Abstract
Diabetic wounds are notoriously difficult to heal due to impaired cell repair mechanisms, reduced angiogenesis, and a heightened risk of infection. Fibroblasts play a vital role in wound healing by producing extracellular matrix (ECM) components and various growth factors, but their function is inhibited in diabetic wounds. Chitooligosaccharides (COS), intermediate products of chitosan degradation, have shown efficacy in promoting tissue repair, yet their role in diabetic wound healing remains underexplored. In a mouse model of diabetic wounds, COS treatment demonstrated substantial bioactivity in accelerating wound healing by enhancing fibroblast proliferation and migration. Additionally, COS increased collagen III deposition and angiogenesis at the wound sites. The COS also mitigated inflammatory responses by controlling leukocyte infiltration and bacterial infection. Mechanistically, COS regulated fibroblast activity via the PI3K/Akt signaling pathway, providing a novel bioactive material for chronic wound healing.
Similar content being viewed by others
Introduction
Diabetes mellitus is a chronic metabolic disease that can lead to severe complications. Elevated blood glucose levels, a hallmark of this condition, affect multiple organ systems and significantly increase health risks1,2. One notable symptom experienced by diabetic individuals is delayed wound healing3. During diabetic wound healing, the inflammation phase is prolonged due to compromised immune function4. Diabetic wounds exhibit aberrant cellular events, such as impaired fibroblast proliferation and migration, reduced recruitment of endothelial progenitor cells, and damage and apoptosis of nerve cells5,6,7,8. Consequently, proinflammatory cytokines, matrix metalloproteinases (MMPs) like MMP-9, and reactive oxygen species (ROS) are overproduced9,10,11, while growth factors essential for wound healing, such as VEGF (vascular endothelial growth factor), TGF-β1 (transforming growth factor-β1), and EGF (epidermal growth factor), are diminished9,10. This unfavorable microenvironment disrupts angiogenesis, ECM remodeling, and re-epithelialization, all essential for tissue repair12,13,14. Moreover, diabetic wounds are more susceptible to bacterial infections than those in non-diabetic participants15. Therefore, reactivating tissue repair cells and improving the regenerative milieu are effective strategies for diabetic wound treatment.
Fibroblasts play a critical role in wound healing by producing collagen and other ECM components during the proliferation phase16. Following injury, fibroblasts proliferate and migrate to the wound site, where they synthesize new connective tissue and facilitate wound closure17,18. Type III collagen and fibronectin, the primary ECM components produced by fibroblasts in the dermal wound area, are essential for early wound healing18. Additionally, the differentiation of fibroblasts into α-SMA-positive myofibroblasts is essential for wound contractility and granulation tissue formation19. However, in diabetic wounds, fibroblast dysfunction, inadequate ECM production, dysregulated fibroblast differentiation to myofibroblasts, and interrupted myofibroblast activity contribute to the pathological process20. Various approaches targeting fibroblasts to improve diabetic wound healing, such as photobiomodulation 21, pulsed electromagnetic field (PEMF) treatment22, or insulin like growth factor (IGF)-123, have shown potential to accelerate wound repair but reveal uncertain efficacies in immunomodulation of diabetic wounds.
Chitosan, a natural polysaccharide, has been successfully used to repair tissue defects, including nerves24, cartilage25, bone26, blood vessels27, corneal28, cardiac tissue29, and skin30. With properties such as biocompatibility, biodegradability, and antimicrobial activity, chitosan is also developed for drug, gene, and vaccine delivery applications31. Chitooligosaccharides (COS), the intermediate products of chitosan degradation, exhibit robust bioactivity as potential growth promoters in nerves32, bones33, and vessels34. Additionally, COS demonstrates significant anti-infection and anti-inflammatory effects, making them highly suitable for tissue repair35,36. Given chitosan’s efficacy in skin wound recovery37, it is hypothesized that COS may enhance diabetic wound healing by facilitating fibroblast activities. In this study, previously determined concentrations of COS were used38 to examine their effects on diabetic wound healing in mice. The findings revealed the promotive function of COS-mediated fibroblasts on wound repair, offering a novel avenue for the clinical treatment of diabetic wounds.
Materials and methods
Preparation of chitooligosaccharides
The synthesis of chitosan oligosaccharides (COS) was conducted following previously established methodologies32,39,40. In summary, chitosan was added into a 15% hydrogen peroxide (H2O2) solution, and the resultant mixture was subjected to microwave irradiation for a duration of 4 min at a power setting of 700 W. The resultant crude COS was subsequently isolated through a filtration process and redissolved in a minimal volume of distilled water. Following this, purification of the crude COS was achieved utilizing a Sephadex-25 column (size of column: 1.6 cm × 85 cm bed volume). The final COS powder was produced via lyophilization of the eluted COS solution under vacuum conditions. An endgroup analysis was performed to ascertain the average molecular weight (MW) of the COS. The purified COS exhibited an average degree of polymerization of approximately 7, corresponding to a molecular weight of around 900 Da, and the degree of deacetylation was found to be approximately 91.8%32.
Establishment of mice diabetic wound model
The animal studies received approval from the Animal Experiment Ethics Committee of Nantong University. Healthy male C57BL/6 mice, aged 8–10 weeks and weighing approximately 26 ± 2 g, were supplied by the Laboratory Animal Center of Nantong University. The mice were adaptively fed for 7 days and given free access to water and food at a room temperature of 24.0 ± 2.0 ℃. Diabetic models were established by intraperitoneal injection of 1% streptozotocin (STZ, pH 4.2–4.5 in citrate and sodium citrate buffer solution, 60 mg/kg) for five consecutive days following 16 h of fasting. Blood glucose levels were measured for three consecutive days to confirm the diabetes model, with successful induction defined by glucose levels exceeding 16.7 mmol/L.
The mice were anesthetized via intraperitoneal injection of 3% sodium pentobarbital (30 mg/kg). The fur around the surgical site on the back was shaved, and the skin was disinfected with iodophor. A perforator was used to create two symmetrical round wounds, each 8 mm in diameter, on the back of each mouse. The mice were then housed individually. Eighteen mice were randomly divided into two groups: one treated with 0.2 mg/ml COS and the other with sodium chloride as the control. The treatment concentration was selected based on previous studies38. The drug was administered once a week by subcutaneous injection, and samples were collected at 7, 14, and 21 days post-injury. All mice were euthanized by cervical dislocation to prevent suffering.
Assessment of wound size
To measure wound size, photographs of the wounds at different time points following COS treatment were taken using a scale ruler as a reference. Adobe Illustrator software outlined the wound boundaries, and the percentage of wound healing was calculated using the formula:
Tissue immunofluorescence
Tissues surrounding the back wounds were collected at various stages, fixed, and embedded in paraffin. Sections were cut to 5 μm thickness, deparaffinized, and rehydrated following standard protocols. They were then blocked with 0.01 M PBS containing 3% BSA, 0.1% Triton X-100, and 10% normal goat serum for 1 h at 37 ℃, and incubated overnight at 4 ℃ with primary antibodies: rabbit anti-collagen I (Servicebio), rabbit anti-collagen III (Servicebio), rabbit anti-α-SMA (Servicebio), rabbit anti-CD45 (Servicebio), or rabbit anti-CD31 (Servicebio). Subsequently, secondary antibody incubation was performed at room temperature for 2 h, followed by staining with Meyer’s hematoxylin and 3,3-diaminobenzidine (DAB; Sigma, St. Louis, Missouri, USA) for 1–2 min (Sorabio, Beijing, P.R.C.), and blocking with neutral gum (Invitrogen, San Diego, CA, USA). Positive signals were observed using a microscope (Leica DMR 3000, Leica Microsystems, Bensheim, Germany).
Cell culture and treatment
The fibroblast NIH/3T3 cell line, obtained from Shanghai Fuheng Biotechnology Co., Ltd., was cultured at 37 °C with 5% CO2 and 20% O2 in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with glucose at a concentration of 4500 mg/L, 10% calf serum (CS), 1% Penicillin/Streptomycin (P/S) and glucose at a concentration of 4500 mg/L. Fibroblasts were treated with 0–2 mg/ml COS for 24 or 48 h, with or without the addition of 10 mM LY294002 (a PI3K/AKT inhibitor).
Cell counting kit-8 assay
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay per the provided protocol. Fibroblasts were seeded in 96-well plates for 24 h (200 µL, 1 × 104 cells/well) before COS treatment for 24 h. Subsequently, 10 μL of CCK-8 was added to each well, and cells were incubated for 2 h. Absorbance at 450 nm was measured with a microplate reader (ThermoFisher, USA). The experiment was repeated three times.
Cell proliferation assay
Fibroblasts were cultured in 96-well plates. After 24 h, cells were treated with COS for 36 h, followed by the determination of cell proliferation using the Cell-Light™ EdU Apollo567 In Vitro Kit (Ribobio). According to the manufacturer’s protocol, EdU solution was added to the cell supernatant and incubated for 2 h. Subsequently, the cells were fixed and washed, and the nuclei were stained. Images were captured using a fluorescence microscope. All experiments were performed in triplicate.
Cell cycle analysis
Fibroblasts were seeded in 6-well plates (3 mL/well, 1 × 106 cells/mL) and treated with COS at concentrations of 0, 0.5 mg/ml, and 1 mg/ml. The cells were resuspended and collected in 50 µL PBS, fixed with ice-cold 75% alcohol, and stored overnight at -20℃. After removing the alcohol, cells were stained with a propidium iodide (PI) solution (1 mg/mL RNase A and 0.05 mg/mL PI; Sigma) for 30 min. The cell cycle was analyzed using a flow cytometer (Attune NxT, Invitrogen) and AttuneTM NxT software (v2.7.0).
Wound healing assay
To assess the effect of COS on fibroblast migration, fibroblasts (3 mL/well, 1 × 106 cells/mL) were seeded into 6-well plates and incubated at 37 °C with 5% CO2. Once a confluent monolayer was formed, a sterile pipette tip was used to scratch the cells. Exfoliated cells and debris were washed away with PBS. The scratch areas were observed and recorded at 0, 24, and 48 h under an optical microscope. ImageJ software was used to analyze COS-mediated cell migration using the formula below:
Transwell assay
Fibroblasts were suspended in DMEM and transferred to the top chambers of each transwell with 8 μm pores (Corning Incorporated). Each well received 300 μL of cell medium (4 × 104 cells/well), followed by the addition of 700 μL of medium containing 10% FBS and COS into the lower chamber to stimulate cell migration. The cells were allowed to migrate at 37 °C for 48 h, washed with PBS, and fixed with 4% paraformaldehyde for 30 min. Cells adhering to the bottom surface of each membrane were stained with 0.5% crystal violet for 20 min, imaged, and counted using a DMR inverted microscope (Leica Microsystems). Assays were conducted in triplicate, and images were analyzed and quantified using ImageJ software.
Tubule formation assay
One day before the experiment, the matrix gel was placed on ice in a refrigerator at 4 °C overnight to melt slowly. On the day of the experiment, the matrix gel was diluted with complete medium at a ratio of 1:1, mixed well, and 50 μL was applied per well to the bottom of a 96-well plate. The plate was placed in a cell culture incubator at 37 °C to solidify for 1 h. Trypsin (without EDTA) was used to digest HUVEC cells, which were then centrifuged, collected, and resuspended in a complete medium (with or without different concentrations of COS). The cell density was adjusted to approximately 3 × 105/mL. A 100 μL aliquot of the cell suspension was added to each well of the 96-well plate (approximately 3 × 104 cells per well). The cells were incubated at 37 °C in a cell culture incubator for 4 h. Tubule formation in the wells was observed under a microscope, and images were taken from three randomly selected fields of view. The images were analyzed and quantified using ImageJ software.
Western blot assay
Total protein was extracted from COS-treated cells using an extraction buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF. Samples were vortexed for 30 min, then centrifuged at 12,000 rpm for 15 min to collect the supernatants. Protein concentrations were determined using the BCA method. Each sample (20 μg) was heated at 95 °C for 5 min, separated on a 10% SDS-PAGE gel, and transferred to a PVDF membrane. Membranes were washed with TBST buffer (50 mM Tris–HCl, 100 mM NaCl, 0.1% Tween-20, pH 7.6), blocked with 5% skimmed dry milk for 2 h, and then incubated overnight at 4 °C with primary antibodies: mouse anti-PNCA antibody (Proteintech), rabbit anti-PI3K antibody (Proteintech), rabbit anti-P-PI3K antibody (Cell Signaling), rabbit anti-AKT antibody (Proteintech), or rabbit anti-P-AKT antibody (Cell Signaling). After washing with TBST, membranes were incubated with a secondary antibody for 2 h. Protein bands were visualized using an Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA), and band intensities were quantified. β-actin served as an internal control.
Disc diffusion assay
The antimicrobial activity of COS against Staphylococcus aureus (provided by the Clinical Center of the Affiliated Hospital of Nantong University) was evaluated using the Kirby-Bauer disk diffusion susceptibility test. A single colony of bacteria was cultured overnight in Mueller–Hinton broth (MHB) at 37 °C and 250 rpm. Bacterial suspension was spread onto Mueller–Hinton Agar (MHA) plates supplemented with COS. After incubating the plates at 37 °C for 24 h, the zones of inhibition (ZOI) around the discs were measured. The antibacterial rate was calculated using the formula:
Statistical analysis
The data were presented as mean ± SEM. Statistical analyses were conducted using GraphPad Prism version 8.4.3 for Windows (GraphPad Software LLC, La Jolla, CA, USA), employing unpaired t-tests and one-way ANOVA. A significance level of p < 0.05 was considered statistically significant.
Results
COS promotes diabetic wound healing of mice following injury
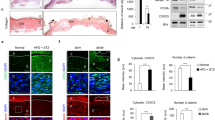
To examine the effects of COS on diabetic wound healing, mice were intraperitoneally injected with 1% STZ (60 mg/kg) for 5 days. One week later, blood samples were taken from the tail vein to measure blood glucose levels, confirming the success of the diabetic model (Table 1). Subsequently, two symmetrical round wounds, each 8 mm in diameter, were created on the back of each mouse using a perforator (Fig. 1A) and treated with 0.2 mg/ml COS once a week. Results indicated that COS significantly promoted wound healing from day 4 onwards compared to the control (Fig. 1B, C). These findings suggest that COS functions as a bioactive material in the repair of diabetic wounds.
Effects of COS on diabetic wound healing. (A) Establishment of diabetic wounds in mice by injecting 1% STZ for 5 days; (B) Effects of 0.2 mg/ml COS on wound healing observed over 2–20 days with weekly treatments; (C) Statistical analysis of (B). Scale bars, 1 mm in (B). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05).
COS potentiates diabetic wound healing by mediating fibroblasts, angiogenesis, and inhibiting inflammatory activation.
To determine whether COS regulates fibroblasts and macrophages during the wound healing process, H&E staining was performed to observe the accumulation of these cells at the lesion site at 7, 14, and 21 days post-wound formation. Tissue sections revealed a significant increase in activated fibroblast numbers and a marked decrease in leukocyte numbers in COS-treated wounds compared to the control (Fig. 2A–F). Additionally, Masson staining showed that COS promoted collagen deposition at the wound sites (Fig. 2G–L).
Skin histology following treatment of wounds with 0.2 mg/ml COS. (A–F) H&E staining of diabetic wound sections after 7, 14, and 21 days of treatment with 0.2 mg/ml COS; (G–L) Masson staining of diabetic wound sections treated with 0.2 mg/ml COS. The rectangle indicates the magnified region. Arrows indicate fibroblast cells, and arrowheads indicate inflammatory cells. Scale bars, 150 μm in (A–L).
To elucidate the roles of COS-mediated fibroblasts and inflammatory cells in diabetic wound healing, immunostaining was used to examine activated fibroblasts, leukocyte infiltration, and angiogenesis with specific markers. The intensity of α-SMA, a marker reflecting fibroblast activation during wound healing, significantly increased following COS treatment at 7, 14, and 21 days (Fig. 3A–C). Collagen I deposition was upregulated in the first two weeks, but in the third week, the change was minimal, and its positive rate was even lower than that of the control group (Figure S1). In contrast, collagen III deposition was markedly upregulated (Fig. 3J–L). Previous studies have shown that the control of cellular contractility during wound healing depends on the composition of the connective tissue matrix, with matrices rich in type III collagen being more contractile than those composed of type I collagen41. Moreover, leukocyte infiltration was attenuated by COS at all three time points, as revealed by CD45 positive signals (Fig. 3G–I). Notably, CD31-positive vascular endothelial cells accumulated at the injury site in greater numbers following COS treatment compared to the control (Fig. 3D–F). Additionally, a tubular formation assay demonstrated that the total length and number of tube formations increased in COS-treated HUVECs compared to the control (Figure S2). These data indicate that COS facilitates diabetic wound healing by mediating fibroblast activation, enhancing angiogenesis, and inhibiting leukocyte infiltration.
Immunostaining of wound healing-related cells and factors following COS treatment of diabetic wounds. (A–C) Immunostaining of α-SMA to detect activated fibroblasts after 7, 14, and 21 days of treatment with 0.2 mg/ml COS. (D–F) Immunostaining of CD31 to detect angiogenesis following COS treatment. (G–I) Immunostaining of CD45 to detect leukocyte infiltration after COS treatment. (J–L) Immunostaining of Collagen III to detect ECM deposition following COS treatment. Scale bars, 150 μm in (A–L).
COS promotes the proliferation of fibroblasts by regulating the cell cycle
To examine COS-mediated cell events in fibroblasts, a CCK-8 assay was initially employed to determine the effects of COS on cell viability. Results demonstrated that COS at a concentration of 1 mg/ml significantly enhanced fibroblast viability (Fig. 4A–C). An EdU assay further revealed that 1 mg/ml of COS significantly promoted cell proliferation (Fig. 4D–M). Flow cytometry analysis indicated that COS at 1 mg/ml facilitated cell cycle progression through the S phase (Fig. 5A–D). These data suggest that COS promotes fibroblast proliferation by regulating the S phase of the cell cycle.
Effects of COS on fibroblast cell proliferation. (A–C) CCK-8 assay detecting fibroblast viability following treatment with 0–2 mg/ml COS for 24, 48, and 72 h; (D–L) EdU assay detecting cell proliferation following treatment with 0–2 mg/ml COS for 36 h; (M) Statistical analysis of (D–L). Scale bars, 200 μm in (D–L). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05).
COS promotes the migration of fibroblasts
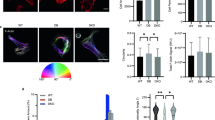
To test the effect of COS on fibroblast migration, a wound healing assay was conducted following treatment with 0, 0.5 mg/ml, 1.0 mg/ml, and 2.0 mg/ml of COS for 24 and 48 h, respectively. Results showed that COS at 1.0 mg/ml efficiently mediated cell migration (Fig. 6A–N). A Transwell assay further confirmed the promoting effects of COS on cell migration (Fig. 6O–R). These results indicate that COS can enhance fibroblast migration.
Effects of COS on fibroblast migration. (A–L) Wound healing assay of fibroblasts following treatment with 0–2 mg/ml COS for 0, 24, and 48 h; (M, N) Statistical analysis of (A–L); (O–Q) Transwell assay of fibroblast migration following treatment with 0–2 mg/ml COS for 0, 24, and 48 h; (R) Statistical analysis of (O–Q). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05). Scale bars, 200 μm in (A–L, O–Q).
COS activates the PI3K/Akt pathway of fibroblasts
To unveil the underlying mechanism of COS-mediated proliferation and migration of fibroblasts, KEGG analysis was performed using previous transcriptome sequencing data of COS-stimulated fibroblasts (Zhang et al. 2022). The analysis revealed significant enrichment of the PI3K/AKT pathway in COS-induced differentially expressed genes (DEGs) in fibroblasts (Figure S3). Western blot analysis showed that the protein levels of phosphorylated PI3K (p-PI3K) and AKT (p-AKT) were markedly increased following 24-h treatment of fibroblasts with 1.0 mg/ml COS (Fig. 7A–C). These data indicate that the PI3K/AKT pathway is regulated by COS in fibroblasts.
COS promotes PI3K/AKT pathway activation in fibroblasts. (A) Western blot analysis of phosphorylated PI3K and AKT in fibroblasts following treatment with 1 mg/ml COS for 24 h. Original blots are presented in Figure S4; (B, C) Quantification of (A). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05).
Interference of the COS-mediated PI3K/AKT pathway affects the proliferation and migration of fibroblasts
To elucidate the regulatory roles of the COS-mediated PI3K/AKT pathway on fibroblast proliferation and migration, cells were treated with the PI3K inhibitor LY294002 at a concentration of 10 mM in the presence of 1 mg/ml COS for 24–72 h. The addition of LY294002 significantly reduced cell viability at 24 h and inhibited fibroblast proliferation by disrupting the S phase of the cell cycle (Fig. 8A–Q). Western blot analysis of PCNA, a specific marker for cell proliferation, corroborated these effects (Fig. 8R–S). Both wound healing and transwell assays revealed that inhibiting the PI3K/AKT pathway markedly reduced cell migration following 1.0 mg/ml COS treatment for 24–72 h (Fig. 9A–O). These findings underscore that COS enhances fibroblast proliferation and migration through the PI3K/AKT pathway.
Effects of the PI3K inhibitor LY294002 on COS-mediated fibroblast proliferation. (A–C) CCK-8 assay of fibroblasts treated with 10 mM LY294002 in the presence of 1 mg/ml COS for 36 h; (D–L) EdU assay of untreated fibroblasts and fibroblasts treated with or without 10 mM LY294002 in the presence of 1 mg/ml COS for 36 h; (M) Statistical analysis of (D–L); (N–P) Cell cycle analysis of untreated fibroblasts and fibroblasts treated with or without 10 mM LY294002 in the presence of 1 mg/ml COS for 36 h; (Q) Statistical analysis of (N–P); (R) Western blot analysis of PCNA in fibroblasts treated with 10 mM LY294002 in the presence of 1 mg/ml COS for 36 h. Original blots are presented in Figure S5; (S) Statistical analysis of (R). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05). Scale bars, 200 μm in (D–L).
Effects of the PI3K inhibitor LY294002 on COS-mediated fibroblast migration. (A–I) Wound healing assay of untreated fibroblasts and fibroblasts treated with or without 10 mM LY294002 in the presence of 1 mg/ml COS for 0, 24, and 48 h; (J, K) Statistical analysis of (A–I); (L–N) Transwell assay of untreated fibroblasts and fibroblasts treated with or without 10 mM LY294002 in the presence of 1 mg/ml COS for 24 h; (O) Statistical analysis of (L–N). Data are expressed as mean ± SEM from three independent experiments (*p < 0.05). Scale bars, 200 μm in (A–I, L–N).
COS plays a crucial role in the anti-bacterial activity of Staphylococcus aureus
Given the role of Staphylococcus aureus as a primary pathogen in diabetic wound infections, the antibacterial properties of COS were evaluated against this bacterial strain. Staphylococcus aureus was inoculated on an agar plate, and paper discs soaked with 1.0 mg/ml COS were placed on the plate. The COS-mediated zone of inhibition (ZOI) was measured after 24 h, displaying an enlarged ZOI in the cultured Staphylococcus aureus following COS treatment (Fig. 10). These results indicate that COS possesses significant antibacterial activity, beneficial for chronic wound healing.
Discussion
Wound healing is a dynamic repair process encompassing three phases: inflammation, proliferation, and remodeling42,43. Delays in the inflammation or proliferation phases prolong healing, resulting in chronic wounds. During diabetic wound healing, growth factors from keratinocytes, fibroblasts, and smooth muscle cells are significantly reduced44. Concurrently, activated leukocytes overexpress proinflammatory cytokines and ROS under oxidative stress, inhibiting wound healing45,46. Reduced growth factors, increased lipid peroxidation products, and aberrant MMP activity hinder the proliferation and migration of reparative fibroblasts and keratinocytes47,48. This impairment significantly reduces ECM production and angiogenesis49, increasing susceptibility to bacterial infection and creating a vicious cycle42,43,50. This study demonstrated that COS can not only significantly enhance diabetic wound healing, but also promote fibroblast proliferation and migration and exhibiting antibacterial properties, suggesting a broad potential for COS in chronic wound repair.
Chitosan, a natural cationic polysaccharide composed of (1 → 4)-2-amino-2-deoxygenate-β-D-glucan51, is widely utilized in clinical practice for its wound healing and antibacterial properties52,53. However, its large molecular weight and water insolubility limit its optimal bioactivity. Conversely, COS, with its smaller molecular weight and water solubility, shows greater efficacy in tissue repair54. For instance, COS promotes angiogenesis55,56, ameliorates systemic inflammation and oxidative stress57, and protects nerve cells from damage58. Therefore, COS represents a more promising bioactive substance for tissue engineering, especially when combined with other biomaterials.
Collagen is the main component of reticular fibers in the skin and plays a pivotal role in wound contraction38. Angiogenesis is essential for successful wound healing59. COS has been shown to promote collagen deposition and angiogenesis in diabetic wounds. However, the mechanisms by which COS activates collagen production and angiogenesis during diabetic wound healing remain unclear. Fibroblasts are critical for supporting wound healing, particularly in collagen structure formation, especially type III collagen, and in promoting angiogenesis through VEGF60,61 and angiopoietin-160,62. This study demonstrated that COS promotes fibroblast proliferation and migration and activates fibroblasts, as indicated by the upregulation of α-SMA. Additionally, COS treatment resulted in increased collagen I deposition during the first two weeks, with minimal changes in the third week, where its positive rate was even lower than the control group. Conversely, collagen III deposition was consistently higher throughout the three weeks. Previous studies have shown that cellular contractility during wound healing depends on the connective tissue matrix composition, with type III collagen-rich matrices being more contractile than those with type I collagen41. This suggests that COS may reduce scar formation after wound healing, resulting in smoother newly formed skin.
The PI3K/AKT signaling pathway is integral to numerous pathophysiological processes, including cell cycle progression, protein synthesis, epigenetic regulation, angiogenesis, apoptosis, and autophagy. Class IA PI3Ks are heterodimers comprising a catalytic subunit (p110) and a regulatory subunit (p85). PI3K activation phosphorylates AKT serine/threonine kinases at Thr-308 and Ser-473, thereby activating downstream effectors63. In idiopathic pulmonary fibrosis (IPF), PI3K/AKT inhibitors mitigate disease progression64, and this pathway is also involved in neurodegenerative diseases65. Moreover, PI3K/AKT signaling modulates tumor cell metastasis and invasion in prostate cancer66, ovarian cancer67, and gastric cancer68, while also facilitating wound healing69,70. The present study demonstrated that the PI3K inhibitor LY294002 diminished COS-mediated diabetic wound healing by inhibiting fibroblast proliferation and migration, underscoring the pathway’s pivotal role in wound repair.
COS enhances fibroblast proliferation and migration through the PI3K/AKT signaling pathway and exhibits antibacterial properties, thus accelerating diabetic wound healing.
COS holds promise as a therapeutic agent for improving diabetic wound healing, necessitating the use of effective drug delivery systems (DDS) to enhance its efficacy. Traditional dosage forms include tablets, capsules, pills, ointments, creams, gels, syrups, and injections or oral liquids71,72,73,74. Advances in science and technology have introduced new DDS, such as nanocarriers, which offer targeted therapeutic effects in complex in vivo environments and protect drugs from excessive degradation. These systems achieve desired therapeutic responses at lower doses, reducing adverse drug reactions75,76. Examples include liposomes77, exosomes78, carbon nanotubes79, and nanoparticles or nanocapsules80. Future research should focus on employing advanced DDS to optimize the efficacy of COS, thereby providing a theoretical foundation for clinical applications.
Data availability
The datasets used or analysed during the current study available from the corresponding author on reasonable request.
References
Sharma, D. K., Pattnaik, G., and Behera, A. Recent developments in nanoparticles for the treatment of diabetes. J. Drug Target. p. 1–12 (2023).
Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 366(9498), 1736–1743 (2005).
Kharaziha, M., Baidya, A. & Annabi, N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater 33(39), e2100176 (2021).
Tehrany, P. M. et al. Multifunctional and theranostic hydrogels for wound healing acceleration: An emphasis on diabetic-related chronic wounds. Environ Res 238(Pt 1), 117087 (2023).
Xiao, Y. et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc Natl Acad Sci USA 113(40), E5792-e5801 (2016).
Shen, T. et al. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater 117, 192–203 (2020).
Zhang, L. et al. Effect of eIF2α in neuronal injury induced by high glucose and the protective mechanism of resveratrol. Mol Neurobiol 60(10), 6043–6059 (2023).
Medras, Z. J. H. et al. Arctigenin improves neuropathy via ameliorating apoptosis and modulating autophagy in streptozotocin-induced diabetic mice. CNS Neurosci Ther 29(10), 3068–3080 (2023).
Wong, S. L. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21(7), 815–819 (2015).
Mirza, R. E. et al. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 62(7), 2579–2587 (2013).
Elraiyah, T., et al. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 63(2 Suppl): p. 37S-45S (2016).
Cho, H. et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev 146, 267–288 (2019).
Kunkemoeller, B. & Kyriakides, T. R. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal 27(12), 823–838 (2017).
Matoori, S., Veves, A., and Mooney, D. J. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 13(585) (2021).
Wu, Y. K., Cheng, N. C. & Cheng, C. M. Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol 37(5), 505–517 (2019).
Hinz, B. The role of myofibroblasts in wound healing. Curr Res Transl Med 64(4), 171–177 (2016).
Abdullahi, A., Amini-Nik, S. & Jeschke, M. G. Animal models in burn research. Cell Mol Life Sci 71(17), 3241–3255 (2014).
Du, J. et al. Direct cellular reprogramming and transdifferentiation of fibroblasts on wound healing-Fantasy or reality?. Chronic Dis Transl Med 9(3), 191–199 (2023).
Hinz, B. & Lagares, D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 16(1), 11–31 (2020).
Wan, R. et al. Diabetic wound healing: The impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regen 29(4), 573–581 (2021).
Mokoena, D. R. et al. Photobiomodulation at 660 nm stimulates fibroblast differentiation. Lasers Surg Med 52(7), 671–681 (2020).
Choi, M. C. et al. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound. Arch Dermatol Res 308(1), 21–29 (2016).
Achar, R. A. et al. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir Bras 29(2), 125–131 (2014).
Jiao, H. et al. Chitosan/polyglycolic acid nerve grafts for axon regeneration from prolonged axotomized neurons to chronically denervated segments. Biomaterials 30(28), 5004–5018 (2009).
Muxika, A. et al. Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol 105(Pt 2), 1358–1368 (2017).
Prakash, J. et al. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int J Biol Macromol 154, 62–71 (2020).
Fiqrianti, I. A. et al. Poly-L-lactic Acid (PLLA)-chitosan-collagen electrospun tube for vascular graft application. J. Funct. Biomater., 2018. 9(2).
Xu, W. et al. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr Polym 192, 240–250 (2018).
Prabaharan, M. et al. Preparation and characterization of poly(L-lactic acid)-chitosan hybrid scaffolds with drug release capability. J Biomed Mater Res B Appl Biomater 81(2), 427–434 (2007).
Pezeshki-Modaress, M., Zandi, M. & Rajabi, S. Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: an in vitro study. Prog Biomater 7(3), 207–218 (2018).
Abourehab, M. A. S., et al. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 23(18) (2022).
Zhao, Y. et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 134, 64–77 (2017).
Rajabi, M. et al. 3D printing of chitooligosaccharide-polyethylene glycol diacrylate hydrogel inks for bone tissue regeneration. J Biomed Mater Res A 111(9), 1468–1481 (2023).
Ei, Z. Z. et al. Chitooligosaccharide prevents vascular endothelial cell apoptosis by attenuation of endoplasmic reticulum stress via suppression of oxidative stress through Nrf2-SOD1 up-regulation. Pharm Biol 60(1), 2155–2166 (2022).
Chotphruethipong, L., et al. Chitooligosaccharide from pacific white shrimp shell chitosan ameliorates inflammation and oxidative stress via NF-κB, Erk1/2, akt and Nrf2/HO-1 pathways in LPS-induced RAW264.7 macrophage cells. Foods, 2023. 12(14).
Ouyang, A. et al. Chitooligosaccharide boosts the immunity of immunosuppressed blunt snout bream against bacterial infections. Int J Biol Macromol 242(Pt 3), 124696 (2023).
Mirhaji, S. S. et al. Design, optimization and characterization of a novel antibacterial chitosan-based hydrogel dressing for promoting blood coagulation and full-thickness wound healing: A biochemical and biophysical study. Int J Biol Macromol 241, 124529 (2023).
Zhang, C. et al. Chitosan degradation products promote healing of burn wounds of rat skin. Front Bioeng Biotechnol 10, 1002437 (2022).
Wang, X. et al. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 128(Pt 8), 1897–1910 (2005).
Wang, Y. et al. Chitosan degradation products promote nerve regeneration by stimulating schwann cell proliferation via miR-27a/FOXO1 axis. Mol Neurobiol 53(1), 28–39 (2016).
Ehrlich, H. P. Wound closure: evidence of cooperation between fibroblasts and collagen matrix. Eye (Lond) 2(Pt 2), 149–157 (1988).
Singer, A. J. & Clark, R. A. Cutaneous wound healing. N Engl J Med 341(10), 738–746 (1999).
Guo, S. & Dipietro, L. A. Factors affecting wound healing. J Dent Res 89(3), 219–229 (2010).
Chen, J. et al. Tissue factor as a link between wounding and tissue repair. Diabetes 54(7), 2143–2154 (2005).
Wetzler, C. et al. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 115(2), 245–253 (2000).
Deng, L. et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev 2021, 8852759 (2021).
Altavilla, D. et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes 50(3), 667–674 (2001).
Doxey, D. L. et al. Platelet-derived growth factor levels in wounds of diabetic rats. Life Sci 57(11), 1111–1123 (1995).
Lerman, O. Z. et al. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162(1), 303–312 (2003).
Shettigar, K. & Murali, T. S. Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur J Clin Microbiol Infect Dis 39(12), 2235–2246 (2020).
Hosseinnejad, M. & Jafari, S. M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol 85, 467–475 (2016).
Kamel, N. A., Abd El-Messieh, S. L., and Saleh, N. M. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2017. 72: 543–550.
Li, P. et al. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr Polym 157, 1383–1392 (2017).
Liaqat, F. & Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr Polym 184, 243–259 (2018).
Huang, X., Jiao, Y. & Zhou, C. Impacts of chitosan oligosaccharide (COS) on angiogenic activities. Microvasc Res 134, 104114 (2021).
Huang, X. et al. Macrophage polarization mediated by chitooligosaccharide (COS) and associated osteogenic and angiogenic activities. ACS Biomater Sci Eng 6(3), 1614–1629 (2020).
Mei, Q. et al. Porous COS@SiO(2) nanocomposites ameliorate severe acute pancreatitis and associated lung injury by regulating the Nrf2 signaling pathway in mice. Front Chem 8, 720 (2020).
Kisadere, I. et al. The protective effects of chitosan oligosaccharide (COS) on cadmium-induced neurotoxicity in Wistar rats. Arch Environ Occup Health 77(9), 755–763 (2022).
Li, W. et al. DNA-based hydrogels with multidrug sequential release for promoting diabetic wound regeneration. JACS Au 3(9), 2597–2608 (2023).
Talbott, H. E. et al. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 29(8), 1161–1180 (2022).
Weng, T. et al. Dual gene-activated dermal scaffolds regulate angiogenesis and wound healing by mediating the coexpression of VEGF and angiopoietin-1. Bioeng Transl Med 8(5), e10562 (2023).
Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care, 2013. 22(8): p. 407–8.
Ersahin, T., Tuncbag, N. & Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst 11(7), 1946–1954 (2015).
Wang, J. et al. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm Sin B 12(1), 18–32 (2022).
Xu, F. et al. Retraction Note to: Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 11(1), 157 (2021).
Chen, H. et al. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 21(5), 1084–1091 (2016).
Ediriweera, M. K., Tennekoon, K. H. & Samarakoon, S. R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin Cancer Biol 59, 147–160 (2019).
Fattahi, S. et al. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci 262, 118513 (2020).
Zhang, W. et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res 370(2), 333–342 (2018).
Jere, S. W., Houreld, N. N. & Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev 50, 52–59 (2019).
Allen, L. V. Jr. Basics of compounding: tips and hints, Part 3: compounding with ointments, creams, pastes, gels, and gel-creams. Int J Pharm Compd 18(3), 228–230 (2014).
Prausnitz, M. R. & Langer, R. Transdermal drug delivery. Nat Biotechnol 26(11), 1261–1268 (2008).
Prausnitz, M. R., Mitragotri, S. & Langer, R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3(2), 115–124 (2004).
Payne, K. A., Roelofse, J. A. & Shipton, E. A. Pharmacokinetics of oral tramadol drops for postoperative pain relief in children aged 4 to 7 years: A pilot study. Anesth Prog 49(4), 109–112 (2002).
Naz, F. & Siddique, Y. H. Nanotechnology: Its application in treating neurodegenerative diseases. CNS Neurol Disord Drug Targets 20(1), 34–53 (2021).
Attia, M. F. et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 71(8), 1185–1198 (2019).
Dimov, N. et al. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci Rep 7(1), 12045 (2017).
Liang, Y. et al. Engineering exosomes for targeted drug delivery. Theranostics 11(7), 3183–3195 (2021).
Zare, H. et al. Carbon nanotubes: Smart drug/gene delivery carriers. Int J Nanomed. 16, 1681–1706 (2021).
Adepu, S., Luo, H., and Ramakrishna, S. Heparin-tagged PLA-PEG copolymer-encapsulated biochanin A-loaded (Mg/Al) LDH nanoparticles recommended for non-thrombogenic and anti-proliferative stent coating. Int. J. Mol. Sci. 22(11) (2021).
Acknowledgements
We would like to thank the Hand Surgery Experimental Center of the Affiliated Hospital of Nantong University for providing the corresponding experimental equipment for this study.
Funding
This study was funded by the Jiangsu Provincial Research Hospital (YJXYY202204-XKB10), China University Industry Academia-Research Innovation (No. 2021JH033), and the Project of Nantong Science and Technology Bureau (JC2021178).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, Z.L. and C. Z; software, Q.Z. and Y.D; visualization, X.S.; validation, W.W; formal analysis, B.W.; data curation, Z.Z.; writing—original draft preparation, review and editing, Z.L. and L.W.; project administration, Y.Z. and K.H.; funding acquisition, resources, Y.Z.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board Statement: The Affiliated Hospital of Nantong University’s Guide-lines for Care and Use of Laboratory Animals were followed, and the Nantong University Animal Ethics Committee gave its approval to all animal treatment operations. (Animal ethics: P20230207-001).
Consent for publication
The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Zhang, C., Wang, L. et al. Chitooligosaccharides promote diabetic wound healing by mediating fibroblast proliferation and migration. Sci Rep 15, 556 (2025). https://doi.org/10.1038/s41598-024-84398-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84398-w