Abstract
Effective prediction of molecular features is crucial for the prognostic assessment of glioma patients. This study aims to develop a nomogram model using fractal analysis and Visually AcceSAble Rembrandt Images (VASARI) features to predict the molecular characteristics of WHO Grade 3–4 diffuse gliomas. Retrospective analysis of clinical data and VASARI features of patients with WHO grade 3–4 diffuse gliomas confirmed by pathology between January 2020 and December 2023 at our institution. Preoperative T1-weighted contrast-enhanced and T2-weighted images were used to delineate the tumor and surrounding edema regions on 3D-Slicer. Fractal dimension (FD) and lacunarity of both the tumor and surrounding edema were extracted using ImageJ software. Univariate and multivariate logistic regression analyses were performed to identify independent predictive factors for the Ki_67 proliferation index (PI), p53, and telomerase reverse transcriptase promoter (TERTp) mutations. Based on these findings, a nomogram prediction model was constructed. Model performance was comprehensively assessed using the receiver operating characteristic curve (ROC), calibration curve (CRC), and decision curve analysis (DCA). Sex, Proportion Enhancing, and Pial invasion were identified as independent predictive factors for the Ki_67 PI. FD of the tumor (FD(T)) was an independent predictor for p53 expression. FD(T), Enhancement Quality, and Definition of the enhancing margin were independent predictors for TERTp mutations. The areas under the ROC for each nomogram model were 0.791, 0.739, and 0.601, respectively. Sensitivities were 68.75%, 78.12%, and 51.43%, and specificities were 81.03%, 64.86%, and 71.00%, respectively. CRC showed a high degree of concordance between predicted probabilities and actual observed values, while DCA demonstrated favorable net benefits for all models. VASARI features and fractal analysis effectively predict the Ki_67 PI, p53, and TERTp mutations in WHO grade 3–4 diffuse gliomas. Furthermore, combining these two approaches enhances the predictive performance for TERTp mutations.
Similar content being viewed by others
Diffuse gliomas originate from glial stem cells or progenitor cells1,2, and are the most common malignant tumors in the adult central nervous system3. According to the 2021 fifth edition of the World Health Organization classification of central nervous system tumors, adult diffuse gliomas are classified into WHO grades 2 to 44. Different grades have different prognostic implications for patients. Grade 2 diffuse gliomas are primarily benign or low-grade malignant tumors, with very low proliferative activity. These tumors can usually be cured through surgical resection alone4. In contrast, grade 3–4 diffuse gliomas are highly malignant, with strong proliferation activity. These tumors often present with early dissemination and metastasis, making complete surgical resection difficult. Additionally, they are prone to recurrence after surgery. Currently, postoperative radiotherapy and chemotherapy are recommended for further precise treatment5.
The Ki_67 proliferation index (PI) is associated with mitosis, the cell cycle, and cellular division activity. It is a commonly used marker that can reflect cell proliferation, tumor differentiation, and patient prognosis6,7, and is now widely used in clinical practice. It is widely used in clinical practice. Telomerase reverse transcriptase (TERT) is an enzyme crucial for maintaining telomeres, the protective caps at the ends of chromosomes8. Telomeres shorten with each cell division, eventually leading to cellular senescence. TERT adds DNA to the ends of telomeres, preventing their shortening and allowing the cell to continue dividing9, making it an important molecular biomarker. TERT promoter (TERTp) mutations are associated with poor prognosis and treatment resistance, making them significant biomarkers in personalized treatment strategies10. p53 is a tumor suppressor gene expression protein that plays a critical role in the human body. It inhibits cancer initiation and progression through multiple mechanisms, including promoting DNA repair and genomic stability, regulating the cell cycle, and inducing apoptosis11,12. Additionally, p53 also regulates cell metabolism, ferroptosis, tumor microenvironment, autophagy and so on, all of which contribute to tumor suppression13. Shi W et al. found that p53 was significantly associated with patient prognosis when investigating survival and prognostic factors in high-grade gliomas14. Therefore, accurately predicting the expression or mutation status of these proteins and genes is critical for developing personalized treatment plans.
Currently, magnetic resonance imaging (MRI) has become an indispensable tool for the diagnosis, treatment, and follow-up of gliomas. With the rapid development of artificial intelligence technology, significant progress has been made in the application of radiomics and deep learning in glioma research. However, the lack of user-friendly software and insufficient interpretability may hinder the widespread clinical application of artificial intelligence in gliomas15. Radiologists assess tumor characteristics through visual evaluation of routine MRI scans. To standardize and systematize the assessment of gliomas, the National Cancer Institute proposed the Visually AcceSAble Rembrandt Images (VASARI) feature set, which is used to evaluate the imaging characteristics of tumors. The VASARI feature set uses standardized terminology to describe common characteristics of primary brain tumors on both pre-contrast and post-contrast MRI scans16. Studies have shown that VASARI features can be used to predict the differential diagnosis of glioblastoma and patient survival rates17,18. Additionally, fractal analysis is a method for quantifying naturally occurring objects with highly complex structures that are difficult to describe using traditional Euclidean geometry19. Fractal dimension (FD) and lacunarity (LAC) are two important parameters in fractal analysis used to describe the complexity and distribution of objects. These parameters have been widely applied in the fields of biology and medicine, particularly in the analysis of central nervous system imaging data, such as grading gliomas20 and exploring their impact on glioma prognosis21,22. Given the clinical value, practicality, reproducibility of the VASARI features and fractal analysis, as well as their accessibility compared to traditional artificial intelligence technologies, this study aims to develop a nomogram using fractal analysis and VASARI features to predict the Ki_67 PI, p53 expression, and TERTp mutation status in WHO grade 3–4 diffuse gliomas. This approach seeks to fill the gap in the current research field and provide clinicians with more accurate and comprehensive treatment information.
Materials and methods
Patients
This study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (Approval No. 2021048). Due to its retrospective nature, the requirement for informed consent was waived by Medical Ethics Committee of Zhongnan Hospital of Wuhan University. The study protocol adhered to the principles of the Declaration of Helsinki, and all procedures conducted complied with the ethical standards established by national and/or institutional research committees.
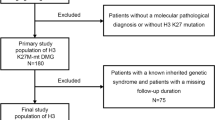
Clinical, pathological, and imaging data from patients diagnosed with WHO grade 3–4 diffuse gliomas, confirmed by pathology between January 2020 and December 2023 at our hospital, were retrospectively collected. Inclusion criteria: (1) Patients diagnosed with grade 3–4 adult diffuse gliomas according to the 2021 WHO classification criteria; (2) Preoperative MRI examination was performed for all patients; (3) No prior intervention for intracranial tumors before diagnosis. Exclusion criteria: (1) Significant image artifacts affecting lesion observation; (2) Tumor lesions too small to delineate. A total of 170 patients with WHO grade 3–4 diffuse gliomas were included, among which 44 patients had grade 3 gliomas (20 male, 24 female, average age 46.48 ± 12.08 years), and 126 patients had grade 4 gliomas (70 male, 56 female, average age 55.82 ± 14.31 years). Details are shown in Fig. 1.
MRI scanning
Patients were scanned supinely using a Siemens Prisma 3.0 T or uMRI 790 3.0T scanner with a phased-array head coil. Initially, non-contrast scans were performed, followed by contrast-enhanced scans. T1-weighted contrast-enhanced (T1C) was performed after the injection of 0.1 mmol/kg Gd-DTPA. Scanning sequences and parameters are detailed in Table 1.
Collection of VASARI features
The VASARI feature set, published by the National Cancer Institute of the United States, consists of 30 quantitative and qualitative imaging features. For this study, 25 commonly used preoperative imaging features were selected for analysis, including tumor ___location (F1), Side of Tumor Epicenter (F2), Eloquent Brain (F3), Enhancement Quality (F4), Proportion Enhancing (F5), Proportion non-enhancement (nCET) (F6), Proportion Necrosis (F7), Cyst(s) (F8), Multifocal or Multicentric (F9), T1/FLAIR RATIO (F10), Thickness of enhancing margin (F11), Definition of the enhancing margin (F12), Definition of the non-enhancing margin (F13), Proportion of Edema (F14), Edema Crosses Midline (F15), Hemorrhage (F16), Diffusion (F17), Pial invasion (F18), Ependymal invasion (F19), Cortical involvement (F20), Deep white matter invasion (F21), nCET tumor Crosses Midline (F22), Enhancing tumor Crosses Midline (F23), Satellites (F24), and Calvarial remodeling (F25). The accurate descriptions of all features can be found in the Cancer Imaging Archive of the National Cancer Institute of the United States, available at the following address: https://wiki.cancerimagingarchive.net/display/Public/VASARI+Research+Project. Two radiologists, each with over five years of experience in brain MRI diagnosis, independently extracted the VASARI features and measured the maximum tumor diameter, while blinded to the patient’s clinical information. When multiple lesions were present, the largest lesion was selected for analysis. In cases of disagreement between the two radiologists, consensus was reached through discussion. If the disagreement persisted, a senior radiologist with over 30 years of diagnostic experience made the final judgment.
Image analysis
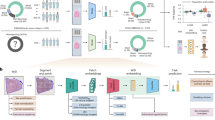
All imaging data from the patients were stored in digital imaging and communication in medicine format, and the lesions were extracted and analyzed. Specifically, the data were imported into the open-source software ITK-SNAP (version 4.2, http://www.itksnap.org/pmwiki/pmwiki.php), where tumor delineation was performed in the axial plane by the two aforementioned radiologists. In cases with multiple lesions, the largest lesion was selected for extraction. Tumor delineation was carried out along the tumor boundary on the slice with the largest cross-section in the T1C sequence, and along the edema boundary on the slice with the largest edema in the T2-weighted imaging (T2WI) sequence. Based on the delineated regions of interest (ROI), MATLAB was used to extract the lesions and perform binarization (Fig. 2). The binarized lesions were then imported into the open-source software ImageJ (https://imagej.net/ij/) for fractal analysis, using the box-counting method from the FracLac plugin. The box sizes within the grid ranged from a minimum of 2 pixels to a maximum of 45% of the image area. Twelve grid positions were defined, and the FD and LAC were calculated for each grid. The average of these values was taken as the final result.
Immunohistochemistry and genetic testing
All postoperative tumor tissues were paraffin-embedded, sectioned, and routinely stained with hematoxylin and eosin. Immunohistochemical analyses of Ki_67 and p53 were performed using monoclonal primary antibodies against mouse anti-human Ki_67 (1:400) and rabbit anti-human p53 (1:200). Ten high-power fields (×400 magnification) were randomly selected, with 100 cells counted in each field. The expression level of Ki_67 was calculated by dividing the number of positive cells by the total number of cells.This study classified the Ki_67 PI into two categories based on the majority of current research in the field and the actual experimental data of this study: PI ≥ 20% was defined as high expression, and PI < 20% as low expression23,24,25. p53 expression was considered negative if fewer than 10% of cells showed expression, and positive if 10% or more of cells expressed p5326. TERTp mutation status was determined through genetic sequencing.
Consistency analysis
The intraclass correlation coefficient (ICC) and Kappa coefficient were used to evaluate the consistency of quantitative and qualitative data measured by two physicians, respectively. An ICC greater than 0.75 for quantitative indicators and a Kappa coefficient greater than 0.60 for qualitative indicators indicate good consistency.
Development and evaluation of predictive models
Univariate and multivariate logistic regression analyses were performed to identify independent factors predicting the Ki_67 PI, p53 expression, and TERTp mutation status. Independent factors selected from these analyses were used to construct nomogram models. The predictive ability of the models was evaluated using receiver operating characteristic curves (ROC), and the area under the curve (AUC), sensitivity, and specificity were calculated based on the maximum Youden index. Additionally, calibration curves (CRC) were used to assess the goodness-of-fit of the models, ensuring their excellent stability and reliability. To further assess the clinical value of the predictive models, decision curve analysis (DCA) was employed to quantify the net benefit of patients at different threshold probabilities, helping to select the optimal model.
Statistical analysis
Statistical analyses were performed using RStudio 4.3.2, MedCalc 20.022, and SPSS 29. In SPSS 29, the Shapiro-Wilk test was first used to assess the normality of continuous variables. Data that followed a normal distribution were expressed as mean ± standard deviation, and comparisons between two groups were conducted using independent samples t-test. Categorical variables were expressed as frequencies, and comparisons between groups were performed using the Chi-square test. Univariate analysis was conducted using either the independent samples t-test or the Chi-square test, and factors with p < 0.05 were included in multivariate logistic regression analysis. ROC curves were plotted using MedCalc. Nomograms and CRC were generated using the “rms” package in R, and DCA were plotted using the “rmda” package. A p-value of < 0.05 was considered statistically significant.
Results
General data
In the high Ki_67 PI expression group, there were 112 patients with WHO grade 3–4 diffuse gliomas, including 68 males and 44 females, with an average age of 55.86 ± 13.90. In the low Ki_67 PI expression group, there were 58 patients with WHO grade 3–4 diffuse gliomas, including 22 males and 36 females, with an average age of 48.66 ± 14.06. In the positive p53 expression group, there were 70 patients with WHO grade 3–4 diffuse gliomas, including 36 males and 34 females, with an average age of 53.11 ± 14.82. In the negative p53 expression group, there were 100 patients with WHO grade 3–4 diffuse gliomas, including 54 males and 46 females, with an average age of 53.60 ± 14.05. In the TERTp mutation group, there were 96 patients with WHO grade 3–4 diffuse gliomas, including 50 males and 46 females, with an average age of 56.68 ± 10.84. In the TERTp wild-type group, there were 74 patients with WHO grade 3–4 diffuse gliomas, including 40 males and 34 females, with an average age of 49.15 ± 17.03. Details are shown in Fig. 3; Table 2.
Inter-group consistency evaluation
ICC was used to assess the consistency of quantitative parameters such as tumor size and FD. The results showed that the ICC values for measurements taken by the two physicians ranged from 0.80 to 0.95, indicating good consistency (ICC > 0.75). Therefore, the average measurements from the two physicians were used for further analysis. Additionally, the Kappa coefficient was used to assess the consistency of qualitative data such as tumor ___location, enhancement degree, and enhancement percentage. The Kappa values for imaging features extracted by the two physicians ranged from 0.82 to 1.0, indicating high agreement, and one feature was randomly removed from further analysis. For the few inconsistent qualitative parameters, consensus was reached through discussion, and these were used for subsequent analysis.
Predictive performance of VASARI features and fractal analysis for Ki_67 PI
Univariate analysis revealed statistically significant differences between the high and low PI groups in terms of age, sex, Enhancement Quality, Proportion Enhancing, Proportion non-enhancement (nCET), Thickness of enhancing margin, Definition of the enhancing margin, Definition of the non-enhancing margin, Proportion of Edema, Pial invasion, and Ependymal invasion (p < 0.05). Multivariate analysis identified sex, Proportion Enhancing, and Pial invasion as independent predictors for the two groups (p < 0.05). Details are shown in Table 3. A predictive model was established based on these three independent predictors, and a nomogram was created using R software (Fig. 4a). The model achieved the highest AUC of 0.791 (0.722–0.849) when the cutoff value was set at 0.4978, with a sensitivity of 68.75% and specificity of 81.03% (Fig. 5a). The CRC demonstrated an average absolute error of 0.033, indicating good consistency between the predicted values and actual clinical outcomes (Fig. 6a). DCA showed that using the model to predict Ki_67 PI provided greater clinical benefit within a certain range, confirming the significant clinical value of this predictive model (Fig. 7a).
Calibration curve (CRC): The x-axis represents the predicted probability, and the y-axis represents the actual observed probability. The diagonal line represents the ideal prediction result. The CRC describes the model’s calibration performance based on the consistency between the actual observations and the predicted results. “Ideal” represents the ideal CRC, where the predicted probability exactly matches the actual observations. “Apparent” represents the model’s raw prediction curve, i.e., the uncalibrated curve, while “Bias-corrected” represents the calibrated prediction curve after bias correction. This curve reflects the performance after adjustment of the raw prediction results, aimed at reducing prediction bias. (a) CRC for predicting the Ki_67 proliferative index, with a mean absolute error of 0.033; (b) CRC for predicting p53 expression, with a mean absolute error of 0.021; (c) CRC for predicting TERT promoter mutations, with a mean absolute error of 0.028.
Decision Curve Analysis (DCA): The x-axis represents the threshold probability, and the y-axis represents the net benefit. The dark gray line represents the net benefit of the assumption “all patients are predicted as high risk (positive).” The black line represents the net benefit of the assumption “no intervention for any patients (negative),” which indicates that the model has no predictive capability. The red line represents the net benefit of the prediction model based on Ki_67/p53/TERT, reflecting the potential value the model brings to clinical decision-making at different thresholds. (a) DCA for predicting the Ki_67 proliferative index, where the prediction model shows good clinical net benefit when the threshold is between 0.17 and 0.78. (b) DCA for predicting p53 expression, where the prediction model shows good clinical net benefit when the threshold is between 0.17 and 0.37. (c) DCA for predicting TERT promoter mutations, where the prediction model shows good clinical net benefit when the threshold is between 0.16 and 0.52.
Predictive performance of VASARI features and fractal analysis for p53
Univariate analysis revealed statistically significant differences between the positive and negative p53 expression groups in terms of tumor long diameter and FD of the tumor (FD(T)) (p < 0.05). Multivariate analysis identified FD(T) as an independent predictor for the two groups (p < 0.05). Details are shown in Table 4. A predictive model was established based on this independent predictor, and a nomogram was created using R software (Fig. 4b). The model achieved the highest AUC of 0.601 (0.523–0.675) when the cutoff value was set at 0.2243, with a sensitivity of 51.43% and specificity of 71.00% (Fig. 5b). The CRC demonstrated an average absolute error of 0.021, indicating good consistency between the predicted values and actual clinical outcomes (Fig. 6b). DCA showed that using the model to predict p53 expression provided greater clinical benefit within a certain range, confirming the potential clinical value of this predictive model (Fig. 7b).
Predictive performance of VASARI features and fractal analysis for TERTp mutations
Univariate analysis revealed statistically significant differences between the TERTp mutation and wild-type groups in terms of tumor long diameter, age, FD(T), Enhancement Quality, Thickness of enhancing margin, Definition of the enhancing margin, Proportion of Edema, Diffusion, Pial invasion, and nCET tumor Crosses Midline (p < 0.05). Multivariate analysis identified FD(T), Enhancement Quality, and Definition of the enhancing margin as independent predictors for the two groups (p < 0.05). Details are shown in Table 5. A predictive model was established based on these independent predictors, and a nomogram was created using R software (Fig. 4c). The model achieved the highest AUC of 0.739 (0.667–0.804) when the cutoff value was set at 0.4299, with a sensitivity of 78.12% and specificity of 64.86% (Fig. 5c). The CRC demonstrated an average absolute error of 0.028, indicating good consistency between the predicted values and actual clinical outcomes (Fig. 6c). DCA showed that using the model to predict TERTp mutation provided greater clinical benefit within a certain range, confirming the significant clinical value of this predictive model (Fig. 7c).
Discussion
This study aimed to explore whether the combination of VASARI features and fractal analysis can effectively predict the Ki_67 PI, p53, and TERTp mutations in patients with WHO grade 3–4 diffuse gliomas. The results indicated that sex and VASARI features could effectively predict the Ki_67 PI; FD(T) could effectively predict p53 expression; and FD(T) combined with VASARI features could effectively predict TERTp mutations. Nomogram models were constructed separately based on the independent predictors, and the results showed that the models had good predictive performance, with high consistency between predicted and actual values, demonstrating significant clinical applicability.
Ki_67 can influence the progression of the cell cycle and is considered one of the most reliable indicators of cell proliferation. It is closely associated with the malignant degree of tumors and is commonly used to assess tumor occurrence, development, metastasis, and prognosis27. The Ki_67 PI is an important reference for evaluating the malignancy and pathological grading of brain tumors28. Several studies have reported that the Ki_67 proliferative index in gliomas can be predicted preoperatively and non-invasively using multimodal MRI techniques, such as DWI, SWI, and DSC-PWI29,30. However, these studies included relatively small sample sizes. Unlike these imaging techniques, this study focuses on combining clinical and imaging features, which not only makes it easier to apply in routine clinical practice but also provides a more comprehensive and personalized assessment of tumor invasiveness and proliferative activity. Additionally, the nomogram model in this study offers another advantage. It visually presents the correlations between various influencing factors and Ki_67, providing clinicians with a more easily interpretable tool. In this study, we found that sex, along with VASARI features such as Proportion Enhancing, and Pial invasion, are independent predictors of high Ki_67 expression in grade 3–4 diffuse gliomas. The nomogram model constructed using these factors demonstrated excellent predictive accuracy, with an AUC value of 0.791. Notably, sex as a predictor may suggest differences in glioma cell proliferation activity between males and females, a phenomenon that warrants further investigation. A higher tumor Proportion Enhancing indicates better blood supply and greater vascular activity within the tumor, which correlates with higher invasiveness. Similarly, when gliomas present Pial invasion, it suggests that the tumor has breached the normal gray matter boundary, signaling a high risk of invasion and dissemination. Since Ki_67 reflects cell proliferation activity, it is closely related to tumor differentiation, invasiveness, and prognosis31. High Ki_67 expression promotes tumor growth, leading to increased infiltration and invasiveness, which results in greater tumor heterogeneity as cancer cells grow rapidly32, consistent with our findings. In future clinical practice, this model could help predict tumor progression in advance, allowing for timely intervention.
The tumor suppressor gene TP53 is the most commonly mutated gene in human tumors33. Tumor development is closely associated with functional impairment caused by mutations in TP5333. The p53 protein primarily functions as a transcription factor, activating the expression of various target genes, playing a key role in regulating the cell cycle, apoptosis, and genomic stability, and serving as a guardian of genome integrity34. The TP53 gene is located on the short arm of human chromosome 17 and consists of 11 exons and 10 introns35. As a common tumor mutation suppressor, p53 is expressed at low levels in normal cells and is activated in response to increased external and internal stress signals36. Upon activation, p53 binds to corresponding transcription factors and target genes (such as p21, MDM2, BBC3, BAX, etc.), inducing cell events such as cell cycle arrest, apoptosis, and DNA repair. Major mutations include point mutations, deletions/insertions, and frame-shift mutations. Mutant p53 can alter DNA binding specificity, disrupt the protein’s three-dimensional structure and thermal stability, leading to functional impairment of p53 activity37, and is correlated with tumor malignancy, playing an important role in tumor progression38. FD is a mathematical parameter used to quantify the irregularity of complex shapes. In medical image processing, FD has been shown to be an effective tool for evaluating tumor morphological complexity39. In our study, we found that FD(T) is an independent predictor of p53 positive expression, which aligns with previous research. When p53 mutations lead to uncontrolled tumor cell proliferation, this disordered cell growth directly increases the morphological complexity and irregularity of the tumor tissue, reflected by an elevated FD(T) value. Furthermore, since p53 mutations are associated with patients’ overall survival14, the FD(T) value may indirectly reflect the prognosis of patients. However, this correlation may also be influenced by other tumor characteristics and intratumoral heterogeneity. Future research should further explore the relationship between these factors and FD(T) in order to comprehensively understand the biological mechanisms underlying FD(T) values and their complex interaction with p53 expression.
TERTp mutation increases gene expression and activates telomerase activity, thereby granting tumor cells the potential for unlimited proliferation and promoting tumor development and dissemination40. Glioblastomas with TERTp mutations typically exhibit higher invasiveness and malignant biological behavior, resulting in higher tumor recurrence rates and lower survival rates for affected patients40,41. In this study, we found that FD(T) is an independent predictor of TERTp mutation. This is because, after the TERTp mutation leads to uncontrolled tumor cell proliferation, the tumor forms a more complex and irregular morphology. The increase in FD(T) values reflects this morphological complexity, making it an effective indicator for predicting TERTp mutation. Therefore, FD(T), as a predictor of TERTp mutation, is biologically reasonable. However, this correlation may be influenced by other tumor characteristics and intratumoral heterogeneity. Future studies should further investigate the relationship between these factors and FD(T). Additionally, we found that the Enhancement Quality, and the Definition of the enhancing margin within the VASARI feature set are independent predictors of TERTp mutation. This further provides imaging evidence that, after TERTp mutations, high-grade gliomas exhibit increased neoangiogenesis and greater tumor invasiveness. These findings offer a deeper understanding of the impact of TERTp mutations on the biological behavior of gliomas.
Nomograms are valuable visualization tools designed to optimize statistical models42. By ingeniously integrating various predictive factors and outcome variables, they provide a straightforward method for estimating event probabilities, thereby addressing the demands of personalized medicine. These tools have already demonstrated extensive clinical utility43,44. This study thoroughly analyzed the independent predictors of Ki_67 PI, p53 expression, and TERTp mutations in WHO grade 3–4 glioma patients. Based on these independent factors, predictive models were constructed and vividly presented in the form of a nomogram. The CRC further confirmed the high concordance between the model’s predicted values and the actual outcomes. Meanwhile, DCA strongly demonstrated the significant clinical value of the model. It provides physicians with a noninvasive, quantitative, and convenient method to make rapid predictions, enabling the development of personalized treatment plans and maximizing patient benefit.
Limitations and future prospects
This study has the following limitations: First, it is a single-center retrospective study, which may introduce some selection bias and limit the widespread application of the model. Secondly, in this study, the predictive efficacy of FD(T) for p53 was relatively poor, which may be related to the relatively small sample size and the inclusion of only one independent influencing factor. Therefore, caution should be exercised when generalizing the results of this study to broader contexts. Finally, due to the limited sample size, this study lacks a validation set. In the future, we will include more samples to validate the model and conduct more detailed exploration across different tumor grades. Additionally, we will investigate other potential influencing factors to build a more comprehensive predictive model, providing more accurate diagnoses and treatment strategies for glioma patients.
Conclusion
In conclusion, we found that VASARI features and fractal analysis can effectively predict the Ki_67 PI, p53 expression, and TERTp mutations, with their combination further enhancing the predictive efficacy for TERTp mutations. By establishing a nomogram predictive model, we achieved accurate, objective, non-invasive, and intuitive predictions for Ki_67, p53, and TERTp mutations. This provides clinicians with a deeper understanding of disease progression and offers a theoretical basis for selecting personalized treatment plans, ultimately maximizing patient benefit.
Data availability
The data analyzed for this study can be accessed from the corresponding author upon reasonable request.
References
Wang, Y. et al. Proteogenomics of diffuse gliomas reveal molecular subtypes associated with specific therapeutic targets and immune-evasion mechanisms. Nat. Commun. 14, 505 (2023).
Karve, A. S. et al. A review of approaches to potentiate the activity of Temozolomide against glioblastoma to overcome resistance. Int. J. Mol. Sci. 25, 3217 (2024).
Price, M. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united States in 2017–2021. Neuro Oncol. 26, vi1–vi85 (2024).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Cordier, D., Krolicki, L., Morgenstern, A. & Merlo, A. Targeted radiolabeled compounds in glioma therapy. Semin Nucl. Med. 46, 243–249 (2016).
Liu, Y. et al. Identifying Ki-67 specific miRNA-mRNA interactions in malignant Astrocytomas. Neurosci. Lett. 546, 36–41 (2013).
Li, F., Wang, D., Wang, N., Wu, L. & Yu, B. A nomogram with Ki-67 in the prediction of postoperative recurrence and death for glioma. Sci. Rep. 14, 20334 (2024).
Stichel, D. et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt Astrocytoma to glioblastoma. Acta Neuropathol. 136, 793–803 (2018).
Amen, A. M. et al. Cancer-specific loss of TERT activation sensitizes glioblastoma to DNA damage. Proc. Natl. Acad. Sci. U S A. 118, e2008772118 (2021).
Śledzińska, P., Bebyn, M. G., Furtak, J., Kowalewski, J. & Lewandowska, M. A. Prognostic and predictive biomarkers in gliomas. Int. J. Mol. Sci. 22, 10373 (2021).
Bykov, V., Eriksson, S. E., Bianchi, J. & Wiman, K. G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer. 18, 89–102 (2018).
Sullivan, K. D., Galbraith, M. D., Andrysik, Z. & Espinosa, J. M. Mechanisms of transcriptional regulation by p53. Cell. Death Differ. 25, 133–143 (2018).
Wang, H., Guo, M., Wei, H. & Chen, Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal. Transduct. Target. Ther. 8, 92 (2023).
Shi, W. et al. A multivariate retrospective analysis of high-grade gliomas: survival and prognostic factors. Cancer Med. 13, e7456 (2024).
Luo, J., Pan, M., Mo, K., Mao, Y. & Zou, D. Emerging role of artificial intelligence in diagnosis, classification and clinical management of glioma. Semin Cancer Biol. 91, 110–123 (2023).
You, W. et al. The combination of radiomics features and VASARI standard to predict glioma grade. Front. Oncol. 13, 1083216 (2023).
Li, L. et al. Magnetic resonance imaging findings of intracranial extraventricular ependymoma: A retrospective multi-center cohort study of 114 cases. Cancer Med. 12, 16195–16206 (2023).
Wan, Y., Rahmat, R. & Price, S. J. Deep learning for glioblastoma segmentation using preoperative magnetic resonance imaging identifies volumetric features associated with survival. Acta Neurochir. (Wien). 162, 3067–3080 (2020).
Di Ieva, A. The fractal geometry of the brain: anoverview. Adv. Neurobiol. 36, 3–13 (2024).
Smitha, K. A., Gupta, A. K. & Jayasree, R. S. Fractal analysis: fractal dimension and lacunarity from MR images for differentiating the grades of glioma. Phys. Med. Biol. 60, 6937–6947 (2015).
Curtin, L. et al. Shape matters: morphological metrics of glioblastoma imaging abnormalities as biomarkers of prognosis. Sci. Rep. 11, 23202 (2021).
Liu, S. et al. Relationship between necrotic patterns in glioblastoma and patient survival: fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci. Rep. 7, 8302 (2017).
Bai, L., Jiang, J. & Zhou, J. Assessment of Ki-67 expression levels in IDH-wildtype glioblastoma using logistic regression modelling of VASARI features. Neurosurg. Rev. 47, 20 (2023).
Xing, Z. et al. Non-invasive prediction of p53 and Ki-67 labelling indices and O-6-methylguanine-DNA methyltransferase promoter methylation status in adult patients with isocitrate dehydrogenase wild-type glioblastomas using diffusion-weighted imaging and dynamic susceptibility contrast-enhanced perfusion-weighted imaging combined with conventional MRI. Clin. Radiol. 77, e576–e584 (2022).
Armocida, D., Frati, A., Salvati, M., Santoro, A. & Pesce, A. Is Ki-67 index overexpression in IDH wild type glioblastoma a predictor of shorter progression free survival? A clinical and molecular analytic investigation. Clin. Neurol. Neurosurg. 198, 106126 (2020).
Hasse, A. et al. Correlation of post-contrast T1-weighted MRI surface regularity, tumor bulk, and necrotic volume with Ki67 and p53 in glioblastomas. Neuroradiology 61, 861–867 (2019).
Xie, Y. et al. Minimal apparent diffusion coefficient in predicting the Ki-67 proliferation index of pancreatic neuroendocrine tumors. Jpn J. Radiol. 40, 823–830 (2022).
Dumke, R. et al. Monocentric evaluation of Ki-67 labeling index in combination with a modified RPA score as a prognostic factor for survival in IDH-wildtype glioblastoma patients treated with radiochemotherapy. Strahlenther Onkol. 198, 892–906 (2022).
Cui, Y. et al. Predicting isocitrate dehydrogenase genotype, histological phenotype, and Ki-67 expression level in diffuse gliomas with an advanced contrast analysis of magnetic resonance imaging sequences. Quant. Imaging Med. Surg. 13, 3400–3415 (2023).
Yang, X. et al. Prediction of Ki-67 labeling index, ATRX mutation, and MGMT promoter methylation status in IDH-mutant Astrocytoma by morphological MRI, SWI, DWI, and DSC-PWI. Eur. Radiol. 33, 7003–7014 (2023).
Ke, X. et al. Apparent diffusion coefficient values effectively predict cell proliferation and determine oligodendroglioma grade. Neurosurg. Rev. 46, 83 (2023).
Xue, C. et al. Apparent diffusion coefficient histogram analysis for the preoperative evaluation of Ki-67 expression in pituitary Macroadenoma. Clin. Neuroradiol. 32, 269–276 (2022).
Levine, A. J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer. 20, 471–480 (2020).
Sipos, T., Kövecsi, C., Ovidiu-Ioan, A. & Zsuzsánna, Ș. General Clinico-Pathological characteristics in glioblastomas in correlation with p53 and Ki67. Med. (Kaunas). 59, 1918 (2023).
Kamada, R., Toguchi, Y., Nomura, T., Imagawa, T. & Sakaguchi, K. Tetramer formation of tumor suppressor protein p53: structure, function, and applications. Biopolymers 106, 598–612 (2016).
Parrales, A. & Iwakuma, T. Targeting oncogenic mutant p53 for Cancer therapy. Front. Oncol. 5, 288 (2015).
Muller, P. A. & Vousden, K. H. p53 mutations in cancer. Nat. Cell. Biol. 15, 2–8 (2013).
Romano, F. J. et al. Del Basso de Caro, M. ATM and p53 combined analysis predicts survival in glioblastoma multiforme patients: A clinicopathologic study. J. Cell. Biochem. 119, 4867–4877 (2018).
Friconnet, G. et al. Advanced MRI shape analysis as a predictor of histologically aggressive supratentorial meningioma. J. Neuroradiol. 49, 275–280 (2022).
Chen, L. et al. Multi-parameter MRI based radiomics nomogram for predicting telomerase reverse transcriptase promoter mutation and prognosis in glioblastoma. Front. Neurol. 14, 1266658 (2023).
Zhang, H. et al. Multiparametric MRI-based fusion radiomics for predicting telomerase reverse transcriptase (TERT) promoter mutations and progression-free survival in glioblastoma: a multicentre study. Neuroradiology 66, 81–92 (2024).
Wang, T. et al. A competing risk model nomogram to predict the Long-Term prognosis of lung carcinoid. Ann. Surg. Oncol. 30, 5830–5839 (2023).
Chen, Y. D. et al. Radiomics and nomogram of magnetic resonance imaging for preoperative prediction of microvascular invasion in small hepatocellular carcinoma. World J. Gastroenterol. 28, 4399–4416 (2022).
Yue, X. et al. Multiparametric magnetic resonance imaging-based radiomics nomogram for predicting tumor grade in endometrial cancer. Front. Oncol. 13, 1081134 (2023).
Acknowledgements
This study was funded by the research fund from the National Natural Science Foundation of China (grant no. 82271960 and 22327901) and Hubei Provincial Natural Science Foundation of China(2024AFB179).
Author information
Authors and Affiliations
Contributions
Changyou Long: Data analysis and draft the manuscript; Changyou Long, Jie Zhou and Shen Gui: Data collection and validation; Dan Xu, Wenbo Sun and Weiqiang Liang: Data collection; Dan Xu and Huan Li: Revise the manuscript; Haibo Xu: Study design and supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Long, C., Xu, D., Sun, W. et al. Nomogram prediction of molecular characteristics in WHO grade 3–4 diffuse gliomas based on fractal analysis and VASARI features. Sci Rep 15, 15485 (2025). https://doi.org/10.1038/s41598-025-00113-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00113-3