Abstract
Multiple myeloma (MM) is the second most common hematological malignancy. Previous studies have validated the prognostic significance of the platelet-to-lymphocyte ratio (PLR) in patients with certain solid tumors. However, the relationship between the PLR and prognosis in myeloma patients has not been clearly demonstrated. In our study, we included 122 newly diagnosed MM patients who were treated with bortezomib-based chemotherapy. These patients were divided into low-PLR and high-PLR groups based on their initial PLR values. We compared the clinical characteristics between the two groups and utilized restricted cubic splines (RCSs) in the regression model to estimate the nonlinear relationship between the initial PLR and overall survival (OS) in MM patients. The results showed that patients in the low-PLR group had significantly worse OS (P = 0.00031) and progression-free survival (PFS) (P < 0.0001) compared to those in the high-PLR group. Furthermore, within the higher-risk MM group, a low PLR was also associated with worse OS (P = 0.0037) and PFS (P = 0.0048). Therefore, a low PLR was identified as an independent predictor of poor OS in MM patients. The RCS curves further confirmed a significant nonlinear relationship between the PLR and OS in patients with MM. The PLR may serve as a significant independent prognostic indicator for MM patients undergoing bortezomib-based chemotherapy, and there exists a crucial nonlinear relationship between the PLR and OS in these patients.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a malignant plasma cell tumor and is the second most common hematologic malignancy, constituting approximately 10% of all hematologic malignancies. MM is characterized by the accumulation of malignant plasma cells in the bone marrow, leading to hypercalcemia, kidney failure, anemia, and osteolytic lesions in bones1,2. Over the past decade, the use of autologous hematopoietic stem cell transplantation (ASCT), followed by the introduction of novel agents such as proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), has significantly improved the survival rates of MM patients3,4,5,6. With continuous improvements in treatment, the life expectancy of MM patients is gradually increasing7,8. However, despite these advancements, almost all MM patients eventually experience relapse, and MM remains an incurable disease. Therefore, there is an urgent need to identify valuable prognostic indicators that can assess patient risk and guide more effective treatment strategies to delay disease progression.
Traditional and classic prognostic factors for MM patients include the stage of the disease, performance status, age, and comorbidities9. Recent studies have demonstrated that a range of genetic, biochemical, and hematological factors are associated with the prognosis of patients with solid cancers as well as other hematologic malignancies10,11,12. Inflammation markers are considered to indirectly reflect the status of the bone marrow microenvironment, which influences the processes that regulate and promote the growth, survival, migration, and even drug resistance of myeloma cells13. Consequently, it is crucial to identify reliable prognostic tools that can predict clinical outcomes and assist clinicians in making informed decisions about treatment options.
However, the relationship between the platelet-to-lymphocyte ratio (PLR) and prognosis has not been clearly demonstrated in myeloma patients. Our study aimed to investigate the role of the PLR in the prognosis of myeloma patients.
Materials and methods
Study Design and patients
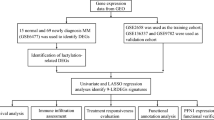
This retrospective study analyzed data from 122 newly diagnosed MM patients who were treated at Huai’an No.1 people’s hospital between October 2014 and December 2021. Patients were followed up until January 2024. We documented the age, sex, immunoglobulin (ig) subtypes, and survival outcomes of all patients. At the time of diagnosis, complete blood count (CBC) data, the proportion of bone marrow monoclonal plasma cells, and biochemical parameters (including serum albumin, lactate dehydrogenase, serum creatinine, calcium, and β2-microglobulin) were collected from all patients. The International Staging System (ISS) was utilized as the staging criterion. The PLR was calculated as the ratio of the platelet count to the lymphocyte count. All relevant parameters were obtained from the patients’ laboratory test results at the time of their initial diagnosis.
Among the 122 patients, 33 (27.05%) had IgA, 55 (45.08%) had IgG, 3 (2.46%) had IgD, 1 (0.82%) had IgM, and 30 (24.59%) had other types of multiple myeloma, including the light chain type, nonsecretory type, and double M-proteinemia type. All patients had undergone at least one cycle of bortezomib-based chemotherapy. Among all the patients, 54 (44%) received the bortezomib/cyclophosphamide/dexamethasone regimen, 36 (30%) received the bortezomib/lenalidomide/dexamethasone regimen, 23 (19%) received the bortezomib/thalidomide/dexamethasone regimen, and 9 (7%) received the bortezomib/doxorubicin/dexamethasone regimen as the induction therapy. A total of 15 (12.3%) patients underwent ASCT as part of their induction regimen. Overall survival (OS) was defined as the time period from the initial diagnosis to death from any cause or the last follow-up, whereas progression-free survival (PFS) referred to the duration from the initial diagnosis to disease progression or death.
This study was approved by the Institutional Review Board (IRB) of Huai’an No.1 People’s Hospital, and the study was conducted in accordance with the principles of the Helsinki Declaration. Due to the retrospective nature of the data collection, all patient identities were anonymized, and informed consent was waived by the IRB.
The inclusion criteria for this study were as follows: (1) Met the diagnostic criteria for MM. (2) Had sufficient medical information available for analysis. (3) Had PLR data available for newly diagnosed patients. (4) Aged between 18 and 80 years inclusive. Exclusion criteria: (1) Patients with significant vital organ impairment have failed to complete ≥ 1 bortezomib treatment cycle. (2) Insufficient medical records or missing critical information necessary for analysis.
Statistical analysis
The data were analyzed using R software. For continuous variables, the mean and standard deviation (Mean ± SD) were reported. Abnormally distributed continuous variable was compared using the Mann–Whitney test while normally distributed continuous variable was compared using Student’s t-test. To explore the role of the PLR as a predictor of survival status, we utilized the R package “survminer” (version 0.4.9) to analyze the covariates of OS and PFS and to determine the optimal cutoff points for the PLR. Subsequently, the Kaplan-Meier (K-M) method was employed to compare survival differences among patient groups stratified based on these cutoff points, thereby evaluating the impact of the PLR on the prognosis of MM patients. K-M survival curves were generated to contrast the prognosis between the low-PLR and high-PLR groups. Furthermore, the corresponding p-value was determined via the log-rank test. Univariate and multivariate Cox proportional hazards models were utilized to identify significant prognostic factors. In the multivariate analysis, we examined multiple variables, including PLR, Durie-Salmon (DS) stage, International Staging System (ISS) stage, sex, age, hemoglobin (Hb) levels, platelet (Plt) count, serum albumin (ALB) levels, lactate dehydrogenase (LDH) levels, red blood cell distribution width standard deviation (RDW-SD), serum creatinine (Cr) levels, calcium (Ca) levels, β2-microglobulin (β2-MG) levels, the proportion of bone marrow monoclonal plasma cells, and ASCT. P-values < 0.05 were considered statistically significant. Restricted cubic splines (RCSs) were incorporated into the regression model to estimate the nonlinear relationship between the PLR and OS in MM patients.
Results
Patient characteristics
The clinical and laboratory characteristics of the patients are summarized in Table 1. A total of 122 patients were included in this retrospective study. The study population was balanced with 50% males and 50% females. The median age of the patients was 63 years (range: 30–77 years). At the end of the follow-up period, the median PFS was 27 months (range: 1–72 months) and the median OS was 34 months (range: 1–146 months). A total of 61 deaths (50%) were recorded. Patients were divided into two groups based on their survival status, and variables from both groups were displayed and compared. The average age of survivors was 60 ± 10 years, which was younger than that of non-survivors at 63 ± 8 years. Significant differences were observed between the two groups in terms of RDW_SD, PLR, Hb, and Plt (P < 0.05). However, there were no significant differences between the two groups in terms of gender, age, DS stage, ISS stage, ALB, LDH, Cr, Ca, β2-MG, the proportion of bone marrow monoclonal plasma cells, or ASCT status (P > 0.05).
Association between PLR and Clinical outcomes
To explore the role of the PLR as a predictor of survival in MM patients, we utilized the R package “survminer” (version 0.4.9) to analyze the two covariates of OS and PFS. The optimal cutoff points for the PLR were determined to be 76.25 (Fig. 1A) and 85.19 (Fig. 1B), respectively. K-M survival curves were generated to compare the prognosis between the low-PLR and high-PLR groups using the log-rank test. The results indicated that patients in the low PLR subgroup exhibited worse OS (P = 0.00031, Fig. 2A) and PFS (P < 0.0001, Fig. 2B).
Subgroup analyses were conducted in the lower risk group (ISS-I + ISS-II) and higher risk group (ISS-III) among MM patients. In the lower-risk MM group, a low PLR was not associated with worse OS (P = 0.099, Fig. 2C), but it was significantly associated with worse PFS (P = 0.0012, Fig. 2D). Conversely, in the high-risk MM group, a low PLR was associated with both worse OS (P = 0.0037, Fig. 2E) and PFS (P = 0.0048, Fig. 2F).
Univariate and multivariate analysis for OS in MM patients
We conducted an exhaustive analysis of PLR utilizing the Cox proportional hazards regression model. This sophisticated model not only assessed the influence of PLR on OS among patients diagnosed with MM but also incorporated survival status as a pivotal evaluation criterion. The comprehensive results of this analysis are presented in Table 2. Univariate Cox regression analysis unveiled that a PLR ≤ 76.25 exerted a statistically significant effect on survival (p = 0.0005), with the risk for this subgroup being 2.47 times greater than that observed in the PLR > 76.25 subgroup. The model demonstrated a satisfactory overall fit, evidenced by a concordance index of 0.609 and a standard error of 0.033. Furthermore, the model successfully underwent rigorous statistical validation through the likelihood ratio test, Wald test, and score (log-rank) test, with all p values falling below 0.001.
To enhance the normality of our dataset, we applied a natural logarithm transformation to the PLR values, yielding a novel variable termed PLR_ln. By taking the natural logarithm of the PLR values, the distribution becomes more approximately normal, which helps to stabilize variance and meet the assumptions for parametric tests. Subsequent analysis of PLR_ln revealed a hazard ratio of 0.52, suggesting that for each unit increment in PLR_ln, the associated risk decreased to approximately 52% of its original magnitude. This finding was statistically significant, with a p value of 0.001. Notably, the model’s fit remained robust, as indicated by a concordance index of 0.605 and a standard error of 0.04.
Subsequently, we embarked on a multivariate analysis, incorporating a total of 14 prognostic covariates. These included DS stage, ISS stage, Sex, Hb, Plt, ALB, LDH, RDW_SD, Age, Cr, Ca, β2-MG, the proportion of bone marrow monoclonal plasma cells and ASCT status. To facilitate visualization of these complex relationships, we utilized multivariate Cox regression forest plots (Fig. 3). Our results indicated that patients within the PLR ≤ 76.25 group exhibited a 2.92-fold increased risk of mortality compared to those in the PLR > 76.25 group (p = 0.005). Additionally, in the multivariate analysis, each one-unit increase in PLR_ln was associated with a reduction in risk to approximately 55% of its baseline value (p = 0.029). Intriguingly, patients with an ALB concentration ≥ 35 g/L were found to have a 2.36-fold higher risk of mortality compared to the reference group (p = 0.017).
Restricted cubic splines
We generated RCS curves to fully demonstrate that there was a significant nonlinear relationship between the initial PLR and OS in MM patients. To optimize the normality of the data, we performed a natural logarithm transformation on the PLR values, obtaining a new variable, PLR_ln. We adjusted for the effects of DS stage, ISS stage, Sex, Hb, PLT, ALB, LDH, RDW_SD, Age, Cr, Ca, β2-MG, the proportion of bone marrow monoclonal plasma cells, and ASCT status (Fig. 4). Through detailed data analysis, we obtained convincing statistical results: the P-overall value of 0.006 indicates the overall significance of the model, while the P-nonlinear value of 0.039 specifically supports the presence of a nonlinear relationship between the initial PLR and OS.
Kaplan‒Meier survival curves of PLR patients. Patients in the low PLR subgroup had worse OS (A) and PFS (B) than those in the high PLR subgroup. Low PLR was not associated with worse OS (C) but worse PFS (D) in the lower-risk MM groups (including ISS-I and ISS-II). A low PLR was associated with worse OS (E) and PFS (F) in the high-risk MM group (ISS-III).
Dose‒response relationship between the PLR and OS in MM patients from the RCS analysis. The odds ratios (the red line) and 95% confidence intervals (the area between the red dashed lines) were based on the RCS for the natural log-transformed PLR (PLR-ln) with 3 knots. The DS stage, ISS stage, sex, Hb, Plt, ALB, LDH, RDW_SD, age, Cr, Ca, β2-MG, proportion of bone marrow monoclonal plasma cells and transplantation status were adjusted in the model.
Discussion
MM is a malignant plasma cell disease characterized by diverse clinical manifestations and insidious onset, which poses significant challenges for early diagnosis and effective treatment. Recent advancements in understanding the tumor microenvironment and immune responses have highlighted the critical role of inflammation in cancer biology. Inflammatory markers are integral to the immune response to tumors and significantly influence the prognosis of cancer patients14,15,16,17,18,19. Key inflammatory markers, such as platelet count, neutrophil count, lymphocyte count, and C-reactive protein, have been shown to impact cancer pathophysiology. Specifically, platelets contribute to tumor progression by promoting cancer cell proliferation, survival, and metastasis20,21,22. Research has indicated that platelets enhance MM progression via IL-1β upregulation23. Another study showed that the half-life of platelets was significantly shortened in MM patients24. A retrospective study verified that lymphocytopenia at diagnosis has an unfavorable influence on the prognosis of Myelodysplastic Syndromes patients, as lymphocyte counts could reflect the immune status of the host25. Both platelets and lymphocytes play roles in the body’s immune inflammatory response.
Some studies have reported that a high PLR is associated with poor prognosis in various solid tumors18,26,27,28, which may be related to indicators of systemic inflammation, platelet-mediated tumor progression, and impaired immune function. However, in contrast, several studies have shown that a low platelet count or a low PLR is a poor prognostic factor in myeloma patients29,30. Our study also found that a low PLR is associated with poor prognosis in MM patients. This difference in MM may be due to the pathology of the disease. Hematological malignancies exhibit distinct pathogenesis compared to solid tumors, being influenced by both genetic mutations within hematopoietic cells and alterations in the bone marrow niches that regulate hematopoiesis and immune cell production31. In MM, there exist complex immune system regulations that modulate bone marrow hematopoiesis and the occurrence and progression of the tumor32. Specifically, the accumulation of malignant plasma cells in the bone marrow inhibits normal thrombopoiesis in MM patients.
Our retrospective study of 122 newly diagnosed MM patients identified the baseline PLR as a significant prognostic factor. By determining the optimal cutoff values for PLR, patients were stratified into high and low PLR groups, revealing significant differences in OS and PFS. K-M curves demonstrated lower survival rates in the low PLR group. Further analysis showed that a low PLR was associated with worse OS and PFS in high-risk MM patients (ISS-III). Multivariate analysis confirmed PLR as an independent prognostic indicator. After logarithmic transformation of PLR into (PLR_ln), every unit increase in PLR_ln was associated with a 55% risk reduction. RCS curves supported a significant nonlinear relationship between initial PLR and OS.
Previous studies have shown clear disagreement and controversy when exploring the relationship between MM and the PLR33,34. Li et al. showed that PLR was not an independent prognostic factor for OS and PFS in MM patients. However, in our study, we found that a low PLR was associated with poor prognosis in MM patients. We employed a unique cutoff value and calculation method for PLR, which may have contributed to our finding that lower PLR is associated with poor prognosis in MM patients. Furthermore, the RCS curves further confirmed the existence of a significant nonlinear relationship between PLR and OS in MM patients. This discrepancy may be related to the characteristics of the study populations, research methods, and the potential biological mechanisms of PLR’s role in different MM subtypes.
Low PLR at diagnosis is linked to poor OS in MM patients, possibly due to multiple factors. First, MM tumor cells express Toll-like receptors (TLRs), which induce IL-6 production and provide proliferation signals for MM cells35,36. Studies have shown that IL-6 levels are negatively correlated with lymphocyte and platelet counts37. Additionally, the pathogenesis of MM is associated with a chronic inflammatory response38. Prolonged inflammatory response can lead to significant depletion or destruction of platelets39,40, thereby indirectly reducing PLR. Concurrently, chronic inflammation may cause immune suppression, compromising normal immune surveillance41. As the PLR serves as an indicator of inflammatory and immune status, a low PLR could signify an immunosuppressed state. In MM, the proliferation of abnormal plasma cells in the bone marrow may impair the production of normal immune cells, leading to decreased immune cell counts42. This decline in immunity may accelerate disease progression and shorten survival duration.
Conclusion
The association between a low PLR at initial diagnosis and poor prognosis in MM patients offers new perspectives for clinical evaluation and treatment planning. However, it should be noted that this study was retrospective, single-center, and involved a relatively small sample size. Consequently, further research is necessary to explore in depth the biological mechanisms underlying the association between low PLR and poor prognosis in MM and to undertake large-scale, multicenter clinical studies to confirm its predictive value. We eagerly anticipate continued efforts in this area, which will ultimately lead to more precise and personalized treatment strategies for MM patients, thereby improving their treatment outcomes and quality of life.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kumar, S. K. Management of multiple myeloma. J. Natl. Compr. Canc Netw. 16 (5S), 624–627 (2018).
van de Donk, N., Pawlyn, C. & Yong, K. L. Multiple myeloma. Lancet 397 (10272), 410–427 (2021).
Kumar, S. K. et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28 (5), 1122–1128 (2014).
Oostvogels, R., Jak, M., Raymakers, R., Mous, R. & Minnema, M. C. Efficacy of retreatment with immunomodulatory drugs and proteasome inhibitors following daratumumab monotherapy in relapsed and refractory multiple myeloma patients. Br. J. Haematol. 183 (1), 60–67 (2018).
Morè, S. et al. Autologous stem cell transplantation in multiple myeloma: where are we and where do we want to go. Cells 11 (4), 606 (2022).
Kegyes, D. et al. Proteasome inhibition in combination with immunotherapies: state-of-the-art in multiple myeloma. Blood Rev. 61, 101100 (2023).
Shah, J. J. et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 126 (20), 2284–2290 (2015).
Hagiwara, M., Panjabi, S., Sharma, A. & Delea, T. E. Healthcare utilization and costs among relapsed or refractory multiple myeloma patients on carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone. J. Med. Econ. 22 (8), 818–829 (2019).
Palumbo, A. et al. Revised International Staging System for multiple myeloma: a Report from International Myeloma Working Group. J. Clin. Oncol. 33 (26), 2863–2869 (2015).
Dolan, R. D., Laird, B., Horgan, P. G. & McMillan, D. C. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit. Rev. Oncol. Hematol. 132, 130–137 (2018).
Szudy-Szczyrek, A. et al. Prognostic value of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in multiple myeloma patients treated with thalidomide-based regimen. Ann. Hematol. 99 (12), 2881–2891 (2020).
Zhang, X. et al. Are the derived indexes of Peripheral whole blood cell counts (NLR, PLR, LMR/MLR) clinically significant prognostic biomarkers in multiple myeloma? A systematic review and Meta-analysis. Front. Oncol. 11, 766672 (2021).
Podar, K., Chauhan, D. & Anderson, K. C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23 (1), 10–24 (2009).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 358 (6367), eaal5081 (2017).
Zhang, H. & Lyden, D. Bone voyage-osteoblasts remotely control tumors. Science 358 (6367), 1127–1128 (2017).
Chae, Y. K., Oh, M. S. & Giles, F. J. Molecular biomarkers of primary and Acquired Resistance to T-Cell-mediated immunotherapy in Cancer: Landscape, Clinical implications, and future directions. Oncologist 23 (4), 410–421 (2018).
Jian, Y. et al. Current advance of Immune Evasion mechanisms and emerging immunotherapies in Renal Cell Carcinoma. Front. Immunol. 12, 639636 (2021).
Yang, S. et al. Molecular mechanisms and cellular functions of liquid-liquid phase separation during antiviral immune responses. Front. Immunol. 14, 1162211 (2023).
Liu, J., Zhang, B., Zhang, G. & Shang, D. Reprogramming of regulatory T cells in inflammatory tumor microenvironment: can it become immunotherapy turning point. Front. Immunol. 15, 1345838 (2024).
Cho, M. S. et al. Platelets increase the proliferation of ovarian cancer cells. Blood 120 (24), 4869–4872 (2012).
Cho, M. S. et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood 130 (10), 1235–1242 (2017).
Wu, B. et al. Megakaryocytes Mediate Hyperglycemia-Induced Tumor Metastasis. Cancer Res. 81 (21), 5506–5522 (2021).
Takagi, S. et al. Platelets enhance multiple myeloma progression via IL-1β Upregulation. Clin. Cancer Res. 24 (10), 2430–2439 (2018).
Fritz, E., Ludwig, H., Scheithauer, W. & Sinzinger, H. Shortened platelet half-life in multiple myeloma. Blood 68 (2), 514–520 (1986).
Saeed, L. et al. Prognostic relevance of Lymphocytopenia, Monocytopenia and lymphocyte-to-monocyte ratio in primary myelodysplastic syndromes: a single center experience in 889 patients. Blood Cancer J. 7 (3), e550 (2017).
Cuello-López, J., Fidalgo-Zapata, A., López-Agudelo, L. & Vásquez-Trespalacios, E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One. 13 (11), e0207224 (2018).
Guan, Y. et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis. 23 (2), 220–231 (2020).
Wang, H. et al. Prognostic value of the platelet-to-lymphocyte ratio in lung cancer patients receiving immunotherapy: a systematic review and meta-analysis. PLoS One. 17 (5), e0268288 (2022).
Shi, L. et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 8 (12), 18792–18801 (2017).
Solmaz, S. et al. Is the platelet-to-lymphocyte ratio a new prognostic marker in multiple myeloma. J. Lab. Physicians. 10 (4), 363–369 (2018).
Méndez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer. 20 (5), 285–298 (2020).
Sharma, N. S. & Choudhary, B. Good cop, bad cop: profiling the Immune Landscape in multiple myeloma. Biomolecules 13 (11), 1629 (2023).
Wongrakpanich, S. et al. The Prognostic significance of Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in patients with multiple myeloma. J. Clin. Lab. Anal. 30 (6), 1208–1213 (2016).
Li, Y. et al. Pretreatment neutrophil/lymphocyte ratio but not platelet/lymphocyte ratio has a prognostic impact in multiple myeloma. J. Clin. Lab. Anal. 31 (5), e22107 (2017).
Mantovani, A. & Garlanda, C. Inflammation and multiple myeloma: the toll connection. Leukemia 20 (6), 937–938 (2006).
Lust, J. A. et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am. J. Hematol. 91 (6), 571–574 (2016).
Sayah, W. et al. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine 141, 155428 (2021).
Zhaoyun, L. & Rong, F. Predictive role of Immune Profiling for Survival of multiple myeloma patients. Front. Immunol. 12, 663748 (2021).
Ho-Tin-Noé, B., Boulaftali, Y. & Camerer, E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood 131 (3), 277–288 (2018).
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E. & Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23 (8), 495–510 (2023).
Brands, X. et al. Immune suppression is associated with enhanced systemic inflammatory, endothelial and procoagulant responses in critically ill patients. PLoS One. 17 (7), e0271637 (2022).
Soekojo, C. Y. & Chng, W. J. The evolution of immune dysfunction in multiple myeloma. Eur. J. Haematol. 109 (5), 415–424 (2022).
Acknowledgements
We would like to acknowledge the participants and investigators of the study.
Funding
This work was funded by the Huai ‘an Municipal Health Commission [grant # HAP202302].
Author information
Authors and Affiliations
Contributions
ZQE and WYF: Conceptualization, Methodology, Formal Analysis, Writing - Original Draft, Writing - Review & Editing. SWT and CY: Formal Analysis, Validation, Investigation, Data curation. HZM: Methodology, Formal Analysis, Investigation. YL and WCL: Supervision, Project Management, Writing - Review Editing. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was approved by the Institutional Review Committee of Huai’an No.1 People’s Hospital and was conducted following the Helsinki Declaration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Wang, Y., Shi, W. et al. The prognostic value of the platelet-to-lymphocyte ratio in multiple myeloma patients treated with a bortezomib-based regimen. Sci Rep 15, 1819 (2025). https://doi.org/10.1038/s41598-024-84343-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84343-x