Abstract
This study aimed to systematically investigate the value of the pre-treatment neutrophil-to-lymphocyte ratio (NLR) in prognosticating the outcome of patients with advanced cancer receiving immunotherapy. We searched Embase, PubMed, Web of Science, and Cochrane Library to identify studies about cancer patients with immunotherapy until November 29, 2024. Retrospective or prospective cohort studies with pretreatment NLR data were included. The odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the predictive value of NLR in prognosis and immunotherapy efficacy. The random effect model was applied for meta-analysis and the risk of bias was assessed by Egger test and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method. A total of 129 articles involving 18780 cases were finally selected. Most cases were advanced cancers with the median follow-up period ranged 2–48.6 months. The high pretreatment NLR level was associated with the significantly reduced OS (HR (95%CI) = 2.26 (2.03, 2.53)), PFS (HR (95% CI) = 1.83 (1.69, 1.98)), ORR (OR (95%CI) = 0.53 (0.46, 0.61)) and DCR (OR (95% CI) = 0.36 (0.29, 0.43)) in patients with advanced cancer receiving immunotherapy. The quality of evidence was low, attributed to the serious risk of bias and incon¬sistency. An elevated NLR before immunotherapy was significantly associated with poor clinical outcomes in patients with advanced cancer.
Similar content being viewed by others
Introduction
Advanced cancer poses a significant global health threat, with its incidence on rise1. Immunotherapy has revolutionized the landscape of cancer treatment2,3, offering unprecedented survival advantages4. Despite its efficacy, immunotherapy is effective only for select patients, with many individuals failing to respond5. Tumor mutational burden, programmed death ligand-1, tumor-infiltrating lymphocytes, and microsatellite instability are gaining importance in the selection of treatment options6. However, these biomarkers have some limitations, such as being unsuitable for dynamic monitoring, high cost, and inconsistent viability7,8. Thus, the urgent need arises for identifying biomarkers that are readily available at a low cost for routine clinical use without specialized genomic technologies. The neutrophil-to-lymphocyte ratio (NLR), defined as the absolute counts of neutrophils and lymphocytes, has been reported to be a predictive biomarker of mortality in all conditions, including cancers9,10,11,12. NLR has been an emerging marker of the association between immune system and diseases13. Considering the interplay among systemic inflammation, immune system, and immunotherapy, NLR may be an attractive biomarker for predicting the efficacy of immunotherapy in cancer patients. Despite some studies14,15,16,17 and meta-analyses18,19,20 have examined the clinical application of NLR for immunotherapy efficacy in patients with advanced cancer, the results are inconsistent. With the development of immunotherapy, and updated clinical data, the performance of the NLR value in prognosticating immunotherapy efficacy deserves further exploration. To obtain objective and comprehensive results, we performed meta-analysis to evaluate the prognostic significance of NLR in patients with advanced cancer receiving immunotherapy. These results may be helpful in guiding the option of immunotherapy in advanced cancer patients.
Materials and methods
Search strategy
Based on the defined search strategy, appropriate literatures were searched from Embase, PubMed, Web of Science, and Cochrane Library databases with the keywords of “neutrophil-lymphocyte ratio,” “NLR,” “immunotherapy,” “immunological therapy,” “immune checkpoint inhibitors,” “neoplasm,” “advanced cancer,” and “tumor.” Keywords were combined with “OR” in the same category and with “AND” in different categories. Both subject headings and free-text terms were used. The retrieval strategy was adjusted based on the database characteristics. Supplementary Table 1 presents the retrieval steps for PubMed. Articles published until November 29, 2024 and limited to the English language were included. Additionally, eligible studies were identified by screening the references of both reviews and included articles.
Literature screening
The inclusion criteria were as follows: (1) Patients with histologically or pathologically confirmed or medical records recorded advanced cancer and received immunotherapy (immune checkpoint inhibitors, ICIs); (2) The study investigated the association of pretreatment NLR levels with overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) and efficacy evaluation was on the basis of the Response Evaluation Criteria in Solid Tumors criteria; (3) The research type was retrospective or prospective cohort study; (4) The hazard ratio (HR) or odds ratio (OR) (95% confidence interval (CI)) of univariate or multivariable adjustment was provided or could be converted according to the frequency and sample size. The exclusion criteria were as follows: (1) non-literary research, such as meeting abstracts, reviews, and comments; (2) studies in which patients had received immunotherapy prior to the study; (3) NLR during and after treatment; and (4) for multiple studies with the same data or repeated publications, the study with the most comprehensive data.
Data extraction and quality evaluation
Two investigators (Jialin Su and Yuning Li) independently screened the relevant literature. Data extraction was conducted independently after confirming the inclusion criteria. Information included publication year, first author, region, study type, basic features (sample size, sex, and age), ICI regimens, follow-up time, NLR threshold, and clinical outcomes. Following data extraction, discrepancies were resolved through discussion between the two investigators until a consensus was reached. The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS), which evaluated selection, comparability, and exposure with eight scoring items and a maximum score of 921. A score of 7–9 was considered high quality, 4–6 as medium quality, and < 4 low quality.
Evidence quality evaluation
GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach is widely used for rating the quality of evidence in meta-analysis and review. In this study, the evidence quality of included studies was assessed based on GRADE system22 by using GRADE Profiler (GRADEpro) Guideline Development Tool (GDT) online tool.
Statistical analyses
To analyze the association between NLR and immunotherapy efficacy in patients with cancer before treatment, patients were divided into high and low NLR group according to the NLR cutoff value of 4 according to the previous description23,24. In addition, our data showed that the heterogeneity was minimal, when NLR was 4. OR and 95% CI served as effect size indices to evaluate whether there were statistically significant differences in ORR and DCR between high NLR vs. low NLR group. HR and 95% CI were used to analyze the association between NLR and survival risk.
Due to the methodological heterogeneity of these studies, a random-effects model was applied to pool the effect values of the meta-analysis. Heterogeneity was tested using the Cochran’s Q and I2 test25. P < 0.05 or I2 > 50% indicated significant heterogeneity among studies. If P > 0.05 and I2 ≤ 50%, no significant heterogeneity was considered. Subgroup analysis was performed according to variables, such as research region, cancer type, confounder correction, and NLR cutoff. A one-by-one exclusion test was used to assess whether a single study significantly affected the results26. Egger test27 and Begg’s test were used to test publication bias. If significant publication bias was present, the stability of the pooled results was analyzed using the trim-and-fill method28. The above statistical analyses were completed using Stata12.0 software.
Results
Literature search
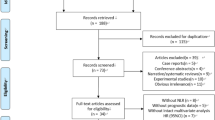
Figure 1 illustrates the literature search process and the PRISMA checklist is listed in Supplementary File 1. A total of 5711 articles were searched (1397 from PubMed, 2497 from Embase, 154 from The Cochrane Library, and 1663 from the Web of Science). After eliminating 2337 duplicate articles, 3374 remained. After browsing the titles and abstracts, 3165 studies that did not meet the inclusion criteria were excluded. Finally, 80 of the 209 studies were eliminated after full-text reading (Supplementary File 2), and finally 129 studies14,24,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158 were included in this analysis.
Research characteristics and quality evaluation
A total of 129 studies published between 2016 and 2024, with 18,780 cases, were included (Table 1). Most participants were patients with advanced cancers, including renal cell carcinoma, non-small cell lung cancer, urothelial carcinoma, head and neck cancer, and liver cancer. The median age of the participants ranged 50–75 years and the median follow-up time ranged 2-48.6 months. The ICI types and grouping thresholds of the NLR are shown in Table 1. The results of the quality evaluation are presented in Supplementary Table 2. The NOS scores were 5–8. Methodological quality was moderate. The main types of biases in the included studies were recall and confounding factors.
Meta-analysis
The forest plot of OS risk in high NLR vs. low NLR before treatment is shown in Fig. 2A. A total of 107 articles were included, and the heterogeneity test results showed I2 = 87.5% (P < 0.001), indicating significant statistical heterogeneity. The pooled result was HR (95% CI) = 2.26(2.02, 2.53) (P < 0.001), indicating that a high level of pretreatment NLR was related with significantly reduced OS. The forest plot of risk of PFS between high NLR and low NLR groups is shown in Fig. 2B, and the heterogeneity test result of the included studies was I2 = 57.8% (P < 0.001). The pooled result was HR (95% CI) = 1.83 (1.69, 1.98), (P < 0.001), indicating that a high pretreatment NLR level was related to a significantly reduced PFS. Thus, an increased pretreatment NLR level might be served as a biomarker for poor prognosis in patients with advanced cancer receiving immunotherapy.
A comparison of the ORR between high and low NLR groups before treatment is shown in Fig. 3A. A total of 38 articles were included, and the heterogeneity test results showed I2 = 0% (P = 0.657), indicating no significant heterogeneity among the studies. Pooled results were OR (95%CI) = 0.54 (0.47, 0.62) (P < 0.001), indicating an obvious correlation between the NLR and ORR. Patients with a high NLR before immunotherapy showed a significantly decreased ORR. A forest plot of the DCR is presented in Fig. 3B, and the heterogeneity test result of the included studies was I2 = 45.2% (P = 0.001). The pooled result was OR (95% CI) = 0.36 (0.29, 0.43) (P < 0.001), indicating that a high pretreatment NLR was related to a significantly decreased DCR. Thus, an increased pretreatment NLR may predict decreased immunotherapy efficacy.
Subgroup analysis
The subgroup analysis results for OS, PFS, ORR, and DCR are shown in Table 2. For OS, the differences between subgroups were not statistically significant after grouping by region, study type, cancer type, multivariate adjustment and NLR cutoff (two studies40,141 did not report the cutoff and were not included in the subgroup analysis) (P > 0.05). Except for metastatic MCC (Merkel cell carcinoma), the pooled results of the other subgroups were in accordance with the original pooled results (P < 0.05). However, none of the above grouping factors were the sources of significant heterogeneity (Figure S1A-1E). For PFS, no significant differences were observed among the subgroups after grouping by region, study type, and multivariate adjustment (P > 0.05); however, subgroup analysis of the cancer type and NLR threshold showed statistically significant differences (P < 0.05). Pooled results for PCS (prospective cohort study), aCRC (advanced colorectal cancer), and metastatic MCC were not significant (P > 0.05), whereas the pooled results for the other subgroups were significant (P < 0.05). Similarly, no significant source of heterogeneity was identified in the subgroup analysis (Figure S2A-2E).
For ORR, although significant results were found in both Asian and non-Asian subgroups (P < 0.001), significant difference was found in the pooled results with regard to region (P = 0.005). There was no significant difference between subgroups based on the study type, cancer type, or NLR cutoff (P > 0.05). In addition, the pooled results of the PCS, aMelanoma (advanced melanoma), aGC (advanced gastrointestinal/gastric Cancer), aUC (advanced urothelial cancer) and UGI (upper gastrointestinal cancer) subgroups were not significant (P > 0.05), and the pooled result of the other subgroups were significant (P < 0.05) (Figure S3A-3D). For DCR, there were no significant differences among the subgroups by region, study type, cancer type, and NLR cutoff (P > 0.05). The pooled results of the PCS, UGI cancer, and aCRC subgroups were not significant (P > 0.05), whereas the pooled result of other subgroups were significant (P < 0.05). None of the above grouping factors was a significant source of heterogeneity (Figure S4A-4D). Thus, an NLR cutoff value of 4 was related to PFS and OS.
Sensitivity analysis and publication bias test
As shown in Table 3, the pooled results for the four indicators showed good stability. Excluding any one study, the pooled results of the other studies were significant (P < 0.05). The Egger results are listed in Table 3 and indicate a significant publication bias in the included studies for OS, PFS, ORR, and DCR. The trim-and-fill method revealed that after including 18 virtual studies on OS (Figure S5A), the pooled result was HR (95% CI) = 2.01 (1.81, 2.24) (P < 0.001), indicating that the original pooled result was stable. For PFS, after including 11 virtual studies (Figure S5B), the pooled results were changed to HR (95% CI) = 1.71 (1.57, 1.86) (P < 0.001), indicating the stability of the original pooled result. For ORR and DCR, no virtual negative results were used to enhance the symmetry of the funnel plot, and the results did not change, suggesting that publication bias in ORR and DCR may be caused by small sample bias. Begg’s test achieved the consistent results (Table 3).
Certainty of evidence
The included studies were observational studies with a low or moderate risk of bias. At the same time, the degree of heterogeneity of studies was high. The GRADE of the evidence was categorized as low quality. The certainty of evidence was low for ORR and was very low for OS, PFS, and DCR (Supplementary Table 3).
Discussion
Our study collected available evidence from 120 articles with 17,969 cases of advanced cancer. The results revealed that the pretreatment NLR was significantly related to OS, PFS, ORR, and DCR. Subgroup analyses by research region, cancer type, study type, and confounder correction remained unchanged. To the best of our knowledge, this is the latest, most comprehensive, and largest meta-analysis on the relationship between the NLR and the efficacy and prognosis of immunotherapy.
Hematological parameters are the most common and easily available for routine clinic monitoring159. The NLR reflects host inflammatory processes, and the relationship between inflammation and human cancer has been extensively explored160. It was gradually found that inflammation is implicated in cancer initiation, invasion, and metastasis161. Inflammation affects host immune response and plays an important role in immunotherapy162. The function of neutrophils in tumor microenvironment is controversial because they contribute to tumor growth, angiogenesis, immune tolerance, and metastasis163. Tumor-associated neutrophils can decrease CD4+/CD8 + T cell and suppress the generation of IFN-γ and TNF-α, leading to an immunosuppressive environment164. Lymphocytes are involved in the antitumor immune response; therefore, elevated lymphocyte infiltration is related to a good prognosis in patients with cancer165. Neutrophils reflect a response to systemic inflammation, whereas decreased lymphocytes represent cell-mediated immune impairments166. The present meta-analysis confirmed that a high NLR before immunotherapy was significantly associated with decreased clinical efficacy and poor prognosis. The cutoff value of the NLR has been reported to be between 2 and 5167. However, the optimal cutoff value remains undetermined. In our study, a cutoff value of 4 was related to prognosis and might serve as a prognostic biomarker. Further large-scale studies are required to validate the clinical value.
Several published meta-analyses have analyzed the prognostic value of pretreatment NLR in patients with advanced cancer168,169,170,171. Templeton18 included 100 articles with 40,559 patients for a meta-analysis, and the pooled results showed that a high NLR was related to poor OS in many solid cancers. Mei et al.20 analyzed 66 studies involving 24,536 patients. The pooled results revealed that an elevated pretreatment NLR correlated with poor clinical outcomes in advanced cancers. However, only these two studies analyzed the prognostic role of the pretreatment NLR in advanced cancers, and it remains unclear whether the NLR has a value in prognosticating immunotherapy efficacy in these patients. A recent study by Jiang19 analyzed the predictive and prognostic value of pretreatment NLR for immunotherapy in patients with advanced cancers. The pooled results revealed that an elevated NLR was significantly associated with poor OS and PFS. However, only 11 studies were included in their meta-analysis. In our study, we analyzed the correlation of pretreatment NLR with not only survival risk but also the efficacy of immunotherapy in patients with advanced cancer. The pooled results indicated that a high NLR before immunotherapy was significantly correlated with poor prognosis and decreased clinical efficacy. Importantly, our study collected available evidence from 120 articles with 17,969 patients, making it the largest and most comprehensive meta-analysis in this field. Overall, combined with previous meta-analyses, more clinical studies are necessary to validate the critical role of the pretreatment NLR in patients with advanced cancer receiving immunotherapy. It is important to determine prognosis and predict biomarkers for immunotherapy efficacy. An elevated pretreatment NLR may be related to clinical outcomes. As a result, it can provide a basis for clinicians to make reasonable medical decisions. This meta-analysis proposes NLR as a new prognostic indicator for patients receiving immunotherapy. The strengths of this meta-analysis include the following: (1) Despite significant heterogeneity in the included studies, the strength of the pooled effect size was large, and NLR was significantly associated with ORR, DCR, OS, and PFS. (2) The included studies were of medium or high quality. Methodological quality evaluation revealed that although the included studies had a certain degree of recall bias and confounding bias, the control for measurement, selection, and withdrawal biases was reasonable. (3) The results of statistical analyses, such as the publication bias test, one-by-one exclusion method, and trim-and-fill method, suggest that the pooled results were reliable and stable.
This study had some limitations. First, the heterogeneity was statistically significant, and no significant source of heterogeneity was identified through subgroup analysis. Heterogeneity may result from clinical and methodological heterogeneity among the studies. Unfortunately, the descriptions of this information in the included studies were not comprehensive or uniform, and their impact on heterogeneity could not be explored quantitatively. Second, the results of the meta-analysis were mostly based on univariate analyses. Although some studies reported no significant differences in age, sex, and earlier treatment regimens, the possibility of incorrect estimation, which exaggerated the association among NLR, ORR, and DCR, could not be ruled out. Additionally, for certain cancer types, there were fewer included studies, and more studies with larger sample sizes are necessary to verify the results. The grouping thresholds of the NLR were inconsistent; therefore, it is recommended to explore and formulate a unified grouping threshold in future studies to facilitate better extrapolation of the research results. Finally, we did not register the protocol for this study. The protocol register may minimize the reporting bias and reduce unplanned duplication172. Thus, we will pay attention to the protocol register in the following review and meta-analysis.
In summary, this meta-analysis indicated that an elevated NLR before immunotherapy was significantly associated with the prognosis of patients with advanced cancer. Pretreatment NLR may be served as a promising marker for immunotherapy efficacy.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Siegel, R. L. et al. Cancer statistics, 2021. CA Cancer J. Clin. 71(1), 7–33 (2021)
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 20(11), 651–668 (2020).
Jiang, T. & Zhou, C. The past, present and future of immunotherapy against tumor. Transl Lung Cancer Res. 4(3), 253–264 (2015).
Nishino, M. et al. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 14(11), 655–668 (2017).
Carretero-González, A. et al. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: A meta-analysis of randomized clinical trials. Oncotarget 9(9), 8706–8715 (2018).
Walk, E. E. et al. The cancer immunotherapy biomarker testing landscape. Arch. Pathol. Lab. Med. 144(6), 706–724 (2020).
Anagnostou, V. et al. Multimodal genomic features predict outcome of immune checkpoint blockade in non-small-cell lung cancer. Nat. Cancer. 1(1), 99–111 (2020).
Savic Prince, S. & Bubendorf, L. Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch. 474(4), 475–484 (2019).
Valero, C. et al. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer 126(5), 994–1003 (2020).
Feng, J. et al. Clinical and prognostic value of neutrophil-lymphocyte ratio for patients with thyroid cancer: A meta-analysis. Medicine 99(20), 0000000000019686 (2020).
Viñal, D. et al. Prognostic value of neutrophil-to-lymphocyte ratio and other inflammatory markers in patients with high-risk soft tissue sarcomas. Clin. Transl Oncol. 22(10), 1849–1856 (2020).
Balta, S. et al. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br. J. Cancer. 109(12), 3125–3126 (2013).
Buonacera, A. et al. Neutrophil to lymphocyte ratio: An emerging marker of the relationships between the immune system and diseases. Int. J. Mol. Sci. 23(7), 3636 (2022).
Ferrucci, P. F. et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 27(4), 732–738 (2016).
Kuzman, J. A. et al. Neutrophil-lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma. BMC Urol. 17(1), 016–0192 (2017).
Alessi, J. V. et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J. Immunother Cancer. 9(11), 2021–003536 (2021).
Kobayashi, T. et al. Pre-pembrolizumab neutrophil-to-lymphocyte Ratio (NLR) Predicts the Efficacy of second-line Pembrolizumab Treatment in Urothelial cancer Regardless of the pre-chemo NLR 1–11 (Cancer Immunology, 2022).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 106(6) (2014).
Jiang, T. et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: A meta-analysis. Cancer Immunol. Immunother. 67(5), 713–727 (2018).
Mei, Z. et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 58, 1–13 (2017).
Yang, M. et al. AIM2 deficiency in B cells ameliorates systemic lupus erythematosus by regulating Blimp-1–Bcl-6 axis-mediated B-cell differentiation. Signal. Transduct. Target. Ther. 6(1), 341 (2021).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336(7650), 924–926 (2008).
Balatoni, T. et al. Biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Pathol. Oncol. Res. 26, 317–325 (2020).
Descourt, R. et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score ≥ 50%) advanced non-small cell lung cancer in the real world: Impact in brain metastasis—A national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol. Immunother. 72(1), 91–99 (2023).
Higgins, J. P. et al. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003).
Aurelio, T. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Tech. Bull., 8(47) (1999).
Egger, M. et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2), 455–463 (2015).
Akyildiz, A. et al. Real-world evaluation of nivolumab in patients with non-nasopharyngeal recurrent or metastatic head and neck cancer: A retrospective multi-center study by the Turkish Oncology Group (TOG). Eur. Arch. Otorhinolaryngol. 281(9), 4991–4999 (2024).
Asano, Y. et al. Novel predictors of immune checkpoint inhibitor response and prognosis in advanced non-small-cell lung cancer with bone metastasis. Cancer Med. (2023).
Ascierto, P. A. et al. Proteomic test for anti-PD-1 checkpoint blockade treatment of metastatic melanoma with and without BRAF mutations. J. Immunother Cancer 7(1), 91 (2019).
Aslan, V. et al. Cachexia index in predicting outcomes among patients receiving immune checkpoint inhibitor treatment for metastatic renal cell carcinoma. Urol. Oncol. 40(11), 494.e1–494.e10 (2022).
Ayers, K. L. et al. A composite biomarker of neutrophil-lymphocyte ratio and hemoglobin level correlates with clinical response to PD-1 and PD-L1 inhibitors in advanced non-small cell lung cancers. BMC Cancer 21(1), 441 (2021).
Bagley, S. J. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 106, 1–7 (2017).
Balatoni, T. et al. Biomarkers associated with clinical outcome of advanced melanoma patients treated with Ipilimumab. Pathol. Oncol. Res. 26(1), 317–325 (2020).
Bamias, A. et al. New prognostic model in patients with advanced urothelial carcinoma treated with second-line immune checkpoint inhibitors. J. Immunother Cancer 11(1) (2023).
Banna, G. L. et al. Neutrophil-to-lymphocyte ratio and lactate dehydrogenase as biomarkers for urothelial cancer treated with immunotherapy. Clin. Transl Oncol. 22(11), 2130–2135 (2020).
Bartlett, E. K. et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 126(1), 76–85 (2020).
Bilen, M. A. et al. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with Nivolumab. Clin. Genitourin. Cancer 16(3), e563–e575 (2018).
Booka, E. et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. 42(6), 2977–2987 (2022).
Capone, M. et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 6(1), 74 (2018).
Chang, L. et al. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J. Immunother. 45(9), 415–422 (2022).
Chen, S. et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Transl Lung Cancer Res. 10(3), 1397–1407 (2021).
Chikuie, N. et al. Baseline neutrophil-to-lymphocyte ratio and glasgow prognostic score are associated with clinical outcome in patients with recurrent or metastatic head and neck squamous cell carcinoma treated with nivolumab. Acta Med. Okayama. 75(3), 335–343 (2021).
Choi, W. M. et al. Kinetics of the neutrophil-lymphocyte ratio during PD-1 inhibition as a prognostic factor in advanced hepatocellular carcinoma. Liver Int. 41(9), 2189–2199 (2021).
Choucair, K. et al. Characterization of age-associated, neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammatory index (SII) as biomarkers of inflammation in geriatric patients with cancer treated with immune checkpoint inhibitors: Impact on efficacy and survival. Cancers (Basel) 15(20) (2023).
Chuma, M. et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: A multicenter analysis. Hepatol. Res. 52(3), 269–280 (2022).
De Giorgi, U. et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin. Cancer Res. 25(13), 3839–3846 (2019).
Diem, S. et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 111, 176–181 (2017).
Dusselier, M. et al. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS One 14(7), e0219060 (2019).
Eso, Y. et al. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr. Oncol. 28(5), 4157–4166 (2021).
Facchinetti, F. et al. Clinical and hematologic parameters address the outcomes of non-small-cell lung cancer patients treated with nivolumab. Immunotherapy 10(8), 681–694 (2018).
Fan, X. et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving Anti-PD-1 therapy. Front. Cell. Dev. Biol. 9, 638312 (2021).
Ferrucci, P. F. et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br. J. Cancer 112(12), 1904–1910 (2015).
Fukui, T. et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: A prospective observational study. Clin. Lung Cancer 20(3), 208–214e2 (2019).
Gou, M. et al. Neutrophil-to-lymphocyte ratio (NLR) predicts PD-1 inhibitor survival in patients with metastatic gastric Cancer. J. Immunol. Res. 2021, 2549295 (2021).
Guo, J. C. et al. Neutrophil-to-lymphocyte ratio and use of antibiotics associated with prognosis in esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitors. Anticancer Res. 39(10), 5675–5682 (2019).
Guven, D. C. et al. The association between early changes in neutrophil-lymphocyte ratio and survival in patients treated with immunotherapy. J. Clin. Med. 11(15) (2022).
Hamai, Y. et al. Ability of blood cell parameters to predict clinical outcomes of nivolumab monotherapy in advanced esophageal squamous cell carcinoma. Onco Targets Ther. 16, 263–273 (2023).
Hasegawa, T. et al. Association of high neutrophil-to-lymphocyte ratio with poor outcomes of pembrolizumab therapy in high-PD-L1-expressing non-small cell lung cancer. Anticancer Res. 39(12), 6851–6857 (2019).
Ho, W. J. et al. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J. Immunother Cancer 6(1), 84 (2018).
Hong, J. Y. et al. Hepatocellular carcinoma patients with high circulating cytotoxic T cells and intra-tumoral immune signature benefit from pembrolizumab: Results from a single-arm phase 2 trial. Genome Med. 14(1), 1 (2022).
Ikoma, T. et al. Inflammatory prognostic factors in advanced or recurrent esophageal squamous cell carcinoma treated with nivolumab. Cancer Immunol. Immunother. 72(2), 427–435 (2023).
Incorvaia, L. et al. Body mass index and baseline platelet count as predictive factors in Merkel cell carcinoma patients treated with avelumab. Front. Oncol. 13, 1141500 (2023).
Inoue, H. et al. Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol. Lett. 24(2), 257 (2022).
Ishihara, H. et al. Predictive impact of peripheral blood markers and C-reactive protein in nivolumab therapy for metastatic renal cell carcinoma. Target. Oncol. 14(4), 453–463 (2019).
Jeyakumar, G. et al. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J. Immunother Cancer. 5(1), 82 (2017).
Jung, M. et al. Ipilimumab real-world efficacy and safety in korean Melanoma patients from the Korean named-patient program cohort. Cancer Res. Treat. 49(1), 44–53 (2017).
Katayama, Y. et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci. Rep. 10(1), 17495 (2020).
Khunger, M. et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS One 13(10), e0197743 (2018).
Kim, C. G. et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 74(2), 350–359 (2021).
Kim, H. S. et al. The presence and size of intrahepatic tumors determine the therapeutic efficacy of nivolumab in advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 14, 17588359221113266 (2022).
Kim, J. H. et al. Real-world efficacy data and predictive clinical parameters for treatment outcomes in advanced esophageal squamous cell carcinoma treated with immune checkpoint inhibitors. Cancer Res. Treat. 54(2), 505–516 (2022).
Kim, N. et al. Prognostic impact of sarcopenia and radiotherapy in patients with advanced gastric cancer treated with anti-PD-1 antibody. Front. Immunol. 12, 701668 (2021).
Kita, Y. et al. Tolerability and treatment outcome of pembrolizumab in patients with advanced urothelial carcinoma and severe renal dysfunction. Urol. Oncol. 40(9), 410e11–410e18 (2022).
Kobayashi, T. et al. Pre-pembrolizumab neutrophil-to-lymphocyte ratio (NLR) predicts the efficacy of second-line pembrolizumab treatment in urothelial cancer regardless of the pre-chemo NLR. Cancer Immunol. Immunother. 71(2), 461–471 (2022).
Ksienski, D. et al. Immune related adverse events and treatment discontinuation in patients older and younger than 75 years with advanced melanoma receiving nivolumab or pembrolizumab. J. Geriatr. Oncol. 13(2), 220–227 (2022).
Ksienski, D. et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res. 10(1), 355–367 (2021).
Lee, J. et al. Comprehensive molecular and clinical characterization of Asian melanoma patients treated with anti-PD-1 antibody. BMC Cancer 19(1), 805 (2019).
Lee, Y. G. et al. Outcomes and biomarkers of Immune checkpoint inhibitor therapy in patients with refractory Head and Neck squamous cell carcinoma: KCSG HN18-12. Cancer Res. Treat. 53(3), 671–677 (2021).
Liu, J. et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 33(8), e22964 (2019).
Liu, S., Zhao, L. & Zhou, G. Peripheral blood markers predict immunotherapeutic efficacy in patients with advanced non-small cell lung cancer: A multicenter study. Front. Genet. 13, 1016085 (2022).
Lu, X., Wan, J. & Shi, H. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios are associated with the efficacy of immunotherapy in stage III/IV non-small cell lung cancer. Oncol. Lett. 24(2), 266 (2022).
Macia-Rivas, L. et al. Real-world data study of the efficacy and toxicity of nivolumab vs. cetuximab and predictors of response to nivolumab in recurrent/metastatic squamous cell carcinoma of the head and neck in a European population. Anticancer Res. 43(4), 1681–1688 (2023).
Matsubara, T. et al. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J. Thorac. Dis. 12(4), 1520–1528 (2020).
Matsuki, T. et al. Hematological predictive markers for recurrent or metastatic squamous cell carcinomas of the head and neck treated with nivolumab: A multicenter study of 88 patients. Cancer Med. 9(14), 5015–5024 (2020).
Matsumura, S. et al. Potential of nutritional markers as predictors after immunotherapy in advanced head and neck squamous cell carcinoma. Anticancer Res. 44(9), 4049–4056 (2024).
Matsuo, M. et al. Inflammation-based prognostic score as a prognostic biomarker in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with nivolumab therapy. In Vivo 36(2), 907–917 (2022).
Morita, M. et al. Immunological Microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer 10(4), 380–393 (2021).
Musaelyan, A. A. et al. Inflammatory and autoimmune predictive markers of response to anti-PD-1/PD-L1 therapy in NSCLC and melanoma. Exp. Ther. Med. 24(3), 557 (2022).
Musaelyan, A. A. et al. Clinical predictors of response to single–agent immune checkpoint inhibitors in chemotherapy–pretreated non–small cell lung cancer. Mol. Clin. Oncol. 20(4), 32 (2024).
Nakamoto, S. et al. Systemic immunity markers are associated with clinical outcomes of atezolizumab treatment in patients with triple-negative advanced breast cancer: A retrospective multicenter observational study. Clin. Exp. Med. 23(8), 5129–5138 (2023).
Nakaya, A. et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int. J. Clin. Oncol. 23(4), 634–640 (2018).
Namikawa, T. et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg. Today 50(11), 1486–1495 (2020).
Nenclares, P. et al. On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J. Immunother. Cancer 9(6) (2021).
Nishiyama, N. et al. The neutrophil-lymphocyte ratio has a role in predicting the effectiveness of nivolumab in Japanese patients with metastatic renal cell carcinoma: A multi-institutional retrospective study. BMC Urol. 20(1), 110 (2020).
Numakura, K. et al. The lymphocyte-to-monocyte ratio as a significant inflammatory marker associated with survival of patients with metastatic renal cell carcinoma treated using nivolumab plus ipilimumab therapy. Int. J. Clin. Oncol. 29(7), 1019–1026 (2024).
Ogata, T. et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: A multicenter retrospective study. Oncotarget 9(77), 34520–34527 (2018).
Ogihara, K. et al. The pretreatment neutrophil-to-lymphocyte ratio is a novel biomarker for predicting clinical responses to pembrolizumab in platinum-resistant metastatic urothelial carcinoma patients. Urol. Oncol. 38(6), 602.e1–602.e10 (2020).
Ota, Y. et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother. Pharmacol. 85(2), 265–272 (2020).
Park, J. H. et al. Prognostic model in patients with metastatic urothelial carcinoma receiving immune checkpoint inhibitors after platinum failure. Curr. Probl. Cancer 46(3), 100848 (2022).
Park, W. et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin. Lung Cancer 19(3), 280–288 (2018).
Pavan, A. et al. Peripheral blood markers identify risk of Immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist 24(8), 1128–1136 (2019).
Peng, L. et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 69(9), 1813–1822 (2020).
Petrova, M. P. et al. Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non-small cell lung cancer. Biosci. Trends. 14(1), 48–55 (2020).
Pozorski, V. et al. Neutrophil-to-eosinophil ratio as a biomarker for clinical outcomes in advanced stage melanoma patients treated with anti-PD-1 therapy. Pigment Cell. Melanoma Res. 36(6), 501–511 (2023).
Prelaj, A. et al. EPSILoN: A prognostic score for immunotherapy in advanced non-small-cell lung cancer—A validation cohort. Cancers (Basel) 11(12) (2019).
Prelaj, A. et al. EPSILoN: A prognostic score using clinical and blood biomarkers in advanced non-small-cell lung cancer treated with immunotherapy. Clin. Lung Cancer 21(4), 365–377 (2020).
Pu, D. et al. Inflammation-nutritional markers of peripheral blood could predict survival in advanced non-small-cell lung cancer patients treated with PD-1 inhibitors. Thorac. Cancer 12(21), 2914–2923 (2021).
Qu, Z. et al. The effect of inflammatory markers on the survival of advanced gastric cancer patients who underwent anti-programmed death 1 therapy. Front. Oncol. 12, 783197 (2022).
Rapoport, B.L. et al. Prognostic significance of the neutrophil/lymphocyte ratio in patients undergoing treatment with nivolumab for recurrent non-small-cell lung cancer. Lung Cancer Manag. 9(3), pLmt37 (2020).
Rebuzzi, S. E. et al. Validation of the meet-URO score in patients with metastatic renal cell carcinoma receiving first-line nivolumab and ipilimumab in the Italian expanded access program. ESMO Open. 7(6), 100634 (2022).
Rebuzzi, S. E. et al. The prognostic value of baseline and early variations of peripheral blood inflammatory ratios and their cellular components in patients with metastatic renal cell carcinoma treated with nivolumab: The ∆-meet-URO analysis. Front. Oncol. 12, 955501 (2022).
Romano, F. J. et al. Neutrophil-to-lymphocyte ratio is a major prognostic factor in non-small cell lung carcinoma patients undergoing first line immunotherapy with pembrolizumab. Cancer Diagn. Progn. 3(1), 44–52 (2023).
Rossi, S. et al. Neutrophil and lymphocyte blood count as potential predictive indicators of nivolumab efficacy in metastatic non-small-cell lung cancer. Immunotherapy 12(10), 715–724 (2020).
Rounis, K. et al. Prediction of outcome in patients with non-small cell lung cancer treated with second line PD-1/PDL-1 inhibitors based on clinical parameters: Results from a prospective, single institution study. PLoS One. 16(6), e0252537 (2021).
Ruan, D. Y. et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol. Rep. (Oxf.) 9(6), 560–570 (2021).
Russo, A. et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): A large retrospective multicenter study. Adv. Ther. 37(3), 1145–1155 (2020).
Sakai, D. et al. Real-world effectiveness of third- or later-line treatment in Japanese patients with HER2-positive, unresectable, recurrent or metastatic gastric cancer: A retrospective observational study. Int. J. Clin. Oncol. 27(7), 1154–1163 (2022).
Shijubou, N. et al. Immunological and nutritional predictive factors in patients receiving pembrolizumab for the first-line treatment of non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 148(8), 1893–1901 (2022).
Shiroyama, T. et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 7(1), 13–20 (2018).
Simonaggio, A. et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol. Immunother. 69(12), 2513–2522 (2020).
Song, P. et al. NLCIPS: Non-small cell lung cancer immunotherapy prognosis score. Cancer Manag. Res. 12, 5975–5985 (2020).
Soyano, A. E. et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J. Immunother. Cancer 6(1), 129 (2018).
Stares, M. et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open. 7(2), 100445 (2022).
Stratmann, J. A. et al. Clinical predictors of survival in patients with relapsed/refractory small-cell lung cancer treated with checkpoint inhibitors: a German multicentric real-world analysis. Ther. Adv. Med. Oncol. 14, 17588359221097191 (2022).
Sue, M. et al. Impact of nutritional status on neutrophil-to-lymphocyte ratio as a predictor of efficacy and adverse events of immune check-point inhibitors. Cancers (Basel) 16(10) (2024).
Suzuki, K. et al. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int. J. Clin. Oncol. 25(1), 135–144 (2020).
Takada, K. et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer. 145, 18–26 (2020).
Takeda, T. et al. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac. Cancer 9(10), 1291–1299 (2018).
Takegawa, N. et al. The impact of nutritional status in nivolumab-treated patients with advanced esophageal cancer. PLoS One 18(5), e0285365 (2023).
Tanaka, K. et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy-the Asahikawa Gastric Cancer Cohort Study (AGCC). Cancer Med. 11(2), 406–416 (2022).
Tanoue, K. et al. Predictive impact of C-reactive protein to albumin ratio for recurrent or metastatic head and neck squamous cell carcinoma receiving nivolumab. Sci. Rep. 11(1), 2741 (2021).
Tokumaru, S. et al. Lymphocyte-to-monocyte ratio is a predictive biomarker of response to treatment with nivolumab for gastric cancer. Oncology 99(10), 632–640 (2021).
Tsutsumida, A. et al. Japanese real-world study of sequential nivolumab and ipilimumab treament in melanoma. J. Dermatol. 46(11), 947–955 (2019).
Tucker, M. D. et al. Association of baseline neutrophil-to-eosinophil ratio with response to nivolumab plus ipilimumab in patients with metastatic renal cell carcinoma. Biomark. Res. 9(1), 80 (2021).
Tural, D. et al. Prognostic factors in patients with metastatic urothelial carcinoma who have treated with Atezolizumab. Int. J. Clin. Oncol. 26(8), 1506–1513 (2021).
Uchimoto, T. et al. Risk classification for overall survival by the neutrophil-lymphocyte ratio and the number of metastatic sites in patients treated with Pembrolizumab-A Multicenter Collaborative Study in Japan. Cancers (Basel) 13(14) (2021).
Ueda, T. et al. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Otolaryngol. 140(2), 181–187 (2020).
Ulas, A., Temel, B. & Kos, F. T. Comparison of prognostic values of seven immune indexes in advanced non-small-cell lung cancer treated with nivolumab: How effective can they be regarding our treatment decisions? Medicina (Kaunas) 60(11) (2024).
Valero, C. et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 12(1), 729 (2021).
Viñal, D. et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin. Transl Oncol. 23(6), 1185–1192 (2021).
Wang, J. H. et al. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancers (Basel) 14(2) (2022).
Wang, L. et al. Prognostic and predictive impact of neutrophil-to-lymphocyte ratio and HLA-I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac. Cancer (2022).
Wang, Y. et al. Assessment of Sarcopenia as a predictor of poor overall survival for advanced non-small-cell lung cancer patients receiving salvage anti-PD-1 immunotherapy. Ann. Transl Med. 9(24), 1801 (2021).
Yamada, T. et al. Impact of the neutrophil-to-lymphocyte ratio on the survival of patients with gastric cancer treated with nivolumab monotherapy. Target. Oncol. 15(3), 317–325 (2020).
Yanazume, S. et al. Potential prognostic predictors of treatment with immune checkpoint inhibitors for advanced endometrial cancer. Jpn. J. Clin. Oncol. (2024).
Yang, K. et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: A multicenter study. Cancer Immunol. Immunother (2021).
Yasumatsu, R. et al. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 41(8), 2610–2618 (2019).
Zahoor, H. et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J. Immunother Cancer 6(1), 107 (2018).
Zaragoza, J. et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br. J. Dermatol. 174(1), 146–151 (2016).
Zer, A. et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 Axis inhibitors in patients with advanced non-small-cell lung cancer. Clin. Lung Cancer 19(5), 426–434 (2018).
Zhang, Y. et al. C-reactive protein levels predict responses to PD-1 inhibitors in hepatocellular carcinoma patients. Front. Immunol. 13, 808101 (2022).
Zhang, Z. et al. Peripheral blood biomarkers predictive of efficacy outcome and immune-related adverse events in advanced gastrointestinal cancers treated with checkpoint inhibitors. Cancers (Basel), 14(15) (2022).
Zhao, Q. et al. Three models that predict the efficacy of immunotherapy in Chinese patients with advanced non-small cell lung cancer. Cancer Med. 10(18), 6291–6303 (2021).
Zhu, Z. F. et al. Predictive role of the monocyte-to-lymphocyte ratio in advanced hepatocellular carcinoma patients receiving anti-PD-1 therapy. Transl Cancer Res. 11(1), 160–170 (2022).
Abuelbeh, I. et al. The predictive value of Peripheral Immune Cell counts for the Presence of Brain metastases in Stage IV Non-small-cell Lung Cancer (NSCLC). Avicenna J. Med. 12(2), 67–72 (2022).
Zhao, N. et al. After treatment with methylene blue is effective against delayed encephalopathy after acute carbon monoxide poisoning. Basic Clin. Pharmacol. Toxicol. 122(5), 470–480 (2018).
Liu, J. et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. 33(8), e22964 (2019).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140(6), 883–899 (2010).
Hussain, S. P. & Harris, C. C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer. 121(11), 2373–2380 (2007).
Elinav, E. et al. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 13(11), 759–771 (2013).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566(7745), 553–557 (2019).
Zhang, D. et al. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: A mechanism by which tumour cells escape immune surveillance. Clin. Immunol. 175, 34–40 (2017).
Gooden, M. J. et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 105(1), 93–103 (2011).
Jiang, T. et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: A meta-analysis. 67(5), 713–727 (2018).
Marchioni, M. et al. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: A systematic review. Clin. Genitourin. Cancer 14(6), 473–484 (2016).
Hu, X. et al. Neutrophil-to-lymphocyte and hypopharyngeal cancer prognosis: System review and meta-analysis. Head Neck 45(2), 492–502 (2023).
Rodrigo, J. P. et al. Neutrophil to lymphocyte ratio in oropharyngeal squamous cell carcinoma: A systematic review and Meta-analysis. Cancers 15(3) (2023).
Li, L. L. & Pan, L. S. Prognostic value of neutrophil-to-lymphocyte ratio in gastric cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Kaohsiung J. Med. Sci. 39(8), 842–852 (2023).
Zhang, W. et al. Neutrophil to lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 14, 1234142 (2023).
Tsujimoto, Y. et al. Majority of systematic reviews published in high-impact journals neglected to register the protocols: A meta-epidemiological study. J. Clin. Epidemiol. 84, 54–60 (2017).
Acknowledgements
This study was supported by the Natural Science Foundation of Hunan Province National Health Commission, No. B202303027655; Natural Science Foundation of Changsha Science and Technology Bureau, No. Kq2208150; Wu Jieping Foundation of China, No. 320.6750.2022-22-59, 320.6750.2022-17-41; Guangdong Association of Clinical Trials (GACT)/Chinese Thoracic Oncology Group (CTONG); and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer, No. 2017B030314120.
Author information
Authors and Affiliations
Contributions
Author Contributions: S.T. and Y.L. contribute to manuscript preparation. L.Z.and J.S. contribute to the study design, manuscript writing. T.C. and Y.L. contribute to data statistics analyze. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, J., Li, Y., Tan, S. et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with immunotherapy efficacy in patients with advanced cancer: a systematic review and meta-analysis. Sci Rep 15, 446 (2025). https://doi.org/10.1038/s41598-024-84890-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84890-3