Abstract
This study aims to evaluate the prevalence of undiagnosed hepatitis delta in southern Spain (Andalusia) and assess the effectiveness and cost-efficiency of implementing reflex testing for hepatitis D detection in HBsAg-positive patients. A multicenter ambispective study was conducted in 17 Andalusian hospitals. The retrospective phase (January 2018–June 2022) analyzed diagnostic processes for hepatitis delta in HBsAg-positive patients. The prospective phase (October 2022–March 2023) implemented reflex testing, performing anti-HDV serology on all HBsAg-positive patients without prior testing. HDV RNA testing followed for those who tested anti-HDV-positive. In the retrospective phase, out of 18,583 HBsAg-positive patients, anti-HDV tests were performed on 3,436 (18%), identifying 205 (6%) positive cases. HDV RNA was tested in 158 (77%) anti-HDV-positive patients, with 69 (44%) testing positive. In the prospective phase, out of 2,384 HBsAg-positive patients without prior anti-HDV testing, 2,293 (96%) were tested, identifying 109 (4.7%) positive cases. HDV RNA was analyzed in 97 (89%) anti-HDV-positive patients, with 30 (31%) testing positive. Reflex testing increased anti-HDV detection by 77%, resulting in a fourfold increase in detecting anti-HDV-positive patients and a threefold increase in detecting HDV RNA-positive patients, reducing undiagnosed HDV RNA-positive cases to 4% compared to 45% with clinical practice. Cost analysis indicated a saving of €265,954 with reflex testing. Reflex testing improves HDV detection, reduces costs, and simplifies diagnosis, making it an efficient strategy for managing chronic hepatitis D patients.
Similar content being viewed by others
Introduction
Infection with the Hepatitis Delta Virus (HDV) requires co-infection with the Hepatitis B Virus (HBV)1. Although the epidemiology of HDV still needs in-depth study, it is estimated that there are between 15 and 20 million infected people worldwide2. Chronic hepatitis D is considered the most severe form of viral hepatitis due to the rapid progression of patients to liver cirrhosis3,4,5,6, hepatocellular carcinoma (HCC)4,6, and ultimately high mortality5,6,7. Approximately 49% of patients develop cirrhosis, of which 13% experience decompensation and 2.5% develop liver cancer8. Moreover, due to these complications, patients utilize more healthcare resources, generating higher costs compared to those with HBV mono-infection9.
These circumstances highlight the need for early and accurate diagnosis of the infection to refer patients to specialists and initiate treatment10. With the recent introduction of bulevirtide, the first antiviral for hepatitis delta treatment, viral load levels have been reduced to almost undetectable, translating to slower disease progression11. This represents a clinical advancement towards the elimination of viral hepatitis12.
In this context, scientific societies differ in their recommendations for HDV testing. The European Association for the Study of the Liver (EASL)13, the Spanish Association for the Study of the Liver (AEEH)14, and the Asian Pacific Association for the Study of the Liver (APASL)15 recommend anti-HDV serology for all HBsAg-positive patients at least once in their lifetime, regardless of risk factors. However, the American Association for the Study of Liver Diseases (AASLD)16 only recommends anti-HDV antibody testing for HBsAg-positive patients with risk factors such as intravenous drug use and migration from endemic areas. Despite these recommendations, HDV infection is underdiagnosed. Preliminary studies conducted in Spain estimate that only 18% of HBsAg-positive patients are tested for HDV17, and of those who test positive, only 46.5% have their viral load measured18.
To address diagnosis, scientific societies urge the use of reflex testing strategies. In this approach, for any HBsAg-positive case, the same blood sample is used to perform anti-HDV serology, and if positive, an HDV RNA test is conducted19. Reflex testing strategies have proven effective in detecting viral hepatitis, including hepatitis D, identifying more infections than multiple blood draws, as typically done in clinical practice10,20,21. Consequently, this increase in diagnosis, along with access to treatment, reduces the cost of the disease22.
Given these circumstances, this study aimed to evaluate the prevalence of undiagnosed hepatitis delta in southern Spain (Andalusia) and estimate the effectiveness of implementing reflex testing for the detection of hepatitis D in HBsAg-positive patients, as well as the costs of this diagnostic strategy to assess its efficiency.
Methods
Descriptive
A multicenter ambispective (retrospective and prospective) study was conducted in 17 hospitals in Andalusia, with almost full coverage of the 9 million population in our region. In the retrospective phase, through the Laboratory Information Systems of the participating centers, a review was carried out, analyzing the diagnostic process of hepatitis delta from January 2018 to June 2022, focusing on HBsAg-positive patients. During this review, HBsAg-positive cases with anti-HDV antibody tests and those with positive anti-HDV tests were identified and tested for HDV RNA. The prospective phase started in October 2022 and continued until March 2023, during which all participating centers implemented reflex testing for hepatitis delta. This reflex test involved performing anti-HDV serology on all HBsAg-positive patients without prior testing and subsequently, if the patient was anti-HDV positive, conducting an HDV RNA test.
Economic evaluation
The strategies compared in the analysis were the implementation of double reflex HDV testing versus non-implementation (clinical practice). To evaluate the diagnostic processes with each strategy, two decision trees were developed simulating the diagnostic care cascade (Figure S1 and Figure S2). In the double reflex testing strategy, HDV RNA testing was performed on anti-HDV-positive patients using the same blood sample, and they were scheduled for consultation to receive the results. In the clinical practice strategy, patients with anti-HDV tests attended a consultation to receive the results, and if positive, HDV RNA testing was requested. After the test, they attended a consultation to receive the results (Fig. 1). The distribution of patients in each care pathway was obtained from the study, starting with a total of 18,583 HBsAg-positive patients in both strategies (Table 1).
The analysis was conducted from the perspective of the Andalusian Public Health System (SSPA). Only direct healthcare costs related to the detection and diagnosis of the disease (anti-HDV serology, HDV RNA test, and specialist visits) were included, excluding treatment costs.
Results were presented by comparing the number of anti-HDV positive and HDV-RNA positive patients detected with each strategy, the number of patients lost to follow-up, the cost of diagnosis, and the cost per HDV RNA-diagnosed patient.
Two sensitivity analyses were performed to evaluate the uncertainty of same parameters of the model. The first equated the prevalence percentages of the retrospective phase in both strategies, and the second equated these percentages to those of the prospective phase.
Results
Descriptive
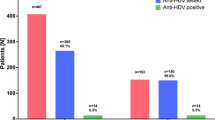
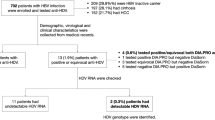
In the retrospective phase, out of a total of 18,583 HBsAg-positive patients, anti-HDV tests were performed on 3,436 (18%), identifying 205 (6%) positive cases. Of the anti-HDV-positive patients, HDV RNA was tested in 158 (77%), of which 69 (44%) were positive, establishing the prevalence of chronic HDV infection at 1.9% (69/3436) of HBsAg-positive patients (Figure S1).
In the prospective phase, of the 3,370 HBsAg-positive patients, 986 (29%) had previously undergone anti-HDV tests. With the implementation of reflex testing, out of 2,384 patients without anti-HDV determination, 2,293 (96%) were tested, with 109 (4.7%) testing positive. Of these, HDV RNA was analyzed in 97 (89%) patients, with 30 (31%) testing positive. The prevalence of viremia among HBsAg-positive patients was 1.3% (30/2293).
Comparing the retrospective and prospective phases, anti-HDV testing in HBsAg-positive patients increased by 77% (19% vs 96%). This resulted in a fourfold increase in the detection of anti-HDV-positive patients and a threefold increase in HDV RNA-positive patients. Thus, with the reflex test strategy, nearly 4% of HDV RNA-positive patients would remain undiagnosed, compared to 45% with clinical practice.
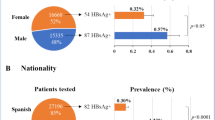
Patients with active hepatitis D virus (HDV) infection were predominantly male (72.5%) with a mean age of 50 years (range: 27–71). A high proportion of them were immigrants 45%, and the most frequent nationalities were Romania and Equatorial Guinea, followed by Morocco, Senegal, Russia, Algeria, among others. A third of the patients had a history of hepatitis C virus (HCV) coinfection, and 20% were coinfected with the human immunodeficiency virus (HIV). Additionally, among patients with available data on hepatic decompensation events, 36% developed portal hypertension, 30% had ascites, and 21% experienced hepatic encephalopathy. Furthermore, 14% were diagnosed with hepatocellular carcinoma as a complication of advanced liver disease.
Economic evaluation
Implementing double reflex HDV testing for the 18,583 HBsAg-positive patients would increase anti-HDV tests to 14,441 patients, detecting 643 additional anti-HDV-positive patients and 595 more HDV RNA-positive patients compared to clinical practice. Without reflex testing, 78% (14,441/18,583) would not be screened. Reflex testing would prevent the loss of 75% (595/753) of anti-HDV-positive cases and 71% (169/238) of HDV RNA-positive cases (Fig. 2).
Regarding costs, double reflex HDV testing for the 18,583 HBsAg-positive patients would quadruple diagnostic test-related costs but reduce specialist visit costs by 79%, (€472,392 vs €99,135), resulting in a total cost saving of €265,954 (Fig. 3).
The cost per HBsAg-positive patient undergoing anti-HDV testing with reflex testing is €13 compared to €145 without intervention. Thus, the cost savings per screened anti-HDV-positive patient would be €132 per HBsAg-positive patient.
Additionally, the cost savings associated with each HDV RNA-positive diagnosed patient would be €6,246 (€978 vs €7,225). Consequently, reflex HDV testing for HBsAg-positive patients would be an efficient strategy.
Sensitivity analyses showed that by matching anti-HDV and HDV RNA prevalences increased the detection of chronic hepatitis D patients and reduced cost differences between the strategies, with the double reflex test strategy remaining less costly than clinical practice (Table S1).
Discussion
Hepatitis D is associated with faster liver disease progression than other viral hepatitis. Although clinical guidelines recommend single-step diagnosis for HBsAg-positive cases, we show that it was not implemented in many hospitals in our geographical area during the retrospective phase of our study. The retrospective phase results showed underdiagnosis of HDV, with only a fifth of hepatitis B patients undergoing anti-HDV testing from 2018 to early 2022. This highlights the need to implement these recommendations to identify undiagnosed infected patients. Similarly, the implementation of reflex testing tripled the detection of chronic hepatitis D patients compared to the previous phase. With the reflex test strategy, the percentage of undiagnosed HDV RNA-positive patients is significantly lower than with clinical practice. This further emphasizes the importance of reflex testing in early HDV infection detection, which can have significant implications for the clinical management of co-infected patients.
Our analysis also underscores the need for accurate prevalence data to formulate effective strategies to identify undiagnosed co-infected patients. Comparing our prevalences with those from another Spanish study that also used reflex testing for HBsAg-positive patients reveals differences (6% vs 8.1% retrospective and 4.7% vs 9.6% prospective)20. In the retrospective phase, our study may have underestimated prevalence as delta determination was only included in 18% of HBsAg-positive patients. Likely, testing was only done for patients with HDV risk factors, justifying the difference.
Additionally, comparing our prospective phase results with those in a recently published article using systematic review and meta-analysis methods to estimate HDV prevalence in 25 countries shows significant variability among European countries23. England, France, and Germany reported anti-HDV prevalences between 2.9% and 3.5%, lower than observed in our study. However, the estimated percentages of HDV RNA-positive cases were higher at 50%, 75%, and 60%, respectively. In the same article, anti-HDV prevalence was reported at 8.3% in Italy and 12.6% in Portugal, with adjusted prevalences of 3.4% and 1.5%, both lower than our study. Data on HDV RNA-positive patients were also significantly higher at 61% and 73%. The prevalences found in this study are higher than those adjusted for risk factors such as population, geographic region, or detection methods23,24. Ignoring these factors can lead to overestimating the actual prevalence and distorting the problem’s magnitude, possibly contributing to the decision not to allocate healthcare resources to effective strategies. Comparing prevalences between countries highlights the importance of understanding HDV epidemiology in each context to improve HBV/HDV co-infection diagnosis.
There is a scarcity of studies analyzing the impact before and after implementing reflex testing on HBV/HDV co-infection detection. Comparing the percentages of HBsAg-positive patients undergoing specific HDV tests in our study with those from another Spanish study shows differences in the previous phase (18% vs 7.6%), but alignment with the later phase (96%)20. This study concluded that reflex HDV testing would increase the number of diagnosed Chronic Hepatitis D cases 9 times over eight years22. The difference from our analysis is primarily due to the higher prevalences used and the longer analysis horizon. Another study that evaluated this strategy’s potential benefits based on a review of published epidemiological data in countries with different prevalence levels, including Spain, concluded that it is effective for identifying undiagnosed individuals, especially in countries with low HBsAg prevalence, such as those in the European Union24. Other studies related to hepatitis C also support the strategy’s effectiveness25.
The economic analysis assessed the cost impact associated with the diagnosis of HDV in HBsAg + patients by incorporating the dual reflex test in southern Spain and does not consider the follow-up of the remaining HBsAg + patients. Given the limited amount of information in published literature, this study contributes epidemiological data and demonstrates the efficiency of its implementation, resulting in a significantly lower cost per detected viremic patient than clinical practice. This is due to avoiding unnecessary visits for result confirmation and duplicate tests. These results align with other HDV and HCV studies22,26.
Moreover, implementing double reflex testing for HDV could change the clinical management of patients in the long term, not only in terms of diagnosis but also in resource planning and public health policies. Double reflex testing would allow early identification of HBV/HDV co-infected patients, giving them a treatment opportunity. Previously, patients were treated with Peg-IFN, a treatment with low response rates and many contraindications, but recently, Bulevirtide, a drug with better tolerability and greater effectiveness, has been approved in many countries11. Early treatment prevents the development of severe liver complications and improves patients’ quality of life27. In addition, a modeling showed that reflex HDV testing would reduce cases of cirrhosis and hepatocellular carcinoma and even liver-related mortality, resulting in savings associated with preventing liver complications22. This underscores the need for strategies that promote diagnosis. Although our study did not analyses the impact beyond diagnosis, increasing the number of HDV-RNA-positive patients detected would improve patient health outcomes. Additionally, identifying and treating the infection helps reduce virus transmission, contributing to preventing new infections. These tests are well-accepted by patients28, which could facilitate detection in hard-to-reach at-risk populations, such as drug users and migrants.
Due to the observational nature of our study, its primary limitation lies in its retrospective design, as well as its “localized” scope, given that the data were collected exclusively from Andalusia. This may influence the prevalence rates and costs included in the analysis. However, sensitivity analyses conducted with the different prevalence found in both phases can provide a broader view of the results. Regarding costs, sensitivity analyses were not performed since the actual costs according to the Andalusian Public Health System were included, aligning with our objective. Lastly, regarding the test methodology, it is noteworthy that currently, the diagnosis and monitoring of treatment response are based on HDV RNA detection and quantification. These tests are not yet standardized for HDV, and results vary between laboratories due to different sensitivities and RNA extraction methodologies, making them non-comparable29,30. Therefore, advancing more sensitive and specific HDV RNA quantification methods is necessary to improve co-infection detection and management. Additionally, longitudinal studies are needed to track the progress and outcomes of patients diagnosed using this strategy. In addition, although our study was conducted in Andalusia rather than across the entire country, and some degree of selection bias cannot be completely ruled out, we believe it is unlikely to significantly affect the generalizability of our findings. Andalusia is the most populous autonomous community in Spain, representing approximately 20% of the national population, which supports the representativeness of the studied population within the broader Spanish context.
In conclusions, the prevalences found indicate a high percentage of HDV-infected patients in southern Andalusia and provide valuable information on the burden of HDV co-infection. The introduction of double reflex testing for HDV in HBsAg-positive patients reduces the underdiagnosis of the infection, significantly increasing the detection of hidden chronic hepatitis delta patients. Moreover, it simplifies infection diagnosis and generates savings for the healthcare system, being an efficient strategy that should be considered for the care of chronic hepatitis B patients.
Data availability
The datasets used and/or analyses during the current study are available from the corresponding author on reasonable request.
References
Lucifora, J. & Delphin, M. Current knowledge on Hepatitis Delta Virus replication. Antiviral Res. 179, 104812 (2020).
Organización Mundial de la Salud (OMS). Hepatitis D. Ginebra: OMS. https://www.who.int/es/news-room/fact-sheets/detail/hepatitis-d, (2023).
Brancaccio, G. et al. The present profile of chronic hepatitis B virus infection highlights future challenges: An analysis of the Multicenter Italian MASTER-B cohort. Dig. Liver Dis. 51(3), 438–442 (2019).
Romeo, R. et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 136(5), 1629–1638 (2009).
Roulot, D. et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J. Hepatol. 73(5), 1046–1062 (2020).
Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). (2000).
Béguelin, C. et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J. Hepatol. 66(2), 297–303 (2017).
Wranke, A. et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: The Hepatitis Delta International Network (HDIN). Liver Int. 38(5), 842–850 (2018).
Elsaid, M. I. et al. Economic and Health Care Burdens of Hepatitis Delta: A Study of Commercially Insured Adults in the United States. Hepatology 72(2), 399–411 (2020).
Parfut, A. et al. Impact of anti-HDV reflex testing at HBs antigen positive discovery in a single center France: Support for primary HDV screening in France. J. Clin. Virol. 171, 105650 (2024).
Buti M, Gonzalez A, Riveiro‐Barciela M, Bourliere M. Management of chronic HBV‐HDV patients chronic HBV‐HDV infection: A review on new management options. UEG J., (2023).
Organización Mundial de la Salud (OMS). Estrategia Mundial del Sector de la Salud contra las Hepatitis Víricas 2016–2021: Hacia el fin de las hepatitis víricas. Ginebra: OMS. https://apps.who.int/iris/bitstream/handle/10665/250578/WHO-HIV-2016.06 spa.pdf?sequence=1&isAllowed=y, (2016).
European Association for the Study of the Liver. Electronic address: [email protected], European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. (2017).
Rodríguez, M. et al. Documento de consenso de la Asociación Española para el Estudio del Hígado sobre el tratamiento de la infección por el virus de la hepatitis B (2020). Gastroenterol Hepatol. 43(9), 559–587. https://doi.org/10.1016/j.gastrohep.2020.03.011 (2020).
Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 10(1), 1–98. https://doi.org/10.1007/s12072-015-9675-4 (2016).
Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67(4), 1560–1599 (2018).
Casado Martín M, Camelo Castillo A, Barrera Baena P, Belén Pérez A, Pinazo Bandera JM, García Barrionuevo A, et al. Co-02. Estado actual de la hepatitis delta en andalucía. Implantacion del diagnostico en un solo paso. Revista andaluza de patología digestiva. (2023).
Gómez González I. Prevalencia de la hepatitis D en la Comunidad Valenciana: búsqueda activa de casos (0890). Santiago de Compostela: XXVI Congreso Nacional de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), (2023).
Crespo, J. et al. Recomendaciones para el diagnóstico integral de las hepatitis virales crónicas en una única extracción analítica. Gastroenterol. Hepatol. 46(2), 150–162 (2023).
Palom, A. et al. Implementation of anti-HDV reflex testing among HBsAg-positive individuals increases testing for hepatitis D. JHEP Rep. 4(10), 100547 (2022).
Martínez-Campreciós, J. et al. Reflex viral load testing in dried blood spots generated by plasma separation card allows the screening and diagnosis of chronic viral hepatitis. J. Virol. Methods 289, 114039 (2021).
Buti, M., Domínguez-Hernández, R., Palom, A., Esteban, R. & Casado, M. Á. Impact of hepatitis D reflex testing on the future disease burden: A modelling analysis. Liver Int. 43(12), 2611–2614 (2023).
Polaris Observatory Collaborators. Adjusted estimate of the prevalence of hepatitis delta virus in 25 countries and territories. J Hepatol. 80(2), 232–242. https://doi.org/10.1016/j.jhep.2023.10.043 (2024).
Razavi, H. A. et al. Hepatitis D double reflex testing of all hepatitis B carriers in low-HBV- and high-HBV/HDV-prevalence countries. J. Hepatol. 79(2), 576–580. https://doi.org/10.1016/j.jhep.2023.02.041 (2023).
Casas MDLP, García F, Freyre-Carrillo C, Montiel N, De La Iglesia A, Viciana I, et al. Towards the elimination of hepatitis C: implementation of reflex testing in Andalusia. Rev. Esp Enferm Dig. (2020).
García F, Domínguez-Hernández R, Casado M, Macías J, Téllez F, Pascasio JM, Casado MÁ, Alados JC. The simplification of the diagnosis process of chronic hepatitis C is cost-effective strategy. Enferm Infecc Microbiol Clin (Engl Ed). https://doi.org/10.1016/j.eimc.2019.03.001. (2019).
Buti M, Calleja JL, Rodríguez MÁ, Domínguez-Hernández R, Cantero H, Espinoza N, Casado MÁ. Clinical and economic value of bulevirtide in the treatment of chronic hepatitis D. Gastroenterol Hepatol. https://doi.org/10.1016/j.gastrohep.2024.502241. (2024).
Palom, A. et al. Community Strategy for Hepatitis B, C, and D Screening and Linkage to Care in Mongolians Living in Spain. Viruses 15(7), 1506 (2023).
Wedemeyer, H. et al. HDV RNA Assays Writing Group at the HBV Forum HDV RNA assays: Performance characteristics, clinical utility, and challenges. Hepatology https://doi.org/10.1097/HEP.0000000000000584 (2023).
Umukoro, E., Alukal, J. J., Pak, K. & Gutierrez, J. State of the Art: Test all for Anti-Hepatitis D Virus and Reflex to Hepatitis D Virus RNA Polymerase Chain Reaction Quantification. Clin Liver Dis. 27(4), 937–954. https://doi.org/10.1016/j.cld.2023.05.008 (2023).
Funding
This study was supported in part by Gilead_ISCIII Grant nº 21_00168.
Author information
Authors and Affiliations
Contributions
AF, MEE, AdS, ERE, NM, MM, JCA, JCA, ABP, PBB, TC, ACC, BP, RGG, IV, JMPB, FFS, MdCL, AG, MdCD, CJM, ERA, PC, FFADL, PDP, ADlIS, DP, AS, MALG, MPLG, JSC, CR, FG, CF, GSR, data collection. RDH, data analysis. JMRZ, RDH, MC, FG, article preparation and review.
Corresponding authors
Ethics declarations
Competing interests
RDH is an employee of Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consulting firm specializing in economic evaluation and health outcomes research that has been paid to provide methodological support for the project and for writing the manuscript. FGG has received funding for research contracts, advisory boards, and conference attendance from Abbvie, Gilead, Hologic, Roche, MSD, Qiagen, and Pfizer. MCM has received funding for research contracts, advisory boards, and conference attendance from Abbvie, Gilead, Roche, MSD, and Advanz Pharma. JMRZ has received funding for research contracts, advisory boards, and conference attendance from Abbvie, Gilead, Roche, and MSD.
Ethical approval
We confirm that this study has been approved by the Ethics Committee of Hospital Costa del Sol. All methods were carried out in accordance with relevant guidelines and regulations. Due to the nature of the study, the Clinical Research Ethics Committee granted an exemption from the requirement to obtain informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fuentes, A., Estévez-Escobar, M., De Salazar, A. et al. Double reflex testing improves the efficacy and cost effectiveness of hepatitis delta diagnosis in southern Spain. Sci Rep 15, 15413 (2025). https://doi.org/10.1038/s41598-025-00101-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00101-7