonstrates a strong attraction, and once they bind, the complex is taken intracellularly by a complex-mediated endocytosis pathway. This internalization enhances the intrcellularly absorption and retention of the complex (Kesharwani et al., 2022).
Despite significant advancements in cancer treatment, cervical cancer therapies such as doxorubicin (DOX) face challenges, including a lack of specific targeting, systemic toxicity, and drug resistance. Current DOX delivery methods are nonspecific, causing notable off-target effects and limited efficacy. Nanoparticle-mediated drug delivery systems hold potential but require further enhancement to selectively target cancer cells while minimizing toxicity to healthy cells. This study aims to address this gap by developing and evaluating a nanoparticle system using HA and Chitosan. The system is designed to deliver DOX specifically to CCa cells overexpressing the CD44 receptor. This targeted approach seeks to optimize DOX delivery to cancerous cells, minimize harm to healthy cells, and improve cervical cancer treatment efficacy, potentially providing a safer and more effective therapeutic option.
Methodology
Materials
Chitosan (Cs) (low molecular weight (50–190 kiloDalton, with 75-80% deacetylation) and tripolyphosphate polyanions were purchased from Scharlau (Germany). Glacial acetic acid was supplied by Merck (Germany). The HA with a high molecular weight of 1500 kDa dialysis membrane with high retention (12000–14000 Mw cut-off) was purchased from Sigma-Aldrich USA by Scientific Worldwide store Islamabad, Pakistan. Phosphate buffer saline, normal saline, and distilled water were provided by the Virology and Immunology Lab, Atta-ur-Rahman School of Applied Biosciences (ASAB), Industrial Biotechnology, National University of Sciences and Technology (NUST), Islamabad, Pakistan. Cell culture media, Fetal bovine serum (FBS) from Gibco Lot Number: 254T19, and Dulbecco’s Modified Eagle Medium (DMEM) from Sigma-Aldrich, Lot Number: 14490803 were purchased from the Asian scientific store in Islamabad, Pakistan. Sodium bicarbonate, Sodium pyruvate, Trypan blue, Trypsin, Triton X-100, and Disodium EDTA solutions were obtained from Gibco. Doxycycline was purchased from the Outpatient pharmacy department of Shifa International Hospital, Islamabad, Pakistan. All the chemicals and solvents utilized were of high analytical quality.
Molecular docking for ligand-receptor binding
In this study, we utilized the crystalline structure of human CD44 which was of high resolution, specifically the 4PZ3 structure, which includes the HA binding ___domain complex. This structure was obtained from the Protein Data Bank (PDB). HA’s structure which is the principal substrate for molecule CD44, was acquired from the PubChem database NCBI using the Compound Identifier (6852395). We employed CB-DOCK2, a powerful tool for structure-based docking, to perform docking studies.
Preparation of blank nanoformulation
HA-coated Chitosan NP production involves an ionic gelation process, which follows environmentally friendly synthesis protocols. This method utilizes a crosslinker called TPP to enable an ionic interaction between chitosan’s amino group which is positively charged and the HA which is negatively charged. Chitosan was dissolved in distilled water to initiate the synthesis. Then, a dropwise addition of 0.1 mg/ml TPP to the chitosan solution, and then HA was added in the end8.
Preparation of drug-loaded nanoformulation
We prepared DOX-loaded NPs in the study using a modified method. Chitosan solution was prepared using different strengths in the range of 0.1 to 1 mg/ml in distilled water to optimize the nanoparticles. In this solution, DOX was added dropwise using a syringe at 1 mg/ml concentration while stirring continuously at 530 rpm for 10 min. The solution was stirred at 530 rpm for 15 min while adding 1 ml of the crosslinker TPP. This process resulted in the formation of Chitosan NPs with different concentrations8. All formulations were then sonicated at 30 mA for 15 min to ensure thorough dispersion and homogenization. Following the sonication process, the nanoparticles containing the drug were coated with HA using a procedure that had been previously explained. The nanoparticles (NPs) of DOX-Loaded Chitosan-HA were subsequently subjected to centrifugation, and lyophilization, and kept at 4 °C for further use (Sultan et al., 2022).
NPs formulation physiochemical characterization
Characterization is crucial in assessing the ability of the NPs formulation to specifically deliver and penetrate the tumor site9.
Morphological analysis of NPs
To analyze the structure of both blank and drug-loaded nanoparticles (NPs), we employed scanning electron microscopy with the MIRA3 instrument from TESCAN, Czech Republic. The dried powder was carefully positioned on aluminum stubs after coating with a thin layer of gold. For SEM imaging, accelerated electrons were employed at a voltage of 15 kV, as described by Silvestro et al.. in 2020. These techniques allowed us to capture detailed images and analyze the structural characteristics of the NPs at a microscale level10,11.
Physiochemical analysis of NPs
We used the Malvern Zetasizer Nanos ZS90 instrument from Malvern Instruments, UK to perform the zeta nano-sizer technique to determine the mean of the nanoparticle size, Polydispersity Index, and zeta potential of both blank NPs and drug-loaded NPs12. To obtain accurate measurements, the NPs were diluted 1:10 with distilled water, readings were documented at a temperature of 25 °C. To ensure reliable data, three separate batches were analyzed. The PDI value of the formulation provides valuable insights into the size distribution of the particles present10.
XRD analysis for NPs
Using an alpha radiation-equipped Bruker D8 ADVANCE X-ray diffractometer from Germany we evaluated crystallographic properties of the dried blank and drug-loaded NP12. XRD is a non-invasive technique that allows for quick analysis and offers valuable information about the crystalline structure of nanoparticles (NPs). XRD patterns were obtained by collecting data in continuous mode at 2θ at an angle range of 10–50°. The patterns were then shown as x-y plots. We acquired the data using a step length of 60 s and a step size of 0.03°2θ (Uz Zaman et al.., 2021).
Functional group identification of NPs
To find out the functional groups and identify the compounds present in the prepared NPs, we employed Fourier transform infrared (FTIR) spectroscopy. The blank and drug-loaded NP was examined by Bruker Alpha-P spectrophotometer (USA), its scanning range was 4000 –500 cm− 1. By examining characteristic peaks of functional groups, we gathered valuable information about the composition of NPs (Mukhtar et al., 2020).
Drug loading and encapsulation efficiency
To determine the quantity of drug enclosed in the NPs, an indirect method was employed. The formulation was centrifuged at 13,500 rpm for 60 mints. This process allowed us to separate the NPs from the supernatant, which contained any unencapsulated drug (DOX)13. The supernatant that remained after the solid particles settled was precisely extracted and filtered through a syringe filter. Then, the filtered supernatant was examined using a UV-Vis spectrophotometer at 274 nm wavelength (NanoDrop 2000c model from Thermo Scientific, Wilmington, DE, USA) to check the amount of the free drug. By quantifying the free drug in supernatant, we calculated the encapsulation efficiency (EE) percentage using the appropriate formula (Silvestro et al.., 2020).
For indirect method:
Where {W1 = Total quantity of DOX, W2 = Free DOX in supernatant}.
Calibration curve in distilled water and phosphate buffer
According to BP guidelines, 1 mg of DOX was dissolved in 50 ml of distilled water and PBS at 7.4 pH. Different drug dilutions, ranging from 0.1 to 1 mg/ml, were prepared using different drug concentrations14. Distilled water or PBS was used as a blank, and all drug dilutions were evaluated at a wavelength of 274 nm using a UV-Vis spectrophotometer. A calibration curve was generated using MS Excel to determine the concentration of unknown DOX in NP formulation samples (Sultan et al.., 2022).
Evaluation of in-vitro drug release from NPs
To evaluate the release of DOX from the NPs, a dialysis bag was submerged in PBS with pH levels of 6.8 and 7.4, kept at 37 °C. 5 mg dried powder was inserted into the dialyzing membrane and thereafter placed in a 50 ml solution of PBS (Shahnaz et al.., 2017). Additionally, the technique was used to assess the release of crude DOX for comparison. The study was performed using triplicate samples to ensure accuracy and reliability. At specified time intervals (0, 0.5, 1, 2, 3, 4, 6, 12, 24, 48, and 72 h), 1.5 ml samples were collected from the dissolution medium and analyzed at 274 nm using NanoDrop 2000c model14.
In vitro anticancer potential of NPs of DOX-loaded chitosan-HA
In vitro analysis
The cytotoxicity of DOX-Loaded Chitosan-HA was assessed in vitro on both the CCa cell line (HeLa) and normal HEK cells. These cell lines were acquired from Dr. Tahir Mehmood, an Associate Professor at the University of Veterinary and Animal Sciences (UVAS) in Lahore, Pakistan. The NPs were administered to them in comparison to crude DOX at equivalent concentrations. ASAB NUST generously supplied HeLa and HEK cells which were cultured in DMEM. This media was enriched with 10% FBS and 1% pen/strep (100 units of penicillin and 100 µg of streptomycin) with a humidity level of 95% air and 5% CO2 at 37 °C. The cells were cultivated in tissue culture flasks with a surface area of 75 cm2 and were utilized for the experiment once they entered the phase of rapid and continuous growth (Jalilian et al., 2019).
Cell culture experimental groups
Three groups were formed for the in-vitro experiment:
-
Group 1: Untreated control HeLa cells in DMEM medium.
-
Group 2: DOX-treated HeLa cells.
-
Group 3: NPs of DOX-Loaded Chitosan-HA treated HeLa cells.
-
Group 4: Untreated control HEK cells in DMEM medium.
-
Group 5: DOX-treated HEK cells.
-
Group 6: NPs of DOX-Loaded Chitosan-HA treated HEK cells.
Cell morphology assay
The characteristic signs of apoptosis include morphological changes such as membrane blebbing, shrinkage, nuclear and cytoplasmic condensation, and cell rounding. To examine morphological changes, HeLa cells were seeded in a 96-well plate at 1 × 106 cells per well, with a volume of 100 µl. Following a period of 24 h, the media was replenished15. The crude DOX and NPs of DOX-Loaded Chitosan-HA were administered to cells at several doses (20, 60, and 100 µg/ml dissolved in PBS). Cell morphology was evaluated using a phase contrast inverted microscope at 12 and 24 h to detect the structural alterations caused by drug and formulation16.
Cell viability via Trypan blue exclusion assay
We employed a Trypan blue exclusion assay on Hela cells to check the effect of formulation on viaable cells. In this experiment, cells were seeded in a 96-well plate, with 130,000 cells per well in 1 ml of growth medium. The supernatant was discarded after a day, and the cells were detached and collected by treating them with Trypsin-EDTA for 5 min at 37 °C. The growth medium was added to inhibit the trypsin reaction, followed by centrifugation at 800 rpm for 4 min to obtain a cell pellet. The cells were washed with PBS and only 1 ml was re-suspended in fresh media. Next, we prepared a mixture of 50 µl of 0.4% trypan blue with varying concentrations of NPs and crude drug15. Specifically, we added 20 and 60 µl of crude drug to 80 and 40 µl of PBS, resulting in a final volume of 100 µl. The third concentration that was taken was 100 µl of pure drug. This mixture was placed for 3 min in an incubator at 37 °C (Madar et al.., 2022). Following incubation, the final mixture was transferred to a hemacytometer for the purpose of cell counting using a phase contrast inverted microscope16. It is important to note that cells should be counted within 5 min of mixing the cells with the dye. By employing this methodology, we could distinguish between viable (white) and dead (blue) cells, providing insights into the cytotoxic properties of NPs. Using the formula below, % cell viability was determined:
Cells cytotoxicity via MTT assay
To determine the cytotoxicity of NPs, we followed a method described previously with some minor modifications. Hela cells were plated in 96-well plates at 1 × 106 cells per well and incubated until they reached desired confluence rate. After 24 h, the media was removed from the cells, and we added different amounts of blank and NPs to the cells. The cells were put in a CO2 incubator at 37 °C with a mixture of 95% air and 5% CO2, along with DMSO (Dimethylsulphoxide) (Sohail et al.., 2016). We used cells in PBS at pH 7.4 with DMEM medium as a negative control. After 48 h, we removed the DMSO and added 10 µl of a stock MTT dye solution with a 5 mg/ml concentration to each cell. The plates were kept in an incubator at 37 °C for 3 to 4 h. After the incubation period, we carefully extracted the medium without causing any disturbance to the cells and introduced 100 µl of anhydrous isopropanol (0.1 N HCl) to each well11. By following these steps, we could assess the cytotoxicity of NPs and compare them to the pure drug.
To continue the experiment, the cells were incubated at 37 °C for 2 h. The absorbance of the cells was measured using a microplate reader from BioTek, Winooski, VT, US at 560 nm after incubation on the 2nd and 4th day (Jalilian et al., 2021). To determine the growth inhibition (GI) and inhibitory concentration (“IC50”), we compared the proportion of viable cells following treatment to control cells not exposed to the drug. We calculated the percentage of cytotoxicity using the following equation:
Cell migration assay
To analyze treated and untreated cell migration, we followed a method where Hela cells were plated in a 6-well plate, with 1.2 × 106 cells per well in 1.5 ml of growth medium11. The cells were then incubated until they reached the desired level of confluence. For the analysis, we utilized the Cell Comb scratch assay. Monolayers of HeLa cells were scratched in a one-direction pattern. To achieve this, we gently dragged 100 µL pipette tips across each row of wells, from top to bottom. After scratching, the wells were rinsed once with 200 µL of warm DPBS. Subsequently, we added 200 µL of pure DOX and DOX-Loaded Chitosan-HA, prepared in media. The plate was incubated at 37 °C for 24 h (Jalilian et al., 2021). After the 24-hour, the plate was imaged, and the results were carefully recorded.
Stability studies
After three months of storage at 4 °C and 37 °C, the stability of the blank and drug-loaded NPs was examined for changes in particle size and shape. Before being evaluated, the NPs were transferred into deionized water after being stored in a lyophilized state17.
Statistical analysis
A one-way analysis of variance (ANOVA) was performed to evaluate the statistical significance of the results. The significance level was established at p < 0.05, implying that any detected differences with a probability less than 0.05 were considered statistically significant. The results were presented as the mean value of three samples and the corresponding standard deviation (mean ± SD).
Results and interpretation
Molecular docking of hyaluronic acid with 4PZ3 (CD44)
The CD44 receptor consists of two binding domains, namely A and B. This study used CB-DOCK2 to dock the CD44 receptor with HA. By performing this docking process, we could evaluate the strength of the binding interaction between the ligand (HA) and the protein receptor CD44 (4PZ3). The obtained binding affinity was − 7.2, indicating a strong interaction between the two. Assessing binding affinity is crucial in drug design and development, as it provides insights into the effectiveness of potential drugs targeting CD44. The negative value of the binding affinity suggests an exothermic reaction and lower affinity values indicate easier binding between the ligand and receptor. The contact amino acid residues of the 4PZ3 molecule that formed conventional hydrogen bonds with HA were; Chain A: ASN25, ILE26, THR27, CYS28, PHE30, HIS35, GLU37, PHE74, GLU75, THR76, CYS77, ARG78, ARG90, CYS97, ARG150. Contact Amino acid residues of chain B were ASN39 GLY40 ARG41 (Table 1; Fig. 1).
Furthermore, hydrogen bonds play a vital role in illustrating the hydrophilicity of molecules involved in the binding. While aromatic bonds are less prevalent, they maintain the three-dimensional structure of nucleic acids and proteins. Hydrophobic interactions, conversely, are more stable and significant for studying molecules’ specificity and affinity. Understanding these molecular interactions helps pave the way for further research and targeted therapeutic development.
Characterization of NPs formulation
Physiochemical characteristics of HA-Chitosan nanoparticles
The results for the optimized blank Chitosan-HA NPs and the DOX-Loaded Chitosan-HA NPs are presented in Table 2; Fig. 2. To ensure accuracy, triplicate readings were taken for each measurement. For the blank Chitosan-HA NPs, the average smallest particle size was determined to be 273.1 nm. These NPs exhibited a zeta potential of + 16.5 ± 6.35 mV and a PDI of 0.274 when prepared with a 1 mg/ml Chitosan concentration. The smallest particle size measured was 284.6 nm for DOX-Loaded Chitosan-HA NPs. The zeta potential for these NPs was + 16.9 ± 5.81 mV, and the PDI was 0.314 at a concentration of 1 mg of DOX. These results provide valuable information about the size and surface charge characteristics of the Chitosan-HA nanoparticles, both in their blank form and when loaded with the drug DOX.
Morphological analysis of NPs
SEM analysis demonstrated the morphological features of blank and drug-loaded NPs at 20Kv, X25,000 in 1 μm. The blank NPs had clumpy appearances with rough surfaces compared to DOX loaded, which showed smooth particle surfaces in the formulation (Fig. 3a and b). The morphology of the prepared NPs appeared regular and round, indicating good dispersion without any adhesion damage. Furthermore, the distribution of nanoparticles was assessed using a zeta sizer, which demonstrated a reasonably even distribution. This information provides insights into the physical characteristics and uniformity of the drug-loaded NPs.
X-ray crystallographic analysis of NPs
To analyze the influence of intermolecular and extra molecular interactions on the crystalline structure of NPs, formulations were subjected to examination using X-ray diffraction (XRD). The results revealed distinct reflections at 2θ = 13°, indicating a prominent feature. Additionally, sharp peaks were observed at 20.5°, 21.5°, and 24° in NP, indicating crystalline material. Minor reflections were also observed at 32° and 42° in both blank and DOX-Loaded Chitosan-HA (Fig. 4). It suggests that the hydrogen bonding of intermolecular and extra-molecular forces, which contribute to the crystalline structure, may have also been retained in the DOX-Loaded Chitosan-HA. When X-rays are scattered by a regular arrangement of atoms, it creates specific diffraction patterns and provides qualitative information about the atomic structure of the crystal lattice.
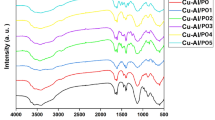
Functional group identification via FTIR
FTIR spectra observed notable peaks indicating specific functional groups and molecular bonds in the formulations (Fig. 5). For instance, Peaks at 2900 to 2880 cm− 1 suggested the stretching of CH bonds, while peaks at 1634 to 1606 cm− 1 indicated the stretching of amide C = O bonds observed in all the formulations. Additionally, peaks at 1419 cm− 1 were due to the CH2 bond, and at 1377–1257 cm− 1 were related to CN bond. Peaks from 1500 to 1550 cm− 1 indicate stretching of N-O bonds. C-O stretching is characterized by peaks at 1050–1085 cm− 1.
Encapsulation efficiency
The UV-visible spectroscopy method was utilized to determine NPs’ encapsulation efficiency (EE). As shown in Table 3, the average EE percentage of DOX-Loaded Chitosan-HA was 89.32 ± 0.59%. Notably, NPs carrying a higher drug load exhibited improved pharmacokinetic properties, resulting in improved effectiveness in in vitro experiments. An increased particles per dose is to elevate the exposure of the drug to both plasma and tumor sites. Figure 6 shows the standard calibration curve of DOX, showing its absorbance at varying concentrations.
In vitro drug release
The experiment aimed to assess DOX’s release from NP using a dialyzing membrane submerged in PBS with pH 7.4 and 6.8 at 37 °C. By analyzing percentage of drug released over time, it was observed that approximately 55% of the drug was released from NPs in pH 7.4 PBS, while approximately 89% was released in pH 6.8 within 72 h (Fig. 7). This sustained release pattern was considered favorable. On the other hand, when pure DOX was administered, 90% drug was released within 2 h at both pH 7.4 and 6.8, and remaining drug was released quickly within 24 h. This suggests that the prepared NPs exhibited a release pattern dependent on the environment’s pH, with a more significant amount of drug being released in an acidic environment that mimics the conditions of a tumor microenvironment.
Cell viability analysis of NPs
Cell viability assay was conducted using various dose concentrations (20, 60, and 100 µg/ml) of pure DOX and DOX-Loaded Chitosan-HA NPs on HeLa cells. The findings were compared with normal HEK cells treated with the same dose. In order to achieve this objective, many techniques were employed including cell morphology analysis, trypan blue exclusion assay, MTT assay, and scratch testing.
Cell morphology analysis
To study the impact of NPs on cell morphology, HeLa cells were subjected to NPs and pure drug for a day. The concentrations (20, 60, and 100 µg/ml) were adjusted for dilution factor. The changes in cell morphology were examined using a 10x lens on an inverted microscope at a duration of 12 and 24 h. Comparing the effectiveness of NPs of DOX-Loaded Chitosan-HA with pure DOX, it was found that the cells exhibited dose- and time-dependent apoptosis. Interestingly, lowest cytotoxicity was measured at 20 µg/ml. At 60 µg/ml, a mild alteration in cell shape was observed, while the maximum toxicity was observed at 100 µg/ml. This higher concentration led to rounding, detachment, and clumping of the treated cells (Fig. 8).
Trypan blue exclusion assay
The cell viability of HeLa cells treated with NPs of DOX-Loaded Chitosan-HA was assessed viaww trypan blue exclusion assay. At 20 and 100 µg/ml, the maximum and minimum percentage cell viability was found to be 41.04 ± 0.037% and 17.22 ± 0.004%, respectively. In comparison, pure DOX exhibited a cell viability of 53.77 ± 0.037% and 32.76 ± 0.0031% at the same concentrations (Fig. 9a). The data indicated a negative correlation between cell viability and mount of drug in cancerous cell lines. The trypan blue exclusion assay was used to assess the cell viability of HEK cells treated with NPs of DOX-Loaded Chitosan-HA. At concentrations of 20 and 100 µg/ml, maximum cell viability was 79.7 ± 0.037% and minimum cell viability was 63.7 ± 0.004%. In comparison, pure DOX exhibited cell viabilities of 52.4 ± 0.037% and 31.5 ± 0.0031% at the same concentrations (Fig. 9b). The data revealed a decline in cell viability as the drug concentration increased in both normal and cancerous cell types.
(a) Percentage cell viability for DOX-Loaded Chitosan-HA NPs compared to pure DOX in HeLa cells at a different concentration by trypan blue exclusion; mean ± SD (n = 3) p < 0.05. (b) Percentage cell viability for DOX-Loaded Chitosan-HA NPs as compared to pure DOX in HEK cells at different concentrations by trypan blue exclusion; mean ± SD (n = 3) p < 0.05.
MTT cytotoxicity assay
The effects of NPs of DOX-Loaded Chitosan-HA on cell viability were examined using the HeLa cell line, comparing it to pure DOX. To assess viability, MTT dye was used to measure absorbance, and a microplate reader was utilized to analyze cells at 520 nm after 2 days exposure. The cytotoxicity of DOX-treated HeLa cells increased proportionally with drug concentration, ranging from 20 to 100 µg/ml. The highest observed effect was 67.2 ± 0.003% at 100 µg/ml, while lowest effect was 46.2 ± 0.004% at 20 µg/ml, resulting in an “IC50” of 26 µg/ml (Fig. 10a). In the case of DOX-Loaded Chitosan-HA NPs, cytotoxicity was measured at 58.81 ± 0.001% for 20 µg/ml. However, as amount of NPs increased to 100 µg/ml, the observed cytotoxicity was significantly to 82.78 ± 0.004%. The “IC50” values for the NPs and pure DOX were 13 µg/ml and 26 µg/ml, respectively. This indicates that DOX-Loaded chitosan nanoparticles demonstrate increased efficacy in combating cervical cancer cells compared to the raw form of DOX.
While taking observations of this experiment, we have seen an unexpected rise in cytotoxic effect with the blank nanoparticles in which no therapeutic moiety was enclosed. This charactistic can be linked with intrinsic behaviouer of carrier material that is chitosan. It interacts with cellular component and cause killing of cancer cells most effectively. In the literature review, it is reported that the chitosan at higher concentration has ability to induce oxidative stress and membrane disruption. So, the nanoformulation of the polymer enhances cellular uptake and amplify their impact on cell viability due to the increased surface charge ratio of particles.
When the pure DOX and NPs of DOX-Loaded Chitosan-HA were applied on HEK cells to check the targeted potential of cytotoxicity, pure drug showed cytotoxicity of 47.5 ± 0.01% and 68.6 ± 0.02%, at 20 and 100 µg/ml respectively, while NP showed 20.2 ± 0.03% and 36.2 ± 0.01% at 20 µg/ml and 100 µg/ml respectively. Interestingly, HA-coated NPs exhibited less toxicity towards normal cells, as they do not bind precisely without the presence of the CD44 receptor. The obtained results conclude that the NPs have a higher potential in targeting and eliminating cancerous cells than the raw drug (Fig. 10b).
Cell migration analysis
HeLa cells were exposed to DOX-Loaded Chitosan-HA and pure drug for 24 h for cell migration analysis. 200 µl of prepared NP and pure DOX were added to separate wells. Wells were examined for cell migration using a 10x lens on an inverted microscope at 24 h. It was exhibited that drug- and NP-treated wells showed less or no significant cell migration, while control wells that contained untreated HeLa cells displayed significant cellular migration (Fig. 11). This demonstrated that prepared Nanoformulation can significantly reduce cell migration, which plays an essential role in cancer invasion and metastasis, thus contributing to rapid treatment of CCa.
Gene expression analysis through RT-PCR
Tumor necrosis factor-α performs significant function in connecting inflammation and cancer, developing the tissue structure needed for tumor growth and spread. It enhances the synthesis of additional cytokines, angiogenic factors, and MMPs, therefore promoting the proliferation and viability of cancerous cells. These activities that promote tumor development suggest that blocking TNF-α can be an effective approach to cancer treatment. Various observations has emphasized the role of TNF-α in the growth, movement, invasion, and formation of blood vessels by tumor cells. TNF-α promotes tumor cell survival by activating genes that encode antiapoptotic molecules dependent on NF-κB (Shishodia and Aggarwal, 2004). Furthermore, TNF-α acts as a growth factor for tumor cells and stimulates the expression of other growth factors like amphiregulin, EGFR, and TGF-α, increasing tumor proliferation.
Additionally, TNF-α contributes to tumor angiogenesis by mediating the production of various angiogenic factors such as IL-8 and VEGF. By inhibiting TNF-α, we can significantly contribute to the treatment of cervical cancer. Notably, DOX-loaded NP has effectively inhibited TNF-α expression (Tables 4 and 5; Fig. 12).
A fold change value of 0.535 was obtained in the case of TNF-Alpha, which indicates a 53% downregulation of TNF-Alpha gene expression compared to the Control/housekeeping gene B-Actin.
Nanoparticle stability analysis
The stability of DOX-Loaded Chitosan-HA was also monitored to record fluctuations in particle size, PDI, and zeta potential over 3 months storage at 4 °C and 37 °C. The characterization of a liquid formulation of DOX-Loaded Chitosan-HA revealed an rise in particle size with decreasing and zeta potential. This indicated that storing NPs in dried form was better.
Discussion
DOX is a second-generation tetracycline derivative frequently used to treat various infections. Numerous studies have shown that it is a pluripotent medication with anti-carcinogenic properties, reducing cell growth and inducing apoptosis in tumor cells. These effects include inhibiting melanoma cell migration and demonstrating an antitumor growth effect on human oral squamous cell carcinoma. Additionally, DOX’s ability to inhibit matrix metalloproteinase (MMPs) affects the stem cells that cause cervical cancer18. Therefore, we used HeLa cells, a human cervical cancer cell line, to investigate the impact of pure DOX and DOX-Loaded Chitosan-HA for treating CCa. pure DOX is an effective antibiotic; however, it has many disadvantages. Its instability in the biological environment and and premature loss are attributed to fast clearance. Moreover, tetracycline resistance is a global issue, and large concentrations of these substances may harm healthy tissues. Nanotechnology enables the efficient delivery of antibiotics to infection sites and helps manage dosage amounts and frequency to infection sites efficiently and manages dosage amounts and frequency, reducing side effects associated with traditional therapy5. Therefore, we considered using biodegradable natural polymers, such as chitosan, to formulate nanoparticles of DOX19.
This research employed DOX to synthesize nanoparticles that were polymeric and surface-functionalized with ligands. HA was used as a ligand to efficiently target CD44 receptors, which are overexpressed in cervical cancer cells. Ligands like HA shield the drug from clearance. With the aid of the crosslinker TPP, the NFs of HA-coated DOX-Loaded Chitosan were formed utilizing the ionic gelation process. Green synthesis protocols prohibited the use of strong chemicals or organic solvents. Hyaluronic acid (HA) has been widely utilized as a targeting ligand due to its specific interaction with CD44 receptors CD44 receptors that are overexpressed on cancer cells. The strong binding affinity between HA and CD44 has been demonstrated20, supporting its efficacy in enhancing nanoparticle uptake and therapeutic efficacy in cancer therapy.
The particle size and PDI of the prepared Chitosan-HA NPs in our study were analyzed using a zetasizer. For the blank Chitosan-HA NPs, the average smallest particle size was determined to be 273.1 nm. These NPs exhibited a zeta potential of + 16.5 ± 6.35 mV and a PDI of 0.274 when prepared with a 1 mg/ml Chitosan concentration21. In the case of the NPs of DOX-Loaded Chitosan-HA, the smallest particle size measured was 284.6 nm. The zeta potential for these NPs was + 16.9 ± 5.81 mV, and the PDI was 0.314. Another study reported the size of two different DOX-chitosan formulations comparable our analysis. The nanospheres varied in size, with DCNP4 ranging from 30 to 220 nm in diameter and DCNP6 ranging from 200 to 320 nm in diameter22. The variation in nanoparticle sizes can be ascribed to parameters such as the molecular weight of the chitosan used, the volume ratio of chitosan to TPP solution, and the specific manufacturing processes, such as stirring and sonication duration, that that differ among various studies23. The size, quantity and distribution of the nanoparticles within the cells determine the applications of NPs and impact the drug’s loading, encapsulation, and release. Studies have reported similar particle sizes (~ 200–300 nm) and zeta potentials (+ 10 to + 20 mV) in HA-coated nanoparticles designed for cancer targeting8,12, emphasizing their role in enhancing tumor penetration and drug delivery.
It is stated that cationic NPs are more readily absorbed through direct permeation than neutral or anionic NPs24. According to Maeda et al.. (2000), nanoparticles ranging in size between 50 nm and 300 nm have been demonstrated the smooth-surfaced particles of the formulation. Furthermore, limited reticuloendothelial system uptake of nanoparticles smaller than 300 nm results in a substantially longer circulation time, which increases tumor cell uptake. As a result, the unique NPs produced in this work have a size of less than 300 nm and are capable of being delivered to CCa cells9. Research has shown that the rate at which NPs are removed from the bloodstream is significantly greater for charged particles than for uncharged particles. Additionally, the presence of a positive zeta potential in formulations in formulations enhances the absorption of by cancer cells, which have a negative charge. The particle dispersion is uniform when the PDI value is less than 0.5. Conversely, a PDI value above 0.5 denotes a heterogeneous distribution in the formulation’s particle sizes25.
SEM analysis demonstrated the smooth surface particles of the formulation. The morphology of the prepared NPs appeared regular and round, indicating good dispersion without any adhesion or subsidence damage. The NPs with the fastest internalization rate are spherical. Thus, out of all the tested NPs with diverse shapes, the spherical NPs exhibited the maximum uptake. A thorough free energy analysis shows that NP shape effect is mostly caused by membrane bending energies during endocytosis. Spherical NPs have a lower membrane bending energy barrier to overcome compared to non-spherical equivalents26. SEM analysis in our study revealed the smooth and spherical morphology of DOX-loaded Chitosan-HA nanoparticles, consistent with observations on HA-functionalized nanoparticles10, highlighting their structural integrity and stability.
FTIR spectra demonstrated the constitution and phase composition of the NPs. The spectra displayed notable peaks indicating specific functional groups and molecular bonds in the formulations. For instance, peaks in the range of 2900 to 2880 cm[-1 suggested the stretching of CH bonds, while peaks at 1634 to 1606 cm[-1 indicated the stretching of amide C = O bonds observed in all the formulations. Additionally, peaks at 1419 cm− 1 were due to the CH2 bond, and those in the range of 1377–1257 cm⁻¹ were related to the CN bond. Peaks from 1500 to 1550 cm− 1 indicate stretching of N–O bonds, while C–O stretching was characterized by peaks at 1050–1085 cm⁻¹27. XRD analysis showed that drug-loaded formulation retained hydrogen bonding of intermolecular and extra molecular forces, which suggests a high degree of crystallinity of NPs. X-ray diffraction patterns are frequently utilized in nanoparticle research as a major characterization tool for acquiring critical parameters such as strain, crystallite size, and crystal structure. Diffraction peaks broaden in nanocrystalline materials due to randomly aligned crystals. This phenomenon can be explained by the fact that no total constructive or destructive X-ray interferences exist in a finite-sized lattice28.
The EE percentage of DOX-loaded NPs in Chitosan-HA was 89.32 ± 0.59%. Notably, NPs carrying a higher drug load exhibited improved pharmacokinetic properties, enhancing efficacy in in vitro experiments. An increased number of particles per dose is known to increase drug exposure to both plasma and tumor sites.
The prepared NPs exhibited a release pattern that was dependent on the environment’s pH, with a more significant amount of drug being released in an acidic environment that mimics the conditions of a tumor microenvironment13. In our study, approximately 89% of the drug was released in a pH 6.8 buffer (representing an acidic environment) within 72 h. This sustained release pattern was considered favorable. On the other hand, when pure DOX was administered, 90% of the drug was released within 2 h, and the remaining amount was quickly released within 24 h. Studies have demonstrated pH-responsive drug release profiles in HA-coated nanoparticles, which align with our study’s findings of sustained drug release in acidic pH environments14, highlighting its importance for effective cancer treatment.
Compared to pure DOX, the DOX-loaded NPs exhibited time- and dose-dependent cytotoxicity against HeLa cells while sparing HEK cells. The in vitro assay’s cell morphology demonstrated apoptosis induction in HeLa cells treated with NPs, confirming the synthesized NPs’ ability to deliver therapeutic agents efficiently while retaining their cytopathic effect29.
Simultaneously, normal cells were protected from the drug’s damaging effects by the NPs’ ability to deliver targeted delivery. Using the trypan blue exclusion assay, it was determined that NPs and pure drug concentrations in normal and malignant cell lines reduced the viability of the cells. The IC50 values for the NPs and pure DOX were 13 µg/ml and 26 µg/ml, respectively30. This indicates that DOX-loaded chitosan nanoparticles demonstrate increased efficacy in combating cervical cancer cells compared to the raw form of DOX.
Previous studies also support that NPs of DOX exhibit greater therapeutic efficacy than pure DOX. For instance, a study found that the anticancer impact of 10 mg/kg of DOX-PNPs surpassed that of 5 and 10 mg/kg of free DOX in reducing the size of solid tumors31. Selective cytotoxicity of HA-targeted nanoparticles towards cancer cells, combined with reduced toxicity to normal cells, has been shown16, validating our findings of an improved therapeutic index and safety profile.
Using nanocarriers as drug delivery vehicles aims to enhance to enhance the therapeutic and pharmacological properties of conventional medications. Incorporating drug molecules into nanocarriers protects them from degradation, providing opportunities for targeted and controlled release. Nanocarriers can operate at a cellular scale and traverse the blood-brain barrier (BBB) due to their small size. Additionally, the NPs used in this study demonstrated the ability to target CD44 through HA-mediated surface functionalization. By reducing off-target toxicity and enabling the sustained release of DOX, the drug’s therapeutic potential was increased17.
Tumor necrosis factor-α is significant in connecting inflammation and cancer, facilitating the tissue structure needed for tumor growth and spread. It promotes the production of other cytokines, angiogenic factors, and MMPs, thereby supporting increased growth and survival of tumor cells. The presence of these actions, which facilitate the growth of tumors, indicates that inhibiting TNF-α can be a successful strategy for treating cancer15. Multiple studies have emphasized the significant role of TNF-α in the growth, movement, invasion, and formation of blood vessels by tumor cells. DOX acts by inhibiting the MAPK and NFkB pathway. MAPKs and NFκB are well-known for their important roles in generating pro-inflammatory cytokines like TNF-α, IL-6, IL-1β, and IL-18. The MAPK signaling pathway is vital for various metabolic processes, including cell differentiation and division, as well as for mediating inflammatory responses. The observed downregulation of TNF-α gene expression in our study aligns with findings on HA-mediated anti-inflammatory effects in cancer cells, indicating potential mechanisms underlying nanoparticle-based cancer therapies32.
The NFκB signaling system, which is activated by the MAPK signaling pathway, has a crucial function in stimulating the expression of pro-inflammatory genes and triggering inflammatory responses. Our findings indicate that DOX suppresses the phosphorylation of MAPK. The assembly of the inflammasome complex is initiated by pathogen-related molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) that are detected by the cell. DOX treatment reduces the activation of the NLRP3 inflammasome. Furthermore, it decreases the activation of caspase-1 in activated macrophages. This suppression of NLRP3 expression and caspase-1 maturation leads to the inhibition of IL-1β and IL-18 release, effectively attenuating the release of pro-inflammatory cytokines. Moreover, DOX inhibits NLRP3 and induces early apoptosis in cancer cells, regardless of the NLRP3 activating capacity of the cancer cells.
Conclusion
Using biodegradable natural polymers as drug delivery vehicles improves the therapeutic and pharmacological properties of traditional cancer therapy. Nanotechnology has revolutionized the dynamics of cancer treatment. The polymeric-based nanoparticle-mediated chemotherapy drug delivery system has gained appeal because of its ease of modification, ability to reduce doses, and prevention of off-target toxicities. The current study achieved a unique sustained/controlled and targeted drug delivery system based on mucoadhesive polymer chitosan, which is biocompatible, biodegradable, and has an outermost coating of HA that functions as a ligand for surface functionalization. Green synthesis protocols were used to synthesize DOX nanoparticles through the ion gelation method. These formulations actively targeted tumor sites by specifically binding to the CD44 receptor, which is overexpressed in cervical cancer. CB-DOCK2 analysis confirmed molecular docking interactions between HA and CD44. The synthesized nanoparticles demonstrated a positive zeta potential and a particle size range of 200–300 nm range, which facilitates the uptake of the particles by the negatively charged cancer cell membrane. Synthesized nanoparticles showed a controlled release of drug in an acidic environment, high encapsulation efficiency, good stability, and crystalline nature. The NPs demonstrated high cytotoxicity in cancer cells compared to pure DOX in a dose and time-dependent manner due to the overexpression of CD44 receptors in cancer cells. NPs displayed no significant cytotoxicity in HEK cells, confirming that the drug was safe for normal cells. Moreover, DOX NPs exhibited a downregulation of TNF-α in DOX-treated HeLa cells. Thus, the objective of this research was to enhance the pharmacological and therapeutic characteristics of pure DOX by employing biodegradable natural polymers as a drug delivery system. When integrated into nanocarriers, DOX-loaded NPs are shielded from degradation, demonstrate targeted and sustained release, and show promise as a therapy for cervical cancer treatment.
Limitation
The morphological features of enclosed moieties were examined by SEM which has provide high resolution images of surface structures and particles shape but it does not provide information about internal sutructures, particle size distribution and surface charge under disffernt physiologica; conditions. For these purpose, Transmission Microscopy should be used. So, future studies wil incorporate this technique for comprehensive characterization.
The other limitation of current study is the use of single cervical cancer cell line (HeLa) for in vitro analysis. Although it’s a widely use cell line for cancer research but other multiple subtypes and different cell lines such as SiHa or C33A with different genetic and phenotypic profiles will be used further for comprehensive analysis.
Additionally, the in vivo experiment are also part of future project to check the translational and cklinical reliance of in vitro results. So, the pharmacokinetics, biodistribution, and therapeutic efficacy of nanoparticles will be assessed in a complex biological system.
Future work will address these gaps and validate the therapeutic potential and safetly profile of nnaoformulatiuons.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hirani, S. et al. Knowledge, awareness, and practices of cervical cancer, its risk factors, screening, and prevention among women in Karachi, Pakistan. Eur. J. Cancer Prev. 30 (1), 97–102 (2021).
Bedell, S. L., Goldstein, L. S., Goldstein, A. R. & Goldstein, A. T. Cervical Cancer screening: past, Present, and Future. Sex. Med. Reviews. 8 (1), 28–37. https://doi.org/10.1016/j.sxmr.2019.09.005 (2019).
Kousar, K. et al. Green synthesis of hyaluronic acid coated, thiolated chitosan nanoparticles for CD44 targeted delivery and sustained release of cisplatin in cervical carcinoma. Front. Pharmacol. 13, 1073004 (2023).
Shen, S. et al. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat. Nanotechnol. 16 (1), 104–113 (2021).
Misra, R. & Sahoo, S. K. Antibacterial activity of doxycycline-loaded nanoparticles. In Methods in Enzymology (Vol. 509, 61–85). Elsevier (2012).
Naseer, F. & Ahmad, M. Green nanoparticles as multifunctional nanomedicines: insights into anti-inflammatory effects, growth signaling and apoptosis mechanism in cancer. F Naseer. Sem. Cancer Biol. https://doi.org/10.1016/j.semcancer.2022.06.014 (2022).
Naseer, F., Ahmed, M., Majid, A., Kamal, W. & Phull, A. R. Green nanoparticles as multifunctional nanomedicines: insights into anti-inflammatory effects, growth signaling and apoptosis mechanism in cancer. Sem Cancer Biol. 86, 310–324. https://doi.org/10.1016/j.semcancer.2022.06.014 (2022).
Jain, A. et al. Hyaluronic acid-conjugated polymeric nanoparticles for targeted cancer therapy. Nanomedicine 14 (7), 2387–2404 (2019).
Anjum, S. et al. Enhancing therapeutic efficacy: sustained delivery of 5-fluorouracil (5-FU) via thiolated chitosan nanoparticles targeting CD44 in triple-negative breast cancer. Sci. Rep. 14, 11431. https://doi.org/10.1038/s41598-024-55900-1 (2024).
Li, X. et al. Morphological characterization of HA-functionalized nanoparticles. J. Microsc. 267 (2), 190–198 (2017).
Anjum, S. et al. Co-delivery of oncolytic virus and chemotherapeutic modality: vincristine against prostate cancer treatment: a potent viro-chemotherapeutic approach. J. Med. Virol. 96, e29748. (2024).
Smith, K. et al. Zeta potential and particle size analysis of HA-coated nanoparticles. J. Nanopart. Res. 22, 1–15 (2020).
Patel, N. et al. Encapsulation efficiency of HA-coated nanoparticles. Eur. J. Pharm. Sci. 145, 105213 (2020).
Gupta, S. et al. pH-responsive drug release from HA-coated nanoparticles. J. Controlled Release. 310, 115–127 (2019).
Zheng, L. et al. TNF-α gene expression modulation by HA-functionalized nanoparticles. J. Nanobiotechnol. 17 (1), 1–12 (2019).
Chen, J. et al. Selective cytotoxicity of HA-targeted nanoparticles. Biomaterials 178, 302–312 (2018).
Naseer, F. et al. Formulation of surface-functionalized hyaluronic acid-coated thiolated chitosan nanoformulation for the delivery of vincristine in prostate cancer: a multifunctional targeted drug delivery approach. J. Drug Deliv. Sci. Technol. 74, 103545. https://doi.org/10.1016/j.jddst.2022.103545 (2022).
Yang, B. et al. Doxycycline induces apoptosis and inhibits proliferation and invasion of human cervical carcinoma stem cells. PloS One, 10(6), e0129138 (2015).
Muxika, A., Etxabide, A., Uranga, J., Guerrero, P. & De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 105, 1358–1368 (2017).
Lee, H. et al. Hyaluronic acid–based nanomaterials for cancer therapy. Nanoscale Adv. 1, 1983–2003 (2018).
Anjum, S. et al. Co-delivery of oncolytic virus and chemotherapeutic modality: Vincristine against prostate cancer treatment: A potent viro-chemotherapeutic approach. J Med Virol. ;96(7):e29748. (2024). https://doi.org/10.1002/jmv.29748. PMID: 38975633.
Cover, N. F., Lai-Yuen, S., Parsons, A. K. & Kumar, A. Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. Int. J. Nanomed., 2411–2419 (2012).
Dzung, N. A., Khanh, V. T. P. & Dzung, T. T. Research on impact of Chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 84 (2), 751–755 (2011).
Khanal, L. N. et al. Green synthesis of silver nanoparticles from root extracts of Rubus ellipticus Sm. and comparison of antioxidant and antibacterial activity. Journal of Nanomaterials, 2022, 1-11 (2022).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10 (2), 57 (2018).
Li, Y., Kröger, M. & Liu, W. K. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale 7 (40), 16631–16646 (2015).
Zhang, Y. et al. TEM analysis of HA-coated nanoparticles. Nano Lett. 21 (8), 3251–3259 (2021).
Akbari, B., Tavandashti, M. P. & Zandrahimi, M. Particle size characterization of nanoparticles–a practical approach. Iran. J. Mater. Sci. Eng. 8 (2), 48–56 (2011).
Mirabelli, P., Coppola, L. & Salvatore, M. Cancer cell lines are useful model systems for medical research. Cancers 11 (8), 1098 (2019).
Song, M. et al. HA-functionalized nanoparticles for cancer therapy. Adv. Drug Deliv. Rev. 176, 113874 (2021).
Gardouh, A. R. et al. Synthesis and antitumor activity of doxycycline polymeric nanoparticles: Effect on tumor apoptosis in solid ehrlich carcinoma. Molecules 25 (14), 3230 (2020).
Park, J. et al. Anti-inflammatory effects of HA-coated nanoparticles in cancer models. J. Experimental Clin. Cancer Res. 41 (1), 1–15 (2022).
Bahrami, B. et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 190, 64–83 (2017).
Gavas, S., Quazi, S. & Karpiński, T. M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 16 (1), 173 (2021).
Naseer, F., Ahmad, T., Kousar, K. & Anjum, S. Advanced Therapeutic Options for Treatment of Metastatic Castration-Resistant Prostatic Adenocarcinoma Front12728054 (Pharmacology, 2021).
Khanal, L. N. et al. Green synthesis of silver nanoparticles from root extracts of Rubus ellipticus Sm. And comparison of antioxidant and antibacterial activity. J. Nanomaterials. 2022, 1–11 (2022).
Zhao, Y., Wang, X., Li, L. & Li, C. Doxycycline inhibits proliferation and induces apoptosis of both human papillomavirus-positive and negative cervical cancer cell lines. Can. J. Physiol. Pharmacol. 94 (5), 526–533. https://doi.org/10.1139/cjpp-2015-0481 (2016).
Kousar, K., Naseer, F., Abduh, S. M. & Ahmad, T. CD44 targeted delivery of oncolytic Newcastle Disease virus encapsulated in thiolated Chitosan for sustained release in cervical cancer: a targeted immunotherapy approach. Front. Immunol. https://doi.org/10.3389/fimmu.2023.1175535 (2023).
Tong, X. et al. Recent advances in natural polymer-based drug delivery systems. Reactive Funct. Polym. 148, 104501 (2020).
Perkins, R. B., Wentzensen, N., Guido, R. S. & Schiffman, M. Cervical cancer screening: a review. Jama 330 (6), 547–558 (2023).
Cohen, P. A., Jhingran, A., Oaknin, A. & Denny, L. Cervical cancer. Lancet 393 (10167), 169–182 (2019).
Grigsby, P., Watson, M., Powell, M., Zhang, Z. & Rader, J. Gene expression patterns in advanced human cervical cancer. Int. J. Gynecol. Cancer. 16 (2), 562–567 (2006).
Manivasagan, P., Senthilkumar, K., Venkatesan, J. & Kim, S. Biological applications of chitin, chitosan, oligosaccharides and their derivatives. Chitin and chitosan derivatives: advances in drug discovery and developments, 223–242 (2013).
Singh, R. & Lillard, J. W. Jr Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 86 (3), 215–223 (2009).
Arbyn, M. et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 8 (2), e191–e203 (2020).
Hamed, I., Özogul, F. & Regenstein, J. M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci. Technol. 48, 40–50 (2016).
Naor, D., Nedvetzki, S., Golan, I., Melnik, L. & Faitelson, Y. CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 39 (6), 527–579 (2002).
Mahapatro, A. & Singh, D. K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 9, 1–11 (2011).
Guo, Q., Yang, C. & Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 289 (24), 7970–7986 (2022).
Holmes, N. E. & Charles, P. G. Safety and efficacy review of doxycycline. Clinical Medicine. Therapeutics, 1, CMT. S2035. (2009).
Imran, H. et al. Optimized DOX Drug Deliveries via Chitosan-mediated nanoparticles and stimuli responses in Cancer Chemotherapy: a review. Molecules 29 (1), 31 (2023).
Peralta-Zaragoza, O. et al. Targeted treatments for cervical cancer: a review. OncoTargets Therapy, 315–328. (2012).
Rimel, B. J., Kunos, C. A., Macioce, N. & Temkin, S. M. Current gaps and opportunities in screening, prevention, and treatment of cervical cancer. Cancer 128 (23), 4063–4073 (2022).
Sadoughi, F., Mansournia, M. A. & Mirhashemi, S. M. The potential role of Chitosan-based nanoparticles as drug delivery systems in pancreatic cancer. IUBMB life. 72 (5), 872–883 (2020).
Wang, L., Guo, H., Lin, C., Yang, L. & Wang, X. (2014). Enrichment and characterization of cancer.
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71 (3), 209–249 https://doi.org/10.55627/pharma.002.01.0299 (2021).
Acknowledgements
The authors gratefully acknowledge technical and financial support from ministry of education and deanship of scientific research(DSR), King Abdulaziz University (KAU), Jeddah, Saudi Arabia.
Funding
This research work was funded by Institutional Fund Projects under grant no.(IFPDP-283-22).
Author information
Authors and Affiliations
Contributions
Ayesha Akhtar: Conceptualization, writing the original draft, in vitro analysis, and lab work. Sadia Anjum and Tahir Ahmad: Supervision, funding, and proofreading. Maryam Sadia: in silico and in vitro analysis. Saleh M. Aldaqal and Maisa S. Abduh: Methodology; investigation; funding resources; data curation. Hammad Ahmad: Mechanistic study, methodology, and review of the manuscript. Faiza Naseer: Formulation development and optimization of nanoparticles. Riaz Mustafa: Provision of raw material and Revision of article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Anjum, S., Akhtar, A., Aldaqal, S.M. et al. Enhanced targeted treatment of cervical cancer using nanoparticle-based doxycycline delivery system. Sci Rep 15, 2318 (2025). https://doi.org/10.1038/s41598-024-84203-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84203-8