Abstract
Understanding how the traits of lineages are related to diversification is key for elucidating the origin of variation in species richness. Here, we test whether traits are related to species richness among lineages of trees from all major biogeographical settings of the lowland wet tropics. We explore whether variation in mortality rate, breeding system and maximum diameter are related to species richness, either directly or via associations with range size, among 463 genera that contain wet tropical forest trees. For Amazonian genera, we also explore whether traits are related to species richness via variation among genera in mean species-level range size. Lineages with higher mortality rates—faster life-history strategies—have larger ranges in all biogeographic settings and have higher mean species-level range sizes in Amazonia. These lineages also have smaller maximum diameters and, in the Americas, contain dioecious species. In turn, lineages with greater overall range size have higher species richness. Our results show that fast life-history strategies influence species richness in all biogeographic settings because lineages with these ecological strategies have greater range sizes. These links suggest that dispersal has been a key process in the evolution of the tropical forest flora.
Similar content being viewed by others
Introduction
Species richness varies across the branches of the tree of life depending both on the traits and biogeographical setting of each lineage1. Traits influence the propensity of a lineage to disperse, adapt and diverge from ancestral populations, as well as survive extinction events1,2,3. In contrast, the biogeographical setting of a lineage relates to the ___location and history of the landscape where the lineage is found, and determines its exposure to processes that influence speciation and extinction, such as mountain building, tectonic movements and environmental change over geological timescales4,5. Understanding the interplay of these extrinsic factors and traits is important for developing a comprehensive understanding of the trajectory of evolution6. This debate is particularly important in the context of the tree flora of the wet tropics, as up to 53,000 of the global total of ca. 73,000 tree species occur in old-growth, closed-canopy wet tropical forests7,8,9.
There are three broad biogeographical settings across wet tropical forests: the Americas, characterised by the largest area of contiguous forest, rapid mountain uplift and shifting drainage patterns during recent geological history10; Africa, characterised by forests that have experienced substantial fluctuations in area during glacial and interglacial cycles11; and SE Asia, characterised by island formation driven by fluctuations in sea level12. These settings are considered to have promoted high speciation rates, in the case of the Americas and SE Asia, and high extinction rates, in the case of Africa. Importantly, these settings are typically assumed to be more important than the traits of any lineage for determining patterns of diversity4,5,13,14. For example, the high species richness of Hirtella compared to other genera of Chrysobalanaceae has been argued to be a result of the colonisation of the Americas by this lineage15: the setting, rather than any specific traits associated with this genus, is thought to have led to high rates of diversification in this group. However, the traits that a lineage possesses can also affect diversification rates. For example, faster demographic rates are linked to high species richness among a range of Amazonian tree lineages16. Here, we therefore test how traits are associated with species richness among genera of the tree flora across the three major biogeographical settings of the lowland wet tropics.
Traits influence diversification by affecting the wide range of processes that underlie speciation and extinction17,18. For example, traits affect the degree to which lineages form isolated populations, which is commonly an initial step towards speciation18,19,20. Traits also influence subsequent stages of speciation, associated with genetic divergence, the emergence of ecological and reproductive isolation, the ability of new species to persist and the interactions among these processes18,19,20,21. Traits may also affect extinction rates by conferring resistance to a specific environmental change that causes populations to decline17 or by influencing the ability of a lineage to disperse and migrate22. A common feature of many of these mechanisms of speciation and extinction is that they are spatial processes. As a result, lineage range size is positively correlated with species richness among plant lineages23,24 because larger ranges increase speciation rates by peripatric and parapatric processes (i.e. allopatric speciation involving geographic isolation25 and selection over environmental gradients26), and decrease extinction rates17. It is therefore important to understand if traits promote higher species richness of lineages because they are associated with large range sizes, or because traits may influence other processes linked to high speciation rates and low extinction rates, such as the rates of ecological differentiation and genetic divergence18.
In tropical wet forests, previous work on understanding variation in species richness among lineages has either focussed on individual groups15, considered a single region16 or excluded traits27. Here, we therefore compare the relationships among traits and species richness of 463 genera across wet forests in the Americas, Africa and SE Asia. We explore the role of three key traits, (1) stem-level mortality rate of genera, as a proxy of generation time, which we expect to correlate positively with species richness, as a ‘live fast, die young’ strategy may allow faster rates of adaptation to novel environmental conditions16; (2) maximum tree size, as a proxy of dispersal ability, which we expect to be negatively correlated with species richness, as limited dispersal may enhance reproductive isolation28; and (3) dioecy, which we expect to be negatively associated with species richness, as it may reduce the ability of isolated populations to establish29. We estimated these traits using demographic and structural data from a pan-tropical network of permanent forest plots30,31, range size estimates based on botanical records32, and information on breeding systems from floras (SI Table S5). We used generalised least squares (GLS) to explore the associations among traits, range size and species richness, and piecewise structural equation models (pSEM)33 to assess whether traits influence species richness directly, or indirectly via variation in range size.
We performed an additional analysis for genera from the Americas, which also incorporated variation in genus population size34, dispersal mode35 and mean species range size within genera36, based on published datasets that are only available for this continent. We expected genus population size to be positively correlated with species richness, given sampling considerations, and dispersal by wind to be associated with lower species richness, as higher dispersal distances may preclude effective reproductive isolation. Finally, we predicted that smaller species-level range sizes would be associated with greater species richness within a genus, as small ranges and high diversification rates are a feature of lineages in endemic hotspots associated with for example, mountain building and island formation, in the tropics37.
In all analyses, we accounted for the phylogenetic relationships among genera in these analyses using a pantropical, DNA-based, genus-level phylogeny, developed from a published genus-level phylogeny for American trees38,39.
Results
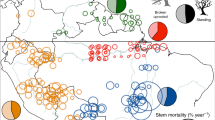
Variation in mortality rates was positively correlated with lineage range size using GLS analysis and in every biogeographic setting using pSEM (Figs. 1 and 2; Table S1). This relationship was also significant for a subset of genera that solely comprise lowland, tropical wet forest trees (Fig. S1) and, among Amazonian genera, mortality rates were positively correlated with mean species range size (Fig. 3). These relationships were driven by lineages from across the phylogeny that share high mortality rates and large range sizes (Fig. 4; e.g. Americas, Inga, mortality rate 2.8% a−1, range size 15.5 million km2; Africa, Uapaca, 1.6% a−1, range size 13.6 million km2; Asia, Elaeocarpus, 2.5% a−1, range size 7.2 million km2). The only major clades that do not contain genera with these linked characteristics are the Dialioideae and Detarioideae subfamilies of legumes (Fig. 4). Overall, these results indicate that fast demography is associated with greater range sizes across the phylogeny and biogeographical settings of the wet tropical tree flora (Fig. 2).
Structural equation models from pSEM analysis showing the relationships between traits, range size and species richness for 463 genera of tropical trees, that occur in the Americas, Africa, Asia or on multiple continents. Standardised effect sizes shown for significant relationships; arrow width is proportional to the standardised effect size. Non-significant relationships are shown with grey dotted lines.
Relationship between (a) range size and species richness, (b) mortality rate and range size and (c) maximum diameter and mortality rate for 463 genera of tropical trees. Genera are grouped by their distribution in American, African or Asian tropical forests or presence in multiple continents. Regression lines show GLS relationships from pSEM models shown in this figure; all relationships are significant and account for the phylogenetic relationships among lineages. Note that y-axes are scaled differently to optimise display of the relationships within each biogeographic setting.
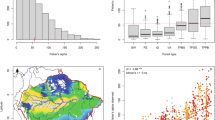
Structural equation models and key univariate relationships from pSEM analysis between traits, mean species range size and species richness for 105 genera of Amazonian trees. Standardised effect sizes are shown for significant relationships and arrow width is proportional to the standardised effect size, apart from for significant quadratic relationship between mean species range size where arrow width where this is not possible to calculate. Boxes that are contiguous have significant correlated errors. Non-significant relationships are omitted for clarity. Univariate relationships between species richness and (A) mean species range size (AOO), and (B) genus population size, (C) mortality rate and mean species range size, (D) maximum diameter and (E) seed mass.
Phylogenetic relationships among species richness, high mortality rates and large range size for 463 genera of tropical trees. Tip circle size is proportional to the species richness of each genus. Coloured bars indicate genera with high mortality rates and/or large range sizes. High mortality rates are classified as > 2% a−1; large range size classified as > 10 M km2 (America), > 8 M km2 (Africa), > 5 M km2 (Asia) and > 20 M km2 (multiple continents). Bar colour indicates the biogeographic setting of each genus. Named genera are those that share both high mortality rates and large range sizes; their distributed pattern illustrates that the association between large range size and high mortality rates is found across the phylogeny and is not restricted to certain clades. The blue segment on the phylogeny highlights the legumes, including the Dialioideae and Detarioideae subfamiles which are the only major clades on the phylogeny that do not demonstrate this association.
In turn, lineage range size was strongly associated with variation in species richness: genera with large range sizes have greater species richness (Figs. 1 and 2, Table S1). The link between large range size and high species richness was found in all biogeographical settings and across the phylogeny (e.g. Americas, Inga (15.5 million km2; 281 species), Protium (18.8 million km2; 152 species); Africa, Uapaca (13.6 million km2; 25 species), Cola (12.9 million km2, 134 species); Asia, Aglaia (6.6 million km2; 119 species), Elaeocarpus (7.2 million km2, 488 species). In contrast to these linear relationships between genus range size and species richness, mean species-level range size within genera had a unimodal relationship with species richness in Amazonia: richness peaked at intermediate values and declined in lineages with the greatest mean species-level range size (Fig. 3A). This quadratic relationship between mean species range size and species richness in Amazonia was independent of a significant positive correlation between population size and species richness (Table S2, Fig. 3B).
Variation in a range of other traits were linked to variation in mortality rates among genera (Fig. 1). There was a consistent negative relationship in all biogeographic settings between mortality rate and maximum size: across the phylogeny, genera with smaller maximum size have high mortality rates (Fig. 2 and Fig. S2). For Amazonian trees, low seed mass was also associated with high mortality rates (Fig. 3). Finally, the presence of dioecy was also associated with high mortality rates for genera that occur on multiple continents and those in Amazonian forests (Figs. S3 and S4). Overall, these relationships indicate that there are linked suites of traits related to stature, dispersal and breeding system that are all associated with fast life-history strategies, larger range sizes of lineages and ultimately, greater species richness (Fig. 1).
In some biogeographical settings, there were direct relationships between traits and species richness that were independent of variation in range size (Fig. 1). For example, species richness is higher in genera with low stature from African forests and those on multiple continents (Fig. S3), but there is no direct association between stature and species richness in Asian forests (Fig. 1 and Fig. S3).
Discussion
This study demonstrates that fast life-history strategies are associated with greater range sizes of lineages of tropical trees in all biogeographical settings, as well as larger mean species-level range sizes amongst Amazonian trees (Figs. 2 and 3). Fast life-history strategies are associated with high dispersal rates, such as low seed mass (Fig. 3), and greater dispersal ability is known to be linked with greater range sizes across a wide variety of groups22. However, links between life-history strategies and range size are poorly documented among plants, particularly within the tropics22. Fast life-history strategies are associated with large range sizes amongst temperate and boreal tree species22,40 and for tropical trees, there is an association between smaller range sizes and slow life-history strategies among 35 species in Costa Rica41, as well as between smaller range sizes and greater fruit mass for palms42. However, our study is the first to identify a link between fast life-history strategies and large range sizes across the entire tropical tree flora.
In turn, this study shows that the link between demographic rates and species richness is mediated by lineage range size (Fig. 1). The link between demographic rates and species richness is consistent with findings for birds and mammals43, a study of North American trees and shrubs44, and previous findings for Amazonian trees, where higher mortality rates were associated with higher species richness among 51 genera16. However, the results here are novel in two respects. First, this study identifies that range size mediates the link between demographic rates and species richness (Fig. 1). Second, we show that the relationship is universal across all three major biogeographical settings of the tropics. Genera with higher mortality rates are associated with greater species richness whether they have evolved and colonised landscapes influenced by geological events that are characterised by high rates of speciation, such as mountain- or island-building in Amazonia or SE Asia, or high rates of extinction, such as the prolonged drier periods that have characterised the African tropics14; the relationship is independent of geological history.
One process that may underpin these relationships may be more rapid colonisation of new landscapes by genera with higher mortality rates, as lineages with fast life-history strategies reproduce more frequently and have traits such as low seed mass that may allow longer-distance seed dispersal45. For Amazonian forests, higher mortality rates are linked to low seed mass among genera (Fig. 3) and at a species-level, to low seed volume46. Faster rates of colonisation and achieving greater range sizes may promote higher speciation rates and lower extinction rates47. For example, larger range sizes are more likely to intersect with a geological event that divides a population, leading to speciation via vicariance, and small populations at the edges of large ranges are more likely to be isolated for sufficient time for peripatric speciation to occur25,26,28. Such processes have been invoked to explain patterns of species richness and diversity among islands in SE Asia48 and have been suggested as a reason why closely related species within genera do not tend to occur together in Amazonia28,49. However, the unimodal relationship between mean species-level range size and species-richness for genera of Amazonian trees (Fig. 3), suggests that high dispersal ability may not be the only process that underpins these patterns. This decline in the species richness of lineages with high mean species-level range sizes is consistent with the idea that speciation rates decline with very high dispersal rates among populations because it becomes harder for ecological and genetic isolation to emerge18.
A second process that therefore may also be important is that higher mortality rates and therefore shorter generation times may be linked with higher rates of genetic change50 that allow faster adaptation to novel environmental conditions. For example, higher rates of genetic change in genera with faster life-history strategies may have promoted the emergence of adaptations to novel environmental conditions, such as new soil types51, over geological timescales. This mechanism is also consistent with predictions from eco-evolutionary theory and experimental studies of insects that indicate that rapid evolution enhances range expansion into novel habitats52,53.
The link between small stature and high species-richness in African, but not Amazonian or Asian lineages, may reflect an association between maximum size and resistance to a specific environmental change that causes extinction: an ability to tolerate drought54,55. The impact of more extended drought periods during the geological history of forests in Africa5,56 may have elevated extinction rates within lineages of large-stature trees, leading to few species-rich, large-stature genera today. Although speculative, this argument suggests that the effect of some traits on species richness at least, may be contingent on the biogeographic setting of the lineage and may be linked to effects on rates of extinction, rather than speciation.
The finding that dioecy is positively associated with species richness in American forests, via associations with high mortality rates and larger range sizes (Fig. 1), contradicts the classic idea that dioecy should be an evolutionary ‘dead-end’29,57. However, these findings are consistent with a broad array of recent work showing that dioecy is common in tropical secondary forests58 and is linked with large geographical range sizes of genera59, species richness is higher in dioecious clades once variation in branch length between non-dioecious and dioecious clades is accounted for60 and genetic diversity is greater and adaptation rates are faster in dioecious compared to monoecious lineages of plants61. The lack of significant associations between dioecy, mortality rates and range size in African and Asian forests may reflect the lower levels of sampling in these regions; significant relationships among these variables are only found in the two groups with the greatest number of genera (Fig. 1). Overall, our results suggest that dioecy in the tropical tree flora, at least for the Americas, may be related to a suite of traits, such as short generation times and high dispersal ability that more than compensate for the difficulty of achieving successful reproduction when sexes are on separate individuals. More generally, our findings demonstrate the need for precise estimates of species’ demographic rates to understand the links between breeding systems and species richness in comparative analyses.
There are uncertainties associated with the estimates of range size, taxonomy and traits that we used in this study. First, range sizes have fluctuated over the evolutionary history of the extant diversity of tropical forests, and time-integrated estimates of range size are required to estimate the land area that has been available for each genus more precisely over geological time62. Second, the number of herbarium records that we used to assess the extant range sizes of the constituent species of genera varies greatly among and within regions. For example, there are more records for each genus for American than for African and Asian forests (Fig. S5), whilst for genera from Africa, most botanical sampling of wet forests is restricted to the Atlantic coast63. Range sizes of some Asian genera are also low, even after acknowledging the island distribution of many taxa (Fig. S6). We expect that estimates of range sizes may increase with more records and coverage, and particularly as African herbaria become digitised. Although these limitations make it difficult to compare the influence of a specific biogeographic setting on species richness today in these analyses, neither increased collections nor integrating changes in biome area over geological time are likely to alter the consistent positive direction of the relationships between range size and species richness, and between mortality rates and range size (Fig. 1).
A third source of uncertainty, are the genus concepts that are used to frame these analyses. Genera are readily identified even within the most diverse tropical flora and therefore provide confidence that we can unite disparate datasets on traits, ecology and phylogenetics64. However, we recognise that the process of re-circumscribing and/or confirming genera as monophyletic using DNA sequence-based phylogenies is an on-going process65,66. We also recognise the lack of equivalence amongst genera because, even using a monophyly criterion, taxonomists may be able to circumscribe one large, or several smaller genera67. However, the tendency either to lump or split genera, will not affect the parameter estimates of our GLS or pSEM analyses as the phylogenetic covariance error structure weights these analyses based on the shared branch length among the tips of the phylogeny. Finally, the ecological traits we use are only proxies for the ability of populations to disperse, adapt and diverge genetically. For example, the use of average mortality rates of trees ≥ 10 cm diameter as a proxy for generation times assumes that the onset of fruit production occurs when trees reach 10 cm diameter and then remains constant until tree death, and that passage time from seed germination to 10 cm diameter is correlated with life expectancies beyond 10 cm diameter16. Studies of juvenile growth and reproductive phenology of tropical trees suggests that these are reasonable simplifications for the tropical tree flora but clearly obscure variation among lineages. For example, the mean minimum diameter of reproduction of 12 tree species in moist forest in Panama was 14.8 cm, but individual species varied from 6.1 to 46.7 cm68. However, across lineages, average lifespan beyond 10 cm diameter correlates with estimates of total generation time because adult survival is such a large and variable component of tree lifespans16. Finally, we note that there are likely other traits that influence diversification that are not included here, such as those associated with pollination and reproductive structures and strategies69. Inclusion of such traits may increase the importance of traits that influence species richness directly, through altering the likelihood and length of transition times for genetic divergence21.
Overall, our results indicate the importance of ecological traits for understanding variation in range size and species richness among genera of tropical trees. Further studies are required to understand the degree of overlap of species ranges within genera, in terms of both geographical and environmental space, and to explore how the signature of founder effects in the genetic structure of species populations varies among lineages with different demographic traits70,71. Phylogenetic analyses of species traits, distributions and demography using comprehensive species-level phylogenies of lineages that vary across the full spectrum of life-history strategies are also required to tease apart the underpinning mechanisms that have driven diversification. Obtaining suitable species-level demographic data means that we need to overcome the challenge that most taxa of tropical trees are rare, and knowledge of the characteristics of this rare majority is crucial for understanding how the high biodiversity of tropical forests evolved. Addressing this challenge involves both making a continued commitment to supporting and expanding on-the-ground monitoring and working to ensure accurate and consistent identifications are used across disciplines64. For tropical trees, the now extensive data across multiple environmental gradients from long-term plot networks30,31 provide a platform for this research and could provide the precise and comparable estimates of demographic rates that are required.
Methods
Plot and genus selection
This study is based on a novel compilation of long-term inventory data from 655 plots located in old-growth and secondary forests from across the tropics (Table S3; Fig. S7). These data are derived from the RAINFOR, AfriTRON, T-FORCES and CTFS-ForestGeo networks and are curated either at ForestPlots.net30 or by CTFS-ForestGeo31. Plot size varied from 0.5 to 50 ha. We selected all individual stems ≥ 10 cm diameter that had two or more measurements where the status of the tree (alive or dead) was recorded at each census. We included genera represented by ≥ 100 individual trees, as this level of sampling is required to obtain robust estimates of the rate of tree mortality72 and maximum tree diameter73. The final dataset comprised 333,540 trees, which had been monitored on average for 14.5 years (maximum 55.8 years; Fig. S8), from 463 genera from 81 plant families that comprise 28,177 species (Fig. S9). The median abundance of the genera in the dataset was 491 stems. All the selected genera contain trees, including palms, that occur in tropical lowland wet forest, but the distribution of the genera may also extend to other biomes and include other life forms such as lianas and shrubs. Analyses were also conducted for a subset of 288 genera (comprising 13,364 species) that solely comprise trees in the tropical lowland wet forest biome, and a subset of 107 genera of Amazonian trees.
Mortality rate and maximum size
We used the plot data to estimate average mortality rates (m), calculated from re-measurements of long-term forest plots, as a proxy for variation in generation times16. Generation time is a key life-history trait that influences processes linked to speciation and extinction, such as higher rates of molecular evolution50. The calculation of mortality rates was based on a maximum likelihood approach based on the survivorship of all individual trees within a genus74. The annual probability of mortality for each tree, \(i,\) was estimated using a logistic transformation that incorporated the U-shaped impact of tree diameter on mortality:
where
These functions were used to calculate the log-likelihood of the dataset of the status of each trees as dead or alive at the end of the period of monitoring for each individual, using Eq. (8) in74. For each genus, the parameter estimates that minimised this function were identified using simulated annealing. Finally, the mortality rate for a median diameter tree of each genus was used as the estimate of genus-level mortality rates.
We assume that the broad extent of the plot network with sample plots widely distributed across the wet tropics (Fig. S7), covers the range of species and environmental conditions that are characteristic of each genus, and that the temporal sampling of the plots (Fig. S8) is sufficient to capture the distribution of the return time of events that cause mortality in wet tropical forests. The mean length of monitoring exceeds the typical return time of two to seven years for El Niño/La Niña events75 which are a major driver of climatic variation and inter-annual variation in tree mortality in this region76. Mean annual mortality rates among genera ranged from 0.1 to 10% a−1 and had a similar distribution among biogeographical regions (Fig. S6).
We used data on the maximum diameter of trees within a lineage calculated from the forest plot data as a measure of dispersal potential, as higher stature is associated with longer seed dispersal distances45. Maximum size data were calculated as the 95th percentile of the tree diameter across all individuals of each genus77,78. Maximum diameter varied from 12 to 200 cm among genera, with similar distributions among continents (Fig. S6).
Species richness, range size, breeding systems, dispersal syndrome and seed mass
We used the list of accepted, species-level names in the World Checklist of Vascular Plants79 to calculate the species richness of each genus. Species richness per genus varied from 1 to > 1000 species (Fig. S6).
We defined range size as the extent of occurrence (EOO; km2) for each genus80. EOOs were calculated using herbarium records for all species within a given genus downloaded from the Global Biodiversity Information Facility. In total, the calculations used 336,832 records; the mean number of records per genus was 727 (Fig. S5). A greater number of genera from African forests had fewer than 100 records, compared to genera from Asian and American forests (Fig. S5). Records were checked for typos and errors, and latitudinal and longitudinal range limits for each genus based on information in floras were used to remove non-native records (Table S4). EOOs were calculated using alpha hulls, by minimising the value of alpha to encompass 99% of all records, using the rangeBuilder package in R81 and clipping these distributions to current land area (Fig. S10).
The regional distribution of each genus was classified from the plot data, and categorised as: the Americas (including Amazonia, the Guiana Shield, Atlantic Forest and Central America; n = 151), Africa (including the forests of west Africa, the Congo basin and wet forests areas in eastern Africa; n = 115), Asia (including the Western Ghats in India, Malesia, NE Australia and Pacific islands; n = 78), or present on multiple continents (n = 119).
We used information on the presence of dioecy in a lineage to assess the role of breeding systems in constraining species richness. Lineages with a dioecious breeding system, where male and female flowers are on different trees, are classically considered to have lower opportunities for successful establishment of new populations29,82 and therefore lower species richness as they require successful dispersal of both male and female plants57. Breeding system was classified for each genus as ‘at least some dioecious species’ or ‘no dioecious species’, based on83 and floras (Table S5). The proportion of genera with at least some dioecious species (approximately 27%) was similar across all biogeographical settings (Fig. S6).
Finally, for taxa from Amazonia we also compiled data on seed mass, dispersal mode, mean species-level range size and population size. Seed mass was calculated for all species within each lineage, as this trait is associated with seed dispersal distances45. Seed mass for Amazonian taxa (n = 92) was calculated as average values for species within genera84; there was insufficient data available to analyse this trait for genera from Africa (n = 8) or Asia (n = 17). The dispersal mode of lineages was classified as wind or non-wind dispersed35. Mean species range size and total population size was calculated using estimates for all species within a lineage34,36.
Statistical analysis
The phylogenetic relationships among genera were estimated using a DNA-based, genus-level phylogeny for the tropics, developed from a published genus-level phylogeny for American trees38,39. Mortality rate, maximum size, range size, species richness all showed significant phylogenetic signal (mortality rate, Pagel’s λ = 0.56, p < 0.005; range size, λ = 0.24, p < 0.05; maximum size, λ = 0.57, p < 0.005; species richness λ = 0.32, p < 0.05), demonstrating the importance of accounting for shared evolutionary branch lengths in our statistical analysis. Links between the phylogeny and trait values were visualised using the ggtree package85.
We used species richness as the response variable in our analyses of diversification, rather than estimating diversification rates for each clade as a function of time. This approach is based on previous work for a subset of the lineages analysed here where a ‘density-dependent’ trajectory of diversification provided a closer fit to the observed distribution of clade ages and species richness data for 51 lineages of Amazonian trees, compared to a model where species accumulate at a constant rate16. This approach is also consistent with general findings that estimates of diversification rates based on assuming a constant rate of species accumulation perform poorly when predicting changes in species richness of lineages through the fossil record, and that variation in clade age can lead to misleading interpretations of differences in diversification rates86. Our approach assumes that the species richness of each lineage has reached a steady-state, and explores which traits determine variation in these steady-state values. We note that clade age is not related to species richness across our data: stem ages of each lineage estimated from the phylogeny vary extremely widely with species richness for these genera (Fig. S11) and therefore the influence of traits on species richness is not confounded by differences in clade age. Overall, our approach is a simplification of the likely wide range of trajectories of change in the number of species within each lineage over geological time. However, in the absence of a detailed fossil record or complete species-level phylogenies for each lineage, this approach represents the only means to gain a macroecological perspective on the traits that influence species richness.
Generalised least squares (GLS) models with phylogenetically correlated errors were used to identify how biogeographical distributions and traits were related to variation in range size across lineages as:
where R is range size, \(\mu\) is mortality rate, D is the biogeographic setting of each genus, S is maximum size, B is a categorical variable denoting either the presence or absence of dioecious species within a genus, and \(\varepsilon\) is distributed as \({\sigma }_{\epsilon }^{2}\mathbf{C}\) where \(\mathbf{C}\) is the variance–covariance of the error term based on the shared branch lengths of the phylogeny.
Variation in species richness was analysed similarly as:
GLS analysis can only test for direct, independent effects of each predictor variable on the response variables. We therefore also used piece-wise structural equation modelling (pSEM) to explore the network of both direct and indirect relationships among these variables33,87.
We hypothesised that the species richness of genera would be influenced by the range size of a lineage, following studies that demonstrate this link for animals and plants23,24 and expectations from allopatric models of speciation that assume that range expansion precedes genetic divergence21. We allowed traits to either influence range size and/or species richness directly, as traits may influence rates of range expansion and contraction and/or other processes that underpin speciation, such as the transition time to genetic divergence following the establishment of a lineage21. Initial pSEM analysis indicated that there were also significant relationships among maximum size and mortality rates within these data, consistent with findings that demographic and structural traits are linked among lineages of tropical trees88,89. We therefore also allowed variation in maximum size and breeding system to be associated with variation in mortality rates in the final pSEM models. For each biogeographical setting, structural equation models based on these networks including only the statistically significant relationships showed a good fit to the data, indicating that significant relationships among the variables were not being excluded (Fig. 1; Americas, Fisher’s C = 6.5, ns; Africa: Fisher’s C = 4.9, ns; Asia, Fisher’s C = 5.8, ns; Multiple continents, Fisher’s C = 5.9, ns; core dataset, all biogeographical settings; pSEM, C = 14.7, ns).
For the analyses focussing on Amazonia, we extended these analyses to include total population size, mean species range size, seed mass and dispersal mode, using a similar statistical approach. For Amazonian taxa, we used GLS to test how traits were related to variation in mean species range size and how traits, mean species range size and total population size were associated with species richness. We included a quadratic term to model the relationship between mean species range size and species richness, based on preliminary exploration of the relationships within the data. Similarly, we also constructed pSEMs to understand the network of direct and indirect relationships among these variables. We explored whether traits determined variation in mean species range size, and how mean species range size is related to species richness. We included total population size as an additional predictor of species richness; correlated errors were permitted between mean species range size and population size. The structural equation model based on this network including only the statistically significant relationships showed a good fit to the data (Fisher’s C = 15.8, ns).
Data availability
All metadata used in the analyses is available as supplementary information and as a Forestplots.net data package from https://doi.org/10.5521/forestplots.net/2025_1
References
Donoghue, M. J. & Sanderson, M. J. Confluence, synnovation, and depauperons in plant diversification. New Phytol. 207, 260–274 (2015).
De Queiroz, A. Contingent predictability in evolution: key traits and diversification. Syst. Biol. 51, 917–929 (2002).
Rabosky, D. L. & Huang, H. A robust semi-parametric test for detecting trait-dependent diversification. Syst. Biol. 65, 181–193 (2015).
Raven, P. H. et al. The distribution of biodiversity richness in the tropics. Science Advances 6, eabc6228 (2020).
Gentry, A. H. Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny?. Ann. Mo. Bot. Gard. 69, 557–593 (1982).
Vamosi, J. C. & Vamosi, S. M. Factors influencing diversification in angiosperms: At the crossroads of intrinsic and extrinsic traits. Am. J. Bot. 98, 460–471 (2011).
Slik, J. F. et al. An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. 112, 7472–7477 (2015).
Beech, E., Rivers, M., Oldfield, S. & Smith, P. GlobalTreeSearch: The first complete global database of tree species and country distributions. J. Sustain. For. 36, 454–489 (2017).
Cazzolla Gatti, R. et al. The number of tree species on Earth. Proc. Natl. Acad. Sci. 119, e2115329119. https://doi.org/10.1073/pnas.2115329119 (2022).
Hoorn, C. et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 (2010).
Maley, J. The African rain forest - main characteristicsof changes in vegetation and climate fomr the Upper Cretaceous to the Quaternary. Proc. R. Soc. Edinburgh 104, 31–74 (1996).
Morley, R. J. Origin and evolution of tropical rain forests. (Wiley, 2000).
Bardon, L. et al. Unraveling the biogeographical history of Chrysobalanaceae from plastid genomes. Am. J. Bot. 103, 1089–1102 (2016).
Hagen, O., Skeels, A., Onstein, R. E., Jetz, W. & Pellissier, L. Earth history events shaped the evolution of uneven biodiversity across tropical moist forests. Proc. Natl. Acad. Sci. 118, e2026347118. https://doi.org/10.1073/pnas.2026347118 (2021).
Bardon, L. et al. Origin and evolution of Chrysobalanaceae: Insights into the evolution of plants in the Neotropics. Bot. J. Linnean Soc. 171, 19–37 (2013).
Baker, T. R. et al. Fast demographic traits promote high diversification rates of Amazonian trees. Ecol. Lett. 17, 527–536. https://doi.org/10.1111/ele.12252 (2014).
Chichorro, F., Juslén, A. & Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 237, 220–229 (2019).
Harvey, M. G., Singhal, S. & Rabosky, D. L. Beyond reproductive isolation: Demographic controls on the speciation process. Annu. Rev. Ecol. Evol. Syst. 50, 75–95 (2019).
Mayr, E. Animal species and evolution. (Harvard University Press, 1963).
Allmon, W. D. & Sampson, S. D. in Species and Speciation in the fossil record 121–167 (2016).
Coyne, H. A. & Orr, J. A. Speciation. Sunderland, MA: Sinauer Associates 545 pages (2004).
Alzate, A. & Onstein, R. E. Understanding the relationship between dispersal and range size. Ecol. Lett. 25, 2303–2323 (2022).
Vamosi, J. C. & Vamosi, S. M. Key innovations within a geographical context in flowering plants: Towards resolving Darwin’s abominable mystery. Ecol. Lett. 13, 1270–1279 (2010).
Hernández-Hernández, T. & Wiens, J. J. Why are there so many flowering plants? A multiscale analysis of plant diversification. Am. Nat. 195, 948–963 (2020).
Couvreur, T. L., Chatrou, L. W., Sosef, M. S. & Richardson, J. E. Molecular phylogenetics reveal multiple tertiary vicariance origins of the African rain forest trees. BMC Biol. 6, 1–10 (2008).
Fine, P. V. A., Daly, D. C., Munoz, G. V., Mesones, I. & Cameron, K. M. The contribution of edaphic heterogeneity to the evolution and diversity of Burseraceae trees in the western Amazon. Evolution 59, 1464–1478 (2005).
Antonelli, A. et al. An engine for global plant diversity: highest evolutionary turnover and emigration in the American tropics. Front. Genet. 6, 130 (2015).
Dexter, K. G. et al. Dispersal assembly of rain forest tree communities across the Amazon basin. Proc. Natl. Acad. Sci. 114, 2645–2650 (2017).
Baker, H. G. Support for Baker’s law - as a rule. Evolution 21, 853–856 (1967).
ForestPlots.net. Taking the pulse of Earth’s tropical forests using networks of highly distributed plots. Biol. Conserv. 108849 (2021). https://doi.org/10.1016/j.biocon.2020.108849
Davies, S. J. et al. ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol. Conserv. 253, 108907 (2021).
GBIF.org. (03 November 2021).
Lefcheck, J. S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016).
Ter Steege, H. et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 1, e1500936 (2015).
Correa, D. F. et al. Geographic patterns of tree dispersal modes in Amazonia and their ecological correlates. Global Ecol. Biogeogr. 32, 49–69 (2023).
Gomes, V. H., Vieira, I. C., Salomão, R. P. & ter Steege, H. Amazonian tree species threatened by deforestation and climate change. Nat. Clim. Change 9, 547–553 (2019).
Fjeldså, J. & Lovett, J. Geographical patterns of old and young species in African forest biota: The significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 6, 325–346 (1997).
Coelho de Souza, F. et al. Evolutionary diversity is associated with wood productivity in Amazonian forests. Nat. Ecol. Evol. 3, 1754–1761. https://doi.org/10.1038/s41559-019-1007-y (2019).
Segovia, R. A. et al. Freezing and water availability structure the evolutionary diversity of trees across the Americas. Sci. Adv. 6, eaaz5373 (2020).
Morin, X. & Chuine, I. Niche breadth, competitive strength and range size of tree species: A trade-off based framework to understand species distribution. Ecol. Lett. 9, 185–195 (2006).
Chacón-Madrigal, E., Wanek, W., Hietz, P. & Dullinger, S. Traits indicating a conservative resource strategy are weakly related to narrow range size in a group of neotropical trees. Perspect. Plant Ecol. Evol. Syst. 32, 30–37 (2018).
Alzate, A. et al. The evolutionary age-range size relationship is modulated by insularity and dispersal in plants and animals. bioRxiv, 2023.2011.2011.566377. https://doi.org/10.1101/2023.11.11.566377 (2023).
Marzluff, J. M. & Dial, K. P. Life history correlates of taxonomic diversity. Ecology 72, 428–439 (1991).
Verdu, M. Age at maturity and diversification in woody angiosperms. Evolution 56, 1352–1361 (2002).
Muller-Landau, H. C., Wright, S. J., Calderón, O., Condit, R. & Hubbell, S. P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 96, 653–667 (2008).
Poorter, L. et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89, 1908–1920 (2008).
Rosenzweig, M. L. Species diversity in space and time. (Cambridge University Press, 1995).
Janssens, S. B. et al. Evolutionary dynamics and biogeography of Musaceae reveal a correlation between the diversification of the banana family and the geological and climatic history of Southeast Asia. New Phytol. 210, 1453–1465 (2016).
Pennington, R. T., Lavin, M. & Oliveira-Filho, A. Woody plant diversity, evolution, and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Evol. Syst. 40, 437–457 (2009).
Smith, S. A. & Donoghue, M. J. Rates of molecular evolution are linked to life history in flowering plants. Science 322, 86–89 (2008).
Fine, P. V. A., Mesones, I. & Coley, P. D. Herbivores promote habitat specialization by trees in Amazonian forests. Science 305, 663–665 (2004).
Szűcs, M. et al. Rapid adaptive evolution in novel environments acts as an architect of population range expansion. Proc. Natl. Acad. Sci. 114, 13501–13506 (2017).
Williams, J. L., Hufbauer, R. A. & Miller, T. E. How evolution modifies the variability of range expansion. Trends Ecol. Evol. 34, 903–913 (2019).
Rowland, L. et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528, 119–122. https://doi.org/10.1038/nature15539 (2015).
Corlett, R. T. & Primack, R. B. Tropical Rain Forests: an Ecological and Biogeographical Comparison. (Wiley-Blackwell, 2011).
Corlett, R. T. & Primack, R. B. Tropical rainforests and the need for cross-continental comparisons. Trends Ecol. Evol. 21, 104–110 (2006).
Heilbuth, J. C. Lower species richness in dioecious clades. Am. Nat. 156, 221–241 (2000).
Réjou-Méchain, M. & Cheptou, P. O. High incidence of dioecy in young successional tropical forests. J. Ecol. 103, 725–732 (2015).
Sabath, N. et al. Dioecy does not consistently accelerate or slow lineage diversification across multiple genera of angiosperms. New Phytol. 209, 1290–1300 (2016).
Käfer, J. et al. Dioecy is associated with higher diversification rates in flowering plants. J. Evol. Biol. 27, 1478–1490 (2014).
Muyle, A. et al. Dioecy is associated with high genetic diversity and adaptation rates in the plant genus Silene. Mol. Biol. Evol. 38, 805–818 (2021).
Fine, P. V. & Ree, R. H. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 168, 796–804 (2006).
Sosef, M. S. et al. Exploring the floristic diversity of tropical Africa. BMC Biol. 15, 1–23 (2017).
Baker, T. R. et al. Maximising synergy among tropical plant systematists, ecologists, and evolutionary biologists. Trends Ecol. Evol. 32, 258–267. https://doi.org/10.1016/j.tree.2017.01.007 (2017).
Ashton, P. & Heckenhauer, J. Tribe Shoreae (Dipterocarpaceae subfamily Dipterocarpoideae) Finally Dissected. Kew Bull. 77, 885–903 (2022).
Cvetković, T. et al. Phylogenomics and a revised tribal classification of subfamily Dipterocarpoideae (Dipterocarpaceae). Taxon 71, 85–102 (2022).
De Queiroz, K. & Gauthier, J. Toward a phylogenetic system of biological nomenclature. Trends Ecol. Evol. 9, 27–31 (1994).
Wright, S. J. et al. Reproductive size thresholds in tropical trees: Variation among individuals, species and forests. J. Trop. Ecol. 21, 307–315 (2005).
Xue, B. et al. Accelerated diversification correlated with functional traits shapes extant diversity of the early divergent angiosperm family Annonaceae. Mol. Phylogenet. Evol. 142, 106659 (2020).
Honorio Coronado, E. N., Dexter, K. G., Hart, M. L., Phillips, O. L. & Pennington, R. T. Comparative phylogeography of five widespread tree species: Insights into the history of western Amazonia. Ecol. Evol. 9, 7333-7345 (2019).
Lowe, A. J. et al. Standardized genetic diversity-life history correlates for improved genetic resource management of Neotropical trees. Diversity Distrib. 24, 730–741 (2018).
Rüger, N., Huth, A., Hubbell, S. P. & Condit, R. Determinants of mortality across a tropical lowland rainforest community. Oikos 120, 1047–1056 (2011).
Poorter, L., Hawthorne, W., Bongers, F. & Sheil, D. Maximum size distributions in tropical forest communities: Relationships with rainfall and disturbance. J. Ecol. 96, 495–504 (2008).
Lines, E. R., Coomes, D. A. & Purves, D. W. Influences of forest structure, climate and species composition on tree mortality across the eastern US. PLoS One 5, e13212 (2010).
Malhi, Y. & Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos. Trans. R. Soc. Lond. (Series B) 359, 311–329 (2004).
Bennett, A. C. et al. Sensitivity of South American tropical forests to an extreme climate anomaly. Nat. Clim. Change 1–8 (2023).
Fauset, S. et al. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 6, 6857 (2015).
Coelho de Souza, F. et al. Evolutionary heritage influences Amazon tree ecology. Proc. Biol. Sci. 283, 20161587. https://doi.org/10.1098/rspb.2016.1587 (2016).
Govaerts, R. (Royal Botanic Gardens, Kew, 2023).
Gaston, K. J. & Fuller, R. A. The sizes of species’ geographic ranges. J. Appl. Ecol. 46, 1–9 (2009).
Rabosky, A. R. D. et al. Coral snakes predict the evolution of mimicry across New World snakes. Nat. Commun. 7, 11484 (2016).
Baker, H. G. Self-compatibility and establishment after “long-distance” dispersal. Evolution 9, 347–349 (1955).
Renner, S. S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588–1596 (2014).
Seed Information Database (SID) Version 7.1. (Royal Botanic Gardens Kew, http://data.kew.org/sid/, 2008).
Yu, G., Smith, D. K., Zhu, H., Guan, Y. & Lam, T. T. Y. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 8, 28–36 (2017).
Rabosky, D. L. & Benson, R. B. Ecological and biogeographic drivers of biodiversity cannot be resolved using clade age-richness data. Nat. Commun. 12, 1–10 (2021).
Shipley, B. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 94, 560–564 (2013).
Aleixo, I. et al. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Change 9, 384–388 (2019).
Esquivel-Muelbert, A. et al. Tree mode of death and mortality risk factors across Amazon forests. Nat. Commun. 11, 5515 (2020).
Acknowledgements
TRB acknowledges funding from a Leverhulme Research Fellowship (‘History or ecology? Untangling the drivers of diversification in the tropics’; RF-2015-653) which supported the conceptualisation, analysis and writing of this manuscript. This paper is a product of the RAINFOR, AfriTRON, T-FORCES, PPBio and CTFS ForestGeo networks. These networks have been supported by multiple funders and grants, including the European Research Council (ERC Advanced Grant 291585 – ‘T-FORCES’), the Gordon and Betty Moore Foundation (#1656 ‘RAINFOR’ and #5349 ‘Monitoring Protected Areas in Peru to Increase Forest Resilience to Climate Change’), the David and Lucile Packard Foundation, the European Union’s Seventh Framework Programme (283080 – ‘GEOCARBON’, 282664 – ‘AMAZALERT’), the Natural Environment Research Council (NERC grants NE/D005590/1 – ‘TROBIT’, NE/F005806/1 – ‘AMAZONICA’, NE/I028122/1 - ‘Niche Evolution', NE/T012722/1 – ‘SECO’, ‘PPFOR’ E/M0022021/1), the NERC/ State of São Paulo Research Foundation (FAPESP) consortium grants ‘BIO-RED’ (NE/N012542/1, 2012/51872-5) and ‘ECOFOR’ (NE/K016431/1, 2012/51509-8), the Royal Society, the Centre for International Forestry (CIFOR), Gabon’s National Parks Agency (ANPN), CNPq, Brazil (305972/2020-0 and 441561/2016-0), FAPESP (2017/50239-0; 2016/50481-3), the Coordenação Programa de Aperfeiçoamento Pesquisa em Biodiversidade (PPBio), the Centro de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 “Estudos Integrados da Biodiversidade Amazônica (CENBAM)” and the French Programme Investissement d’Avenir (CEBA, ref. ANR-10-LABX-25-01; TULIP, ref. ANR-10-LABX-0041). We also thank the National Council for Science and Technology Development of Brazil (CNPq) for support to the Cerrado/Amazonia Transition Long-Term Ecology Project (PELD/403725/2012-7), the PPBio Phytogeography of Amazonia/Cerrado Transition project (CNPq/PPBio/457602/2012-0) and a Productivity Grant. Funding for plots in the Udzungwa Mountains (Tanzania) was obtained from the Leverhulme Trust under the Valuing the Arc project. The BCI forest dynamics research project was founded by S.P. Hubbell and R.B. Foster and is now managed by R. Condit, S. Lao, and R. Perez under the Center for Tropical Forest Science and the Smithsonian Tropical Research in Panama. Numerous organizations have provided funding, principally the U.S. National Science Foundation, and hundreds of field workers have contributed. We acknowledge our use of the large-scale forest plot at Pasoh Forest Reserve which is an ongoing project of the Malaysian Government, directed by the Forest Research Institute Malaysia. Plot data were also included from the Tropical Ecology Assessment and Monitoring (TEAM) Network, a collaboration between Conservation International, the Missouri Botanical Garden, the Smithsonian Institution and the Wildlife Conservation Society, and partly funded by these institutions, the Gordon and Betty Moore Foundation, and other donors. We acknowledge individual financial support from long-term research development project No. RVO 67985939 of the Czech Academy of Sciences (RH), NERC Independent Research Fellowship NE/S01537X/1 (TJ), a Royal Society University Research Fellowship, ERC Advanced Grant and a Phillip Leverhulme Prize (SLL), a UK an ERC Advanced Grant, a Royal Society Wolfson Research Merit Award, and a Royal Society Global Challenges Award (‘FORAMA’, ICA/R1/180100) (OLP), Grant ANID FONDECYT/Iniciacion 11200967 and Grant ANID PIA/BASAL FB210006 (RS), and grant INTER-TRANSFER LTT19018 from the Ministry of Education, Youth and Sports of the Czech Republic (MS). Data from RAINFOR, AfriTRON and T-FORCES are stored and curated by ForestPlots.net, a cyber-infrastructure initiative developed at the University of Leeds that unites permanent plot records and their contributing scientists from the world’s tropical forests. The development, management and implementation of ForestPlots.net has been funded by grants to OLP (principally from NERC NE/B503384/1, NE/N012542/1 BIO-RED, ERC AdG 291585 T-FORCES’, and Gordon and Betty Moore Foundation #1656, ‘RAINFOR’), EG (‘GEOCARBON’, and NE/F005806/1 ‘AMAZONICA’), TRB (Gordon and Betty Moore Foundation #5349, ‘Monitoring Protected Areas in Peru to Increase Forest Resilience to Climate Change’, NERC NE/I028122/1 ‘Niche Evolution’; Institutional Links project 414694929 under the UK/Peru Newton-Paulet Fund partnership funded by the UK Department for Business and Energy and Industrial Strategy and CONCYTEC and delivered by the British Council; NERC Impact Accelerator award), SLL (Royal Society University Research Fellowship; NERC New Investigators Award; Phillip Leverhulme Prize; NERC Large Grant NE/R016860/1, ‘CongoPeat’) and DG (NERC NE/N004655/1, ‘TREMOR’). We thank E. Manson, D. Coenen, and C. Pertusi for help mining genetic sequences from GenBank, J. Lloyd for plot data and G. Hidalgo Pizango for assisting in assembling the information about genus-level traits. We acknowledge the pioneering work of T. Lovejoy to establish long-term forest monitoring of as part of the Biological Dynamics of Forest Fragments project; this study is contribution number XXX to the Technical Series (TS) of the BDFFP (INPA – STRI).
Author information
Authors and Affiliations
Contributions
T.R.B. conceived and designed the study, performed the analyses and wrote the paper. K.G.D., R.S., T.P., F.C., W.M., D.F.R.P.B., S.A.I., S.Q., O.L.P, R.P. and D.S. contributed to discussions that developed the analyses. R.S., K.G.D. and T.P. contributed phylogenetic data; S.A.I. and S.Q. contributed trait data; all other co-authors contributed plot data. All other authors (S.A.B., K.A.B., S.A., P.A., M.A., E.A., E.A. de O., E.A.D., C.A., A.A., L.A., A.A.-M., E.A., L.A., P.A., S.A.A.I., G.A.A.C., M.B., W.B., M.B., L.F.B., O.B., C.B., J.B., J.-F.B., H.B., S.B., N.N.B., N.B., W.B., V.B., L.B., P.B., D.B., F.B., F.Q.B., R.B,F.B, M.BtN.,B.B, D.F.R.P.B., P.C., J.C. ,W.C. ,C.C. ,V.C.M., C.C. ,J.C., E.C. ,M.C. ,J.C., D.C., F.C.V., F.C., A.C., L.dC. ,D.C.D., M.D., A.D., G.D., S.D., C.dD., T.dH., J.d.A.P., G.D. , K.D., T.d.F.,M.. N.D.K., J.L.D., V.D., G.E., T.E., J.E., B.Y.E., F.E., C.E., S.F., T.R.F., M.F., G.F.L., E.G.F., G.F., D.G., M.G., E.G., C.G., R.G.V., J.H., K.H., A.H., O.H., T.H., R.H., R.H., N.H., C.M.H., E.N.H.C., I.H.C., W.H.H., W.H., M.I., S.A.I., K.J., E.J., T.J., E.K., L.K., T.K., K.K., W.L., S.L., M.L., S.L., S.L., J.L., G.L.G., J.L., R.L., W.E.M., J.R.M., Y.M., B.M., B.M.J. ,A.M., C.M., F.M., C.M., I.M.P., F.M., V.M., A.M.M., S.M., P.M., J.M., P.M., L.N., P.N., D.N., A.N.L., P.N.V., L.O., W.P., N.P.C., A.P.G., J.P., K.S.H.P., A.P., C.P., T.P., M.C.P.M., P.P., O.L.P., J.P., N.P., A.D.P., A.T.P., A.P., R.P., L.Q., S.Q., C.Q., F.R.A., H.R.A., J.R., M.R., M.A.R., F.R., E.R., K.A.S., R.S., I.S., M.S.S., J.S., R.A.S ,J.S., R.S., D.S., N.S., J.S.E., M.S., M.S., J.S., B.S., J.S., R.S.,T.S,M.S., M.S, H.T., J.T., S.T., J.T., D.T., H.t.S., J.T., A.T.L., J.T.M. D.T., P.v.d.M., G.v.d.H., P.v.d.H., M.v.N., B.v.U., R.V.M., R.V., B.V., S.V., I.C.G.V., E.V.T., J.V., L.W., S.W., M.W., J.W., T.L.Y., I.Y., R.Z., L.Z. commented on the manuscript. Management of the plot networks that are used in this study is led by O.L.P. (RAINFOR, T-FORCES), S.L.L. (AfriTRON, T-FORCES), S.D. (CTFS-ForestGeo) and F.C. and W.M. (PPBio). E.N.H.C., B.M., T.R.B., C.E., S.L.L., L.Q. and O.L.P contribute to managing the ForestPlots.net repository as members of the ForestPlots.net steering committee.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baker, T.R., Adu-Bredu, S., Affum-Baffoe, K. et al. Large range sizes link fast life histories with high species richness across wet tropical tree floras. Sci Rep 15, 4695 (2025). https://doi.org/10.1038/s41598-024-84367-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84367-3