Abstract
Respiratory tract infections caused by various pathogens remain a significant public health concern due to their high prevalence and potential for severe complications. This study systematically analyzed the epidemiological characteristics of six common respiratory pathogens—Chlamydia pneumoniae (CP), Mycoplasma pneumoniae (MP), Adenovirus (AdV), Influenza A virus (FluA), Influenza B virus (FluB), and Respiratory Syncytial Virus (RSV)—in patients from Sichuan Jinxin Xinan Women and Children’s Hospital between April 2023 and March 2024. Throat swab samples were collected from a total of 22,717 individuals. Each sample was processed using the AUTOMOLEC 3000 analyzer and the PCR-fluorescent probe method. The results showed that 10,171 (44.8%) individuals tested positive for at least one pathogen. MP had the highest overall positive rate (21.83%), followed by FluA (17.50%) and FluB (14.84%). MP showed the highest mean monthly (average) positive rate (16.84% ± 8.41). Significant differences were found between MP and AdV, CP and RSV in average positive rate (p < 0.05). Co-infection analysis revealed frequent associations between MP and AdV, MP and CP, and FluB with MP. Seasonal analysis indicated distinct peaks: FluA and FluB in winter, RSV in spring, and MP in summer, autumn and winter. Age-stratified analysis showed higher positivity rates of RSV in children aged 0–6 years, MP and CP in the 7–17 years group. Gender-based differences were only observed in RSV positive samples. These findings provide crucial insights into the prevalence and seasonal distribution of respiratory pathogens in Chengdu, offering valuable data to inform public health strategies in the post-COVID era.
Similar content being viewed by others
Introduction
Respiratory tract infections are among the most common and widespread diseases in the population, leading to a significant number of outpatient visits and hospitalizations. These infections not only cause acute symptoms but can also result in severe complications1. Symptoms of respiratory infections include coughing, fever, shortness of breath, and general malaise, which in severe cases can develop into more complex conditions such as pneumonia and bronchitis2. Among the pathogens involved, Chlamydia pneumoniae (CP), Mycoplasma pneumoniae (MP), Adenovirus (AdV), Influenza A virus (FluA), Influenza B virus (FluB), and Respiratory Syncytial Virus (RSV) are the most common causative agents3,4. Studies have shown that RSV is a leading cause of hospitalization in infants, while FluA/FluB account for a high number of cases during seasonal outbreaks5,6. In recent years, the emergence of the novel coronavirus (SARS-CoV-2) has further exacerbated the public health challenges associated with respiratory infections7. As the COVID-19 pandemic subsides, we now find ourselves in a “post-COVID era”8. Understanding the epidemiological characteristics of pathogens is crucial for the effective prevention and control of related diseases, especially in the post-COVID era, where public health strategies and vaccination programs play a key role.
Existing research indicates that the prevalence of respiratory pathogens varies across different seasons, age groups, and geographical regions, with epidemiological data specific to certain areas remaining relatively limited9,10. Moreover, the phenomenon of co-infections in respiratory infections has gained increasing attention. Co-infections with different pathogens not only potentially increase the severity of the disease but may also influence the choice of treatment strategies. For instance, co-infection with FluA/FluB and RSV can lead to more complex clinical presentations, increasing hospitalization rates and treatment difficulties11,12. In China, epidemiological studies on respiratory pathogens have been conducted in various regions. For example, a pathogen detection study conducted in Xiamen from January 2020 to October 2022 found that 56.36% of acute respiratory tract infection patients tested positive for at least one respiratory pathogen13. Research in Beijing revealed that the detection rate of common respiratory pathogens in children reached 41.71%, with FluA/FluB and RSV being particularly prominent14. Additionally, a study on the epidemiological characteristics of common respiratory pathogens in children in central Shandong, China, found that the prevalence of different pathogens varies across age groups and seasons15. These studies provide valuable data for understanding the epidemiological characteristics of respiratory pathogens. However, systematic research on respiratory pathogen prevalence in southwestern China, particularly in Chengdu, remains relatively scarce16,17. As one of the largest cities in southwestern China with a population exceeding 20 million, understanding the prevalence of these pathogens in Chengdu can help develop more effective prevention and control measures.
Therefore, this study aims to systematically analyze the epidemiological characteristics of six common respiratory pathogens—CP, MP, AdV, FluA, FluB, and RSV—based on sample data collected from Sichuan Jinxin Xinan Women and Children’s Hospital between April 2023 and March 2024. By comprehensively analyzing positive rates, monthly trends, and co-infection rates of these pathogens, we aim to provide scientific evidence for regional respiratory disease prevention and control, while also offering data support for future related research.
Materials and methods
Sample preparation
Throat swab samples between April 2023 and March 2024 were collected from each participant using polypropylene fiber-tipped plastic rod swabs. Both sides of the tonsils and the posterior pharyngeal wall were simultaneously swabbed. The swab head was then immersed in 3 ml of sampling solution, and the tail of the swab was discarded. The tube cap was tightly closed to ensure sample integrity. The study included all samples tested for CP, MP, AdV, FluA, FluB, and RSV from various departments in our hospital. The majority of the samples were collected from the emergency department or fever clinics. The study included a total of 22,717 participants, comprising 10,774 males and 11,943 females. The average age of the participants was 8.87 ± 11.20 years. The age distribution analysis revealed a minimum age of 0 years, with the 25th percentile at 2 years, the median age at 5 years, the 75th percentile at 9 years, and a maximum age of 91 years. The clinical diagnoses were diverse, encompassing conditions such as acute respiratory infections, gastrointestinal dysfunction, mastitis, enteritis, fever, dermatitis, tumors or others. Informed consent was obtained from all subjects and/or their legal guardian(s) involved in the study. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and received approval from the Medical Ethics Committee of Sichuan Jinxin Xinan Women and Children’s Hospital.
Sample processing
Prior to nucleic acid extraction, the samples were thoroughly shaken to ensure even distribution of the sample material. All sample processing, including opening the tubes, was conducted within a biological safety cabinet to prevent contamination. The samples were then loaded onto the AUTOMOLEC 3000 automated nucleic acid extraction and real-time fluorescent PCR analyzer.
Instrumentation and detection method
The AUTOMOLEC 3000 analyzer was used for both nucleic acid extraction and PCR analysis. The detection method employed was the PCR-fluorescent probe method, which enables simultaneous qualitative detection of multiple respiratory pathogens. The following pathogens were targeted in the assay: FluA, FluB, RSV, MP, CP and ADV.
Result interpretation
The interpretation of PCR results was based on the cycle threshold (Ct) values as follows: FluA, FluB, RSV: Positive if Ct < 36; negative if Ct ≥ 36 or No Ct. MP, CP: Positive if Ct ≤ 36; negative if Ct > 36 or No Ct. ADV: Positive if Ct ≤ 35; negative if Ct > 35 or No Ct. The detection of FluA, FluB and RSV, was based on RNA targets, while MP, CP, and ADV were detected based on DNA targets.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (version 8.0). Descriptive statistics were calculated to summarize the data, including the number of detected individuals, monthly distribution, and age. For each pathogen, the number of detected samples, overall positive rate, mean monthly positive rate, and monthly positive rate distribution were calculated. Quantitative data are presented as mean ± standard deviation (SD). Comparative analyses were conducted to evaluate the differences in positive rates among the respiratory pathogens. For comparisons across multiple groups, one-way analysis of variance (ANOVA) was employed, with Bonferroni correction applied for multiple comparisons. For pairwise comparisons, an unpaired t-test was used if the data were normally distributed; otherwise, the Mann-Whitney test was applied. A p-value of less than 0.05 was considered statistically significant. Data visualization was carried out using Cytoscape for co-infection analysis, while all other graphs were generated using GraphPad Prism.
Results
Prevalence and monthly distribution of respiratory pathogens and co-infections in the study population
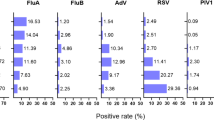
Among the 22,717 individuals, 10,171 (44.8%) tested positive for at least one pathogen. The overall positive rates of respiratory pathogens revealed MP as the most prevalent (21.83%), followed by FluA (17.50%), FluB (14.84%), and RSV (13.84%). CP and AdV had lower rates at 1.31% and 6.18%, respectively (Fig. 1A). The mean monthly positive rates showed MP at 16.84% ± 8.41, RSV at 13.43% ± 12.56, and FluA at 10.42% ± 10.01. Significant differences were observed between RSV and CP (p < 0.01), and CP and MP (p < 0.0001) (Fig. 1B).
Co-infections occurred most frequently between MP and AdV, followed by MP with CP and FluB with MP. RSV co-infections with FluA and AdV were moderately frequent, while co-infections involving FluA or FluB with MP were less common. CP co-infections were rare (Fig. 1C). We also evaluated the co-infection positivity rates of various viruses and bacteria. The results showed that the five most common co-infection combinations were: MP and AdV, with a positivity rate of 1.08%; followed by FluB and MP, at 0.70%. Other combinations included FluA and RSV, with a positivity rate of 0.67%; RSV and FluB, at 0.42%; and FluA and MP, at 0.41% (Fig. 1D). These findings indicate that the co-infection of MP and AdV was the most significant in the sample, highlighting its importance in respiratory infections.
Monthly distribution analyses revealed variability in pathogen positivity rates. CP positivity was consistently below 2%, peaking in March (1.57%). MP positivity rates increased after June, with the highest in December (26.39%). AdV peaked in March (9.42%) and January (6.39%). FluA positivity was highest in December (29.05%), while FluB peaked in January (26.89%). RSV exhibited its highest rate in April (50.67%), with a secondary peak in October (13.79%) (Fig. 1E).
Overview of respiratory pathogen. (A) Bar graph showing the overall positive rates of CP, MP, AdV, FluA, FluB, and RSV. (B) Violin plot showing the anverage monthly positive rates of CP, MP, AdV, FluA, FluB, and RSV. (C) Network diagram illustrating the co-infection relationships among CP, MP, AdV, FluA, FluB, and RSV. The size of the circles, the thickness of the lines, and the intensity of the line colors represent the frequency of co-infections. (D) Bar Chart of Co-infection Positivity Rates for CP, MP, AdV, FluA, FluB, and RSV. (E) Monthly distribution of the number of tested samples and corresponding positive rates for CP, MP, AdV, FluA, FluB, and RSV. CP (Chlamydia pneumoniae), MP (Mycoplasma pneumoniae), AdV (Adenovirus), FluA (Influenza A virus), FluB (Influenza B virus), and RSV (Respiratory syncytial virus). ** indicates p < 0.01, **** indicates p < 0.0001.
Gender-based differences in positive rates of respiratory pathogens
The analysis of positive rates across different sexes revealed that MP had the highest positive rates in both males (22.83%) and females (20.14%). Notably, FluA exhibited similar positive rates between males (17.93%) and females (18.05%), while FluB showed slightly higher rates in females compared to males (Fig. 2A). A comparison of the average monthly positive rates between different sexes showed no noticeable differences for any of the six pathogens (Fig. 2B).
Furthermore, we analyzed the average gender differences in positive samples for each pathogen over the course of a year. Our findings revealed no significant gender differences in positive rates for CP, MP, AdV, FluA, and FluB. Interestingly, we observed that among RSV-positive samples, the proportion of males was significantly higher than that of females (Fig. 2C). This result suggests that males may have a higher susceptibility to RSV infection. Additionally, we presented the monthly distribution of RSV-positive samples by gender throughout the year (Fig. 2D).
Gender-specific analysis of respiratory pathogen positivity rates. (A) Heatmap showing the positive rates of CP, MP, AdV, FluA, FluB, and RSV across different genders. (B) Violin plot showing the average monthly positive rates of CP, MP, AdV, FluA, FluB, and RSV across different genders. (C) Violin plot showing the average of gender proportion in positive samples of CP, MP, AdV, FluA, FluB, and RSV. (D) Monthly distribution of gender proportion in RSV positive samples. CP (Chlamydia pneumoniae), MP (Mycoplasma pneumoniae), AdV (Adenovirus), FluA (Influenza A virus), FluB (Influenza B virus), and RSV (Respiratory syncytial virus). ** indicates p < 0.01.
Seasonal variation in pathogen positivity rates
The analysis of seasonal positive rates for respiratory pathogens revealed distinct patterns across the year. MP exhibited the highest positive rates during autumn (September to November, 22.56%) and winter (December to February, 24.37%). RSV showed a significant peak in spring (March to May, 27.30%), while FluA and FluB had elevated positive rates in winter (December to February), at 22.02% and 23.45%, respectively. In contrast, CP and AdV maintained relatively stable positive rates across the seasons, with minor fluctuations observed (Fig. 3A).
Analysis of average positivity rates for respiratory pathogens across different seasons revealed notable seasonal variations. For CP, the average positivity rate was significantly higher in Autumn (1.37%) compared to Summer (0.40%) (p < 0.05). The highest positivity rate was observed in Winter, reaching 14.25%. MP showed a significantly higher average positivity rate in Autumn (21.02%) compared to Spring (2.30%) (p < 0.001), with Summer also exhibiting elevated rates (14.96%). AdV had a significantly higher average positivity rate in Winter (6.13%) compared to Summer (4.11%) (p < 0.05). The rates in Spring and Autumn were relatively close, averaging 5.80% and 5.63%, respectively. FluA had the highest average positivity rate in Winter (18.74%), with a notably lower rate in Summer (0.08%). FluB demonstrated a significantly higher average positivity rate in Winter (21.54%) compared to Spring (2.16%) (p < 0.05). The rates in Summer (0.25%) and Autumn (6.79%) were notably lower. RSV showed a significantly higher average positivity rate in Autumn (11.55%) compared to Summer (5.42%) (p < 0.05). Spring had the highest rate at 27.08% (Fig. 3B). These results highlight the significant seasonal variations in pathogen positivity rates, reflecting the varying impact of respiratory pathogens throughout the year.
Seasonal analysis of respiratory pathogen positivity rates. (A) Heatmap showing the positive rates of CP, MP, AdV, FluA, FluB, and RSV across different seasons. (B) Average monthly positive rates of CP, MP, AdV, FluA, FluB, and RSV across different seasons. CP (Chlamydia pneumoniae), MP (Mycoplasma pneumoniae), AdV (Adenovirus), FluA (Influenza A virus), FluB (Influenza B virus), and RSV (Respiratory syncytial virus). Spring, Summer, Autumn, and Winter correspond to March–May (month 3–5), June–August (month 6–8), September–November (month 9–11), and December–February (month 12 − 2), respectively. * Indicates p < 0.05.
Pathogen positivity rates across different age groups
For CP, the 7–17 years group had the highest positivity rate (3.26%), compared to the 0–6 years (0.60%) and ≥ 18 years (0.36%) groups. MP showed a peak positivity in the 7–17 years group (40.71%), with lower rates in the 0–6 years (16.19%) and ≥ 18 years (6.77%) groups. AdV positivity was highest in the 0–6 years group (6.95%), followed by the 7–17 years (6.14%) and ≥ 18 years (2.14%) groups. FluA and FluB rates were higher in the 7–17 years group (21.50% and 30.92%, respectively) compared to the 0–6 years (15.76% for FluA, 8.07% for FluB) and ≥ 18 years (19.23% for FluA, 19.50% for FluB) groups. RSV was most prevalent in the 0–6 years group (20.84%) and lower in the 7–17 (3.63%) and ≥ 18 (4.66%) groups (Fig. 4A).
Significant differences were noted in positivity rates for CP and MP, which were higher in the 7–17 and ≥ 18 years groups compared to the 0–6 years group (CP: p < 0.01; MP: p < 0.0001). AdV rates were significantly higher in the 0–6 years group compared to the ≥ 18 years group (p < 0.05), while FluA and FluB showed no significant differences across groups. RSV positivity was significantly higher in the 0–6 years group compared to older groups (p < 0.001) (Fig. 4B).
Monthly distribution analyses showed seasonal peaks for CP in the 7–17 years group (April and September) and for MP in July and August across the 7–17 and ≥ 18 years groups. AdV peaked in March for both the 0–6 and 7–17 years groups. FluA rates were highest in December for the 0–6 years group and in April for older groups. FluB and RSV peaked in January and April, respectively, with the highest RSV positivity in the 0–6 years group (Fig. 4C).
Analysis of respiratory pathogen positivity rates across different age groups. (A) Heatmap showing the positive rates of CP, MP, AdV, FluA, FluB, and RSV across different age groups. (B) Average monthly positive rates of CP, MP, AdV, FluA, FluB, and RSV across different age groups. (C) Monthly distribution of positive rates for CP, MP, AdV, FluA, FluB, and RSV across different age groups. CP (Chlamydia pneumoniae), MP (Mycoplasma pneumoniae), AdV (Adenovirus), FluA (Influenza A virus), FluB (Influenza B virus), and RSV (Respiratory syncytial virus). * Indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Discussion
This study provides a comprehensive analysis of the epidemiological characteristics of six common respiratory pathogens—CP, MP, AdV, FluA, FluB, and RSV—based on data collected from Chengdu Jinxi Women’s and Children’s Hospital. Our findings reveal significant insights into the prevalence, seasonal variation, and co-infection patterns of these pathogens, offering valuable data for regional disease prevention and control strategies.
The overall prevalence rates of respiratory pathogens in our study reflect notable variability. MP emerged as the most predominant pathogen, followed by FluA and FluB. Several studies have reported similar findings. Chen et al. found that the positivity rate for Mycoplasma MP in children with acute respiratory infections was 25.9%18. Zhang et al. found that the positivity rate for MP was 18.41% among 4,213 children in Chengdu from 2014 to 202017. This rate is slightly lower than our findings, which we attribute to the impact of COVID-19 prevention measures, including prolonged periods of staying at home. This suggests a significant impact of MP on the respiratory disease burden in Chengdu. The observed lower prevalence rates of CP and AdV indicate that these pathogens are less common in the study population, though they still contribute to the overall disease landscape19,20.
The mean monthly positive rates revealed that RSV and MP exhibit the highest average rates, indicating persistent and significant activity of these pathogens. The statistical significance observed between MP and CP, and CP and RSV underscores the differential prevalence and activity levels of these pathogens across different months. The higher mean monthly positive rates for RSV and MP suggest that these pathogens have a more consistent presence throughout the study period compared to others. Similar findings have been reported in other studies. For instance, a retrospective analysis of acute respiratory infections in children in Suzhou showed that RSV and MP were the primary contributors to the infection rates during the study period21. Another analysis of 23 nationwide hospitals in South Korea found that MP and RSV are the two most common pathogens causing community-acquired pneumonia22.
Co-infection patterns highlight complex interactions between respiratory pathogens. In an epidemiological study of respiratory infections, the co-infection rate of AdV and MP was 19.51%23. In another study on children with community-acquired pneumonia, the co-infection rate of MP and AdV reached as high as 90.1%24. The frequent co-infections between MP and AdV in our study, suggest that these pathogens may have synergistic effects or shared transmission pathways. The less frequent co-infections involving RSV may indicate more isolated transmission dynamics or varying clinical presentations. These findings emphasize the need for comprehensive diagnostic approaches to accurately identify and manage co-infections in clinical settings.
Seasonal variations in pathogen positivity rates reveal distinct patterns. FluA and FluB exhibit peaks during winter months, aligning with the well-documented seasonal surge in influenza cases25. This may be due to low temperatures and low humidity, which facilitate the spread of the influenza virus26. During the same period, data released by the Chinese National Influenza Center showed that both FluA and FluB were predominantly observed in winter. However, FluA also peaked in April, with a positivity rate exceeding 40% during the first week of April 2023, compared to a peak positivity rate of approximately 23% for FluB during the second week of January 2024 (data not shown). Our findings for FluB align with the data from the Chinese National Influenza Center, but there is a slight discrepancy in the results for FluA. We hypothesize that the nationwide FluA outbreak in April may not have occurred in Chengdu. RSV shows a significant peak in spring, while MP has notable high rates in summer, autumn and Winter. Our findings are consistent with the results reported by Wang27 et al. and Yu28 et al. The stability of CP and AdV positivity rates across seasons suggests a more constant presence of these pathogens throughout the year. These seasonal trends are crucial for informing public health interventions, including vaccination and surveillance strategies.
The analysis of pathogen positivity rates across different age groups reveals specific patterns of susceptibility. CP and AdV show higher positivity in older children, while MP and FluA have higher rates in adolescents. RSV, with its peak in the 0–6 age group, underscores the vulnerability of young children to this pathogen. Our findings are supported by multiple studies. For example, one study indicated that the positive rate of RSV in children aged 0–3 years is significantly higher than in other age groups29. Another analysis revealed that nearly 50% of RSV-related hospitalizations involve children under 6 months of age30. According to the Dawson, most children are infected with RSV before the age of 2, and younger children appear to be more susceptible31. These age-specific findings highlight the need for targeted preventive measures and treatment approaches tailored to different age groups.
Gender-based differences in positivity rates were generally minimal, though some pathogens exhibited slight variations. For instance, FluB positivity was higher in females, while MP positivity was higher in males during certain months. However, none of these findings reached statistical significance. No significant differences in positive rates were found in other studies between males and females for RSV, FluA, FluB, or AdV32. These differences may reflect broader epidemiological trends or require further investigation to understand underlying factors.
This study has several limitations that should be acknowledged. First, the study is retrospective in nature, which inherently limits the ability to establish causality between the factors analyzed and the outcomes observed. Additionally, the data were collected from a single hospital, which may limit the generalizability of the findings to other populations or regions. The sample size, though sufficient for initial analysis, may not be large enough to detect smaller effects or to explore all possible interactions between pathogens and demographic factors. Moreover, seasonal variations and potential environmental factors influencing the incidence of respiratory pathogens were not comprehensively controlled for in this study. Lastly, while PCR-based detection methods are highly sensitive, they cannot distinguish between active infection and colonization, which might lead to overestimation of pathogen prevalence. These limitations highlight the need for further prospective, multicenter studies with larger sample sizes to validate our findings and provide a more detailed understanding of the epidemiology of respiratory pathogens.
In conclusion, our study enhances our understanding of the epidemiological characteristics of key respiratory pathogens in Chengdu. The insights gained from the prevalence, seasonal variations, and co-infection patterns provide valuable information for developing targeted public health strategies and improving disease management practices. Future research should focus on exploring the underlying mechanisms driving these patterns and evaluating the effectiveness of intervention strategies in reducing the burden of respiratory infections.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Soriano, J. B. et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Respiratory Med. 8 (6), 585–596 (2020).
Kuchar, E. et al. Pathophysiology of clinical symptoms in acute viral respiratory tract infections. Pulmonary Infect. 857, 25–38 (2015).
Ma, J. et al. Analysis of common respiratory infected pathogens in 3100 children after the coronavirus disease 2019 pandemic. Curr. Med. Sci. 42 (5), 1094–1098 (2022).
Charlton, C. L. et al. Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections. Clin. Microbiol. Rev. 32 (1). https://doi.org/10.1128/cmr (2018). 00042 – 00018.
Obando-Pacheco, P. et al. Respiratory syncytial virus seasonality: a global overview. J. Infect. Dis. 217 (9), 1356–1364 (2018).
Coultas, J. A., Smyth, R. & Openshaw, P. J. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax 74 (10), 986–993 (2019).
Lamers, M. M. & Haagmans, B. L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 20 (5), 270–284 (2022).
Lee, S. M. & Lee, D. Opportunities and challenges for contactless healthcare services in the post-COVID-19 era. Technol. Forecast. Soc. Chang. 167, 120712 (2021).
García-Arroyo, L. et al. Prevalence and seasonality of viral respiratory infections in a temperate climate region: a 24‐year study (1997–2020). Influenza Other Respir. Viruses. 16 (4), 756–766 (2022).
Chadha, M. et al. Human respiratory syncytial virus and influenza seasonality patterns—early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir. Viruses. 14 (6), 638–646 (2020).
Pott, H., LeBlanc, J. & ElSherif, J. S. Clinical features and outcomes of influenza and RSV coinfections: a report from Canadian immunization research network serious outcomes surveillance network. BMC Infect. Dis. 24 (1), 147 (2024).
Cong, B. et al. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: a systematic review and meta-analysis. J. Glob. Health 17, 12 (2022).
Hong, S. et al. Epidemiology of respiratory pathogens in patients with acute respiratory tract infection in Xiamen, China: a retrospective survey from 2020 to 2022. Heliyon 9(11), e2230 (2023).
Xiuli, G. et al. Analysis of the prevalence of common respiratory pathogens in children in Beijing in 2020. Chin. J. Microbiol. Immunol. 12, 141–147 (2022).
Lv, G. et al. Epidemiological characteristics of common respiratory pathogens in children. Sci. Rep. 14 (1), 16299 (2024).
Li, Q. et al. Pediatric respiratory pathogen dynamics in Southern Sichuan, China: a retrospective analysis of gender, age, and seasonal trends. Front. Pead. 12, 1374571 (2024).
Zhang, L. et al. Analysis of mycoplasma pneumoniae infection among children with respiratory tract infections in hospital in Chengdu from 2014 to 2020. Translational Pediatr. 10 (4), 990 (2021).
Chen, L. et al. Epidemiology of Mycoplasma pneumoniae in children with acute respiratory infections in Hangzhou during January 2021 to June 2023. J. Lab. Med. 48 (3), 121–127 (2024).
Di Pietro, M. et al. Chlamydia trachomatis and Chlamydia pneumoniae interaction with the host: latest advances and future prospective. Microorganisms 7 (5), 140 (2019).
Gu, J. et al. Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection 49, 1–13 (2021).
Yinying, R. et al. Retrospective analysis of viral pathogen of acute respiratory tract infection in children in Suzhou area. Chin. J. Appl. Clin. Pediatr. 24, 254–259 (2019).
Lee, E. et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect. Dis. 20, 1–10 (2020).
Liang, J. et al. Epidemiology and co-infection patterns in patients with respiratory tract infections in southern China between 2018 and 2020. J. Infect. 83 (3), e6–e8 (2021).
Li, F. et al. Mycoplasma pneumoniae and adenovirus coinfection cause pediatric severe community-acquired pneumonia. Microbiol. Spectr. 10, e0002622 (2022).
Zhu, G. et al. Epidemiological characteristics of four common respiratory viral infections in children. Virol. J. 18, 1–5 (2021).
Shaman, J. et al. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 8 (2), e1000316 (2010).
Wang, L. et al. Disruption in seasonality, patient characteristics and disparities of respiratory syncytial virus infection among young children in the US during and before the COVID-19 pandemic: 2010–2022. medRxiv (2022).
Yu, J. et al. Respiratory syncytial virus seasonality, Beijing, China, 2007–2015. Emerg. Infect. Dis. 25 (6), 1127 (2019).
Duan, Y. et al. Incidence, hospitalization, and mortality in children aged 5 years and younger with respiratory syncytial virus-related diseases: a systematic review and meta‐analysis. Influenza Other Respir. Viruses. 17 (5), e13145 (2023).
Bourdeau, M. et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Netw. Open. 6 (10), e2336863–e2336863 (2023).
Dawson-Caswell, M. & Muncie, H. L. Jr Respiratory syncytial virus infection in children. Am. Family Phys. 83 (2), 141–146 (2011).
Chamekh, M. et al. Differential susceptibility to infectious respiratory diseases between males and females linked to sex-specific innate immune inflammatory response. Front. Immunol. 8, 1806 (2017).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Ma, J., Li, Y. et al. One-year epidemiological patterns of respiratory pathogens across age, gender, and seasons in Chengdu during the post-COVID era. Sci Rep 15, 357 (2025). https://doi.org/10.1038/s41598-024-84586-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84586-8