Abstract
Obstructive sleep apnea (OSA) is associated with an increased prevalence of aortic aneurysm, but the impact of OSA on the subclinical damage of large thoracic vessels remains controversial. Short sleep duration and poor sleep quality has been reported to be related with higher arterial stiffness. In the current study, we aimed to investigate the association of sleep disturbance and sleep apnea with the size of the ascending aorta (AA), descending thoracic aorta (DTA), and main pulmonary artery (MPA). One hundred and fifty-five newly diagnosed OSA patients free of cardiovascular disease and medication were included. In-laboratory polysomnography (PSG) and chest computed tomography (CT) scanning were performed. The sleep duration was defined as total sleep time (TST) as recorded during overnight PSG, with TST < 6 h defined as short sleep duration. Sleep latency (SL), sleep efficiency (SE) and wake after sleep onset (WASO) were used to assess the objective sleep quality, and the Epworth Sleepiness Scale (ESS) was used to assess EDS (excessive daytime sleepiness). The diameter of AA was positively associated with age (B = 0.199, P < 0.001) and diastolic blood pressure (DBP) (B = 0.103, P < 0.001), and was negatively associated with mean pulse oxygen saturation (SpO2mean) (B = − 0.176, P = 0.022). The diameter of DTA was positively associated with age (B = 0.112, P < 0.001), body mass index (BMI) (B = 0.184, P = 0.041), and DBP (B = 0.033, P = 0.024), and was negatively associated with TST (B = − 0.006, P = 0.023). Neither nocturnal hypoxia nor TST were associated with the diameter of MPA or the ratio of MPA to AA (PA/A). The aortic or MPA measurements were not associated with SL, SE, WASO or ESS. In patients with OSA, nocturnal hypoxia and sleep duration were associated with the diameter of AA and DTA, respectively. It is suggested that sleep apnea and sleep disturbance may exert effects on the remodeling and enlargement of thoracic large vessels through distinctive mechanisms.
Similar content being viewed by others
Introduction
Aortic diseases, including aortic aneurysms or aortic dissection, remain fatal nowadays. Despite advances in the management of acute vascular events, the short and long-term prognoses are still poor. Aortic dilation can evolve in aneurysm, with a greater risk of rupture or dissection. Sleep disorders, including sleep disordered breathing (SDB) and sleep disturbances, have been shown to play an important role in the pathogenesis of vascular dysfunction and hypertension, conditions which may promote dilation and subsequent aortic dissection and rupture1. Besides, SDB has the potential to increase pulmonary arterial pressure (PAP) not only during sleep, but also in the waking state, and patients with pulmonary arterial hypertension (PAH) are at risk of developing SDB2.
Obstructive sleep apnea (OSA) is characterized by repeated episodes of complete or partial collapse of the upper airway during sleep, resulting in intermittent hypoxia, sleep fragmentation, sympathetic activation and fluctuation of intrathoracic pressure. Although the association between OSA and cardiovascular diseases has been the hot topic over the past 30 years, limited studies have been focused on the potential impact of OSA on aorta. Several observational studies have reported that OSA is highly prevalent among patients with aortic aneurysms and aortic dissections. Patients with co-occurring OSA and Marfan’s syndrome as well as patients at the more severe end of the spectrum of OSA seem to be especially vulnerable to aortic disease3,4. However, the impact of OSA on subclinical aorta damage, that is the aorta dilation or aorta size remains controversial. OSA has also been shown to be an aggravating factor for pulmonary hypertension and responsible for a small increase in PAP over the long term, but its association with the dimension of pulmonary artery has rarely been investigated5.
Sleep disturbance is being increasingly recognized as a risk to cardiovascular (CV) health. While most of the studies have focused on SDB, a limited number of studies have recently addressed other aspects of sleep such as sleep duration, sleep quality and excessive daytime sleepiness (EDS)6,7,8. Several studies have reported that sleep disturbances, such as sleep deprivation, EDS and insomnia, are associated with insulin resistance, visceral obesity, arterial stiffness and hypertension9,10. However, no previous studies have reported the association of these sleep disturbances with subclinical changes of large arteries. In the current study, we aimed to investigate the association between sleep disturbances with CT-measured size of the thoracic aorta and main pulmonary artery (MPA), taking into account of SDB.
Materials and method
Study population
This was an observational, prospective study of patients with a new diagnosis of moderate to severe OSA free of cardiovascular disease and medication, enrolled in sleep center of the National Respiratory Disease Research Center at the China-Japan Friendship Hospital from May 2021 to December 2022. All patients underwent questionnaire assessment, anthropometry and comorbidities assessment, PSG monitoring, and chest CT scans. Patients with significant cardiopulmonary diseases such as chronic obstructive pulmonary disease, interstitial lung disease, pulmonary vascular diseases, chronic heart failure with reduced ejection fraction, current medical condition of chronic sleep disruption such as pain, psychiatric disorders, or current use of hypnotics, anxiolytics, antidepressants, and any other antipsychotics were excluded from the study. The study was approved by the Ethic Committee of the China-Japan Friendship Hospital, and written informed consent was obtained from all participants.
Anthropometry, symptoms, and comorbidity
Anthropometric indices including body mass index (BMI), neck circumference (NC), waist circumference, and hip circumference were collected. EDS was assessed using the Epworth Sleepiness Scale (ESS). Blood pressure (BP) was measured according to standard methods and recorded BP was the average of 3 consecutive readings during a 5-min period following at least 10 min of rest in the supine position. The comorbid conditions of cardiovascular disease, hypertension, type 2 diabetes mellitus were examined based on medical history, biochemical profiles, and medications.
Polysomnography (PSG) study
All subjects underwent one night of full overnight diagnostic PSG (Alice 6, Philips Respironics, Murrysville, Pennsylvania, United States). Subjects were allowed to follow their habitual sleep time. All data were analyzed by experienced PSG technologists based on the 2012 American Academy of Sleep Medicine criteria11. The sleep duration was defined as total sleep time (TST) as recorded during overnight PSG, with TST < 6 h defined as short sleepers and TST ≥ 6 h as normal sleepers. Sleep efficiency (SE) is the ratio of the amount of total time asleep versus the total time in bed. Sleep latency (SL), also referred to as sleep onset latency is the time it takes to transition from the state of wakefulness to sleep. Wake after sleep onset (WASO) is the total amount of wake time after sleep onset until final awakening. Heart rate variability (HRV) parameters SD1 and SD2 are derived from Poincaré plots. SD1 and SD2 correspond to the standard deviations perpendicular and along the line of identity in the Poincaré ellipse, respectively. SD1 (short-term variability) reflects instantaneous RR-interval differences. SD2 (long-term variability) represents overall RR-interval dispersion. The SD1/SD2 ratio reflects autonomic nervous balance: lower values indicate sympathetic dominance.
Chest computed tomography scans and vascular measurements
All eligible patients underwent a chest CT scan using low-dose 128-slice scanner (GE Medical Systems). Scanning was conducted when the subject was awake and supine. The scans were acquired at a 0.5 mm collimation/interval and were reconstructed at a 1 mm thickness/interval, with 120 kV, 100 mA, and a rotation time of 0.5 s. Vascular measurements were performed by an investigator who was unaware of the participants’ clinical characteristics. The sizes of AA, DTA, and MPA were calculated as described in previous studies12,13. At the level of the main pulmonary artery’s bifurcation, we corrected the plane along the sagittal and coronal reconstructions respectively. Then, find the oblique plane perpendicular to the ascending aorta to obtain the real transverse area of the vessel. A similar technique was applied to measure the dimensions of the DTA and the MPA using the same image, as shown in Fig. 1. The normative value for PA:A ratio (PA/A) of 0.9 for both sexes was used to define pulmonary arteria enlargement (PAE) according to the Framingham Heart Study.
Statistical analysis
Comparisons of continuous variables between groups were determined by the unpaired t test and the chi-square test was used for categorical data. Pearson correlation was used to assess the possible relations between demographic parameters and sleep variables with vascular dimensions. Univariate regression analysis was used to determine the association of potential predictor variables with the vascular dimensions. Predictor variables with P < 0.10 according to the bivariate analysis were selected for multivariate regression analysis. A value of P < 0.05 was considered statistically significant. All analyses were conducted with SPSS 22.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Study population characteristics
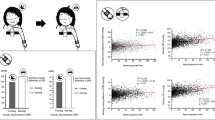
One hundred and fifty-five patients with OSA were enrolled in this study and were divided into two groups according to TST. The anthropometric metrics, sex distribution, smoking status, mean ESS score, sleep structure, severity of OSA, nocturnal hypoxia, blood pressure and heart rate variability (SD1/SD2) were comparable between short sleepers and normal sleepers. However, short sleeper tended to be older (49.55 ± 13.92 vs 43.05 ± 11.75, P = 0.021), had lower sleep efficiency (70.1% ± 10.3% vs 86.4 ± 8.2%, P < 0.001) and larger diameters of DTA (25.27 ± 3.25 vs 23.80 ± 2.52, P = 0.007). There were no differences in the diameters of AA and MPA, as well as the frequency of PAE between the two groups (Table 1). AA, DTA and MPA measures as a function of sleep duration categories are depicted in Fig. 2.
Correlates of vascular dimensions in patients with OSA
The size of AA was correlated with age, SBP, DBP, T90, ODI, SD1/SD2, and SpO2mean. The size of DTA was correlated with age, SBP, DBP, and TST. The size of MPA was correlated with BMI and SpO2mean. PA/A was not correlated with patient characteristics or sleep-relevant parameters, except age. No significant correlations were found between vascular dimensions and EDS, SL, SE or WASO (Table 2, Fig. 3).
Correlates of vascular dimensions in patients with OSA. (A) The diameter of AA was negatively correlated with SpO2mean (B = − 0.168, P = 0.037); (B) The diameter of DTA was negatively correlated with TST (B = − 0.170, P = 0.034); (C) The diameter of MPA was negatively correlated with SpO2mean (B = − 0.167, P = 0.037).
Possible predictors of vascular dimensions
Multiple regression analysis was performed with age, sex, BMI, blood pressure, TST, SD1/SD2, sleep structure profiles and OSA severity parameters included as independent variables, and AA, DTA, MPA and P/AA included as the dependent variables. The diameter of AA was positively associated with age (B = 0.199, P < 0.001) and DBP (B = 0.103, P = 0.001), and was negatively associated with SpO2mean (B = − 0.176, P = 0.022). The diameter of DTA was positively associated with age (B = 0.112, P < 0.001), BMI (B = 0.184, P < 0.001), and DBP (B = 0.033, P = 0.024), and was negatively associated with TST (B = − 0.006, P = 0.023). Neither nocturnal hypoxia parameters nor TST were associated with the diameter of MPA except BMI (B = 0.194, P = 0.002). P/AA was negatively associated with age (B = − 0.005, P < 0.001) and DBP (B = − 0.002, P = 0.033). Parameters of sleep quality (SL, SE, WASO, ESS) were not associated with any vessel measurements (Table 3).
Discussion
Aortic diseases, which mainly include aortic dilatation, aortic aneurysm and aortic dissection, have high morbidity and mortality14. Multiple genetic and environmental factors may be involved in these complex and multifactorial diseases, except for some well-established risk factors including older age, male sex, smoking, hyperlipidemia and hypertension, recent studies proposed a possible link between OSA and aortic diseases15,16. The high prevalence of SDB among subjects with pulmonary hypertension is a clue to the possible influence of OSA on the remodeling of pulmonary artery. Also, observational studies have suggested strong associations between sleep disturbance and many cardiovascular diseases17,18. In the current study, we found that nocturnal hypoxia is associated with the diameter of AA, sleep duration is associated with the diameter of DTA, while neither nocturnal hypoxia nor sleep disturbance parameters were associated with the diameter of MPA.
Previous studies investigating the association of OSA and aortic subclinical damage among the general population have showed a somewhat mixed body of evidence. Some studies reported ascending aortic diameter was significantly greater in OSA patients than in those controls, and the aortic root diameter was significantly correlated to parameters of OSA severity (either AHI or oxygen desaturation index). By contrast, two prospective and one retrospective study did not establish the afore mentioned relationships19,20,21. Some previous studies have suggested that chronic intermittent hypoxia may induce vascular remodeling through multiple interconnected pathways. For example, oxidative stress mediated by NADPH oxidase and excessive reactive oxygen species production impairs endothelial nitric oxide bioavailability, while hypoxia-inducible factor-1α activation upregulates vascular endothelial growth factor and endothelin-1 expression, promoting vascular smooth muscle proliferation. Nuclear factor-κB-mediated inflammatory responses enhance endothelial adhesion molecule expression and pro-inflammatory cytokine release, facilitating leukocyte infiltration. Sympathetic nervous system hyperactivity contributes to hemodynamic stress, and matrix metalloproteinase activation disrupts extracellular matrix integrity through collagen-elastin imbalance. Experimental models demonstrate characteristic aortic remodeling featuring medial thickening with fragmented elastin fibers and collagen deposition. These mechanisms collectively drive vascular structural alterations through synergistic oxidative, inflammatory, and biomechanical disturbances19. The limited research data and the methodological differences in aortic measuring may contribute to the heterogeneity of the findings. Transthoracic echocardiography (TTE) and multidetector CT are the two most widely used tools to measure the dimensions of thoracic large vessels, however, CT scan measurements are considered to be more reliable because TTE is highly performer-dependent and unable to represent the vessel lesions fully. In the current study, we recruited a relatively large sample size of untreated patients with OSA and used chest CT to assess the dimension of the thoracic large arteries, we found that except age and DBP, SpO2mean (B = − 0.176, P = 0.014) was negatively associated with the size of AA, which suggests that hypoxia may play an important role in vascular remodeling and enlargement of AA. It may add evidence to the view that nocturnal intermittent hypoxia can lead to varieties of hemodynamic and metabolic alterations, including hypertension, activated oxidative stress, endothelial dysfunction, and systemic inflammation22. Basic research has suggested that intermittent hypoxia and reoxygenation can activate reactive oxygen and hypoxia inducible factor-1, thus making a contribution to the pathogenesis of vascular damage23. Proliferation and activation of macrophages were found in the aortic walls of mice exposed to intermittent hypoxia24.
Sleep disturbance has become global health concerns. Poor sleep quality has significant adverse health outcomes. Sleep quality has four attributes: sleep efficiency, sleep latency, sleep duration, and wake after sleep onset25. Most studies on sleep disturbance used questionnaires to measure the perceptions of sleep quality, depth, and restoration, such as perceived difficulties falling asleep and staying asleep, as well as sleep satisfaction. However, recent studies found that objective measurements are more accurate than the subjective perception to predict the risk of cardiovascular and/or cerebrovascular disease. Our study was innovative for evaluating the association between the PSG-derived objective sleep quality parameters, containing sleep duration, SE, SL and WASO, and thoracic large arteries dimensions. We found the diameter of DTA were greater in those exhibiting TST < 6 h and the DTA size was negatively associated with TST. Short sleep duration is usually defined as TST < 6 h. Previous studies have demonstrated that short sleep duration was closely related to the occurrence and development of obesity, hypertension, atherosclerosis and type 2 diabetes26. Recent prospective studies have also reported that short sleep duration was independently associated with increased risks of CVD onset and death27.
OSA and short sleep may increase the risk of cardiovascular events through some shared psychological and physiologic pathways. They are both associated with increased sympathetic activity and reduced parasympathetic activation during sleep, and have higher prevalence of unhealthy behaviors, such as higher energy intake and poor overall diet quality, which are associated with weight gain and obesity, and higher incidence of metabolic syndrome and type 2 diabetes28,29. Habitual short sleep can also disrupt circadian rhythmicity and lead to circadian misalignment30. Chronic inflammation may also play a part in affecting cardiovascular health in OSA and short sleep31. A study showed shorter sleep duration was associated with increased brachial artery diameter32. However, fewer studies investigated the association between short sleep and thoracic aortic size among patients with OSA. In this study, we found that different from the AA size, which was associated with the severity of OSA, the DTA size was more related with TST. Fifty to 65 percent of aortic intimal tears originate in the ascending aorta within the sinotubular junction and extend to involve the remaining portions of the thoracoabdominal aorta. Approximately 20 to 30 percent of intimal tears originate in the vicinity of the left subclavian artery and extend into the descending thoracic and thoracoabdominal aorta33. The commonality of these two predominant locales for the development of the aortic tear is hypothesized to be related to higher shear forces (dP/dT) in these regions34,35. The differences in the association of the severity of OSA and the size of AA or DTA may be due to the tracing of the aorta, especially the aortic arch, which may mitigate the surge of shear forces associated with the fluctuation of intrathoracic pressure companied with upper airway collapse. The legions of DTA may be more influenced by atherosclerosis. The heart rate variability attenuation, a marker of autonomic perturbations, which was calculated as the ratio of SD1 to SD2 (SD1/SD2) from PSG, was not significantly different between short sleepers and normal sleepers. Also, SD1/SD2 was not found to be associated with the size of DTA in the current study. Short sleep duration increases the risk of cardiovascular remodeling through the synergistic effect of multiple pathways and the specific physiological mechanisms remain to be further explored.
EDS is classically viewed as a consequence of insufficient sleep or a symptom of sleep disorders. Some epidemiological and clinical studies have shown that patients with EDS have greater cardiovascular risk than those without EDS36. However, the results of our study showed that EDS was independent of all vascular dimensions, which was consistent with one previous study. The study has also demonstrated that EDS is not associated with larger aortic diameters37. We also did not find the association between other objective measurements of sleep qualities, such as SL, SE, WASO, with the vascular dimensions. Current research on the associations of sleep disturbance and large vascular damage among patients with OSA is rare, which deserved further investigation.
Sleep apnea is well recognized as a cause of hypoxia and hypercarbia with the potential to increase pulmonary arterial pressure during sleep but also in the waking state. It is reported that pulmonary hypertension is present in 12–34% of obstructive sleep apnea38. Conversely, 25% of patients with pulmonary hypertension by right heart catheterization turn out to have sleep apnea. Marta Marin-Oto used a pulmonary artery diameter of more than 29 mm in men and more than 27 mm in women or as a PA:Ao ratio of more than 0.9 to define pulmonary artery enlargement (PAE) and found that nocturnal hypoxemia was associated with PAE in smokers with or without COPD39. Roberto Castellana studied 60 patients with OSA and found that time of oxygen saturation < 90% (T90) influenced MPA sizes12. Paul Stark investigated the concurrence of sleep apnea and pulmonary hypertension in a Veteran population and no significant difference in pulmonary artery diameters had be found between the group without sleep apnea and the group with sleep apnea, which was in line with our study40. Currently, “pure” OSA is considered to be responsible for a small increase in PAP whose clinical impact has not been demonstrated in general population and the relations of OSA or sleep disturbance with pulmonary artery remodeling have not been clarified.
Several limitations of our study should be mentioned. First, the current study was cross-sectional and could not show causal relations between variables. Second, the patients were from a single medical center without severe comorbidities, besides, the study did not include a control group of patients without OSA. Validation of our findings in a wider variety of populations is needed. Third, we used PSG to measure sleep duration and objective sleep quality parameters, however, continuous sleep tracking with actigraphy for more days may provide complementary information. Finally, calculating a TST threshold to predict the risk of cardiovascular remodeling may be more clinically informative, however, it is difficult to be done according to our current sample size.
Conclusions
Our study suggested nocturnal hypoxia and sleep duration were associated with the diameter of AA and DTA, respectively, while the above sleep parameters were not found to be associated with the diameter of MPA. It is suggested that sleep apnea and sleep disturbance may exert effects on the remodeling and enlargement of thoracic large vessels through distinctive mechanisms. As to the paucity of available data, larger prospective and well-designed studies are needed to shed light on this topic.
Data availability
The datasets of the current study cannot be made openly available to protect the medical information of participants. However, the corresponding author can provide the dataset on a reasonable request.
Abbreviations
- AA:
-
Ascending aorta
- AHI:
-
Apnea–hypopnea index
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- DTA:
-
Descending thoracic aorta
- EDS:
-
Excessive daytime sleepiness
- ESS:
-
Epworth Sleepiness Scale
- MPA:
-
Main pulmonary artery
- MS:
-
Metabolic syndrome
- T90:
-
Time of oxygen saturation < 90%
- TST:
-
Total sleep time
- ODI:
-
Oxygen desaturation index
- OSA:
-
Obstructive sleep apnea
- SpO2mean:
-
Nocturnal oxygen saturation
- SpO2min:
-
Minimum nocturnal oxygen saturation
References
Gaisl, T. et al. Prevalence of obstructive sleep apnea in patients with thoracic aortic aneurysm: A prospective, parallel cohort study. Respiration 99(1), 19–27. https://doi.org/10.1159/000502892 (2020).
Adir, Y., Humbert, M. & Chaouat, A. Sleep-related breathing disorders and pulmonary hypertension. Eur. Respir. J. 57(1), 2002258. https://doi.org/10.1183/13993003.02258-2020 (2021).
Floras, J. S. Sleep apnea and cardiovascular disease: An enigmatic risk factor. Circ. Res. 122(12), 1741–1764. https://doi.org/10.1161/CIRCRESAHA.118.310783 (2018).
Sowho, M. et al. Association of sleep apnoea risk and aortic enlargement in Marfan syndrome. BMJ Open Respir. Res. 8(1), e000942. https://doi.org/10.1136/bmjresp-2021-000942 (2021).
Gaisl, T., Bratton, D. J. & Kohler, M. The impact of obstructive sleep apnea on the aorta. Eur. Respir. J. 46(2), 532–544. https://doi.org/10.1183/09031936.00029315 (2015).
Erden, I. et al. Poor-quality sleep score is an independent predictor of nondipping hypertension. Blood Press Monit. 15(4), 184–187. https://doi.org/10.1097/MBP.0b013e32833a23a0 (2010).
Nagai, M., Hoshide, S., Nishikawa, M., Shimada, K. & Kario, K. Sleep duration and insomnia in the elderly: Associations with blood pressure variability and carotid artery remodeling. Am. J. Hypertens. 26(8), 981–989. https://doi.org/10.1093/ajh/hpt070 (2013).
Yamaki, M., Sato, T. & Fujii, H. Lower ankle-brachial index is associated with poor sleep quality in patients with essential hypertension. Am. J. Cardiovasc. Dis. 5(1), 77–82 (2015).
Xu, H. J. et al. Interaction between obstructive sleep apnea and short sleep duration on insulin resistance: A large-scale study: OSA, short sleep duration and insulin resistance. Respir. Res. 21(1), 151. https://doi.org/10.1186/s12931-020-01416-x (2020).
Haghayegh, S. et al. Sleeping difficulties, sleep duration, and risk of hypertension in women. Hypertension 80(11), 2407–2414. https://doi.org/10.1161/HYPERTENSIONAHA.123.21350 (2023).
Berry, R. B. et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 8(5), 597–619. https://doi.org/10.5664/jcsm.2172 (2012).
Castellana, R. et al. Effects of obstructive sleep apnea on the thoracic aorta and the main pulmonary artery: Assessment by CT. J. Clin. Sleep Med. 17(1), 3–11. https://doi.org/10.5664/jcsm.8770 (2021).
Raymond, T. E., Khabbaza, J. E., Yadav, R. & Tonelli, A. R. Significance ofmain pulmonary artery dilation on imaging studies. Ann. Am. Thorac. Soc. 11(10), 1623–1632. https://doi.org/10.1513/AnnalsATS.201406-253PP (2014).
Bossone, E. & Eagle, K. A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 18(5), 331–348. https://doi.org/10.1038/s41569-020-00472-6 (2021).
Chou, E., Rirruccello, J., Ellinor, P. T. & Lindsay, M. E. Genetics and mechanisms of thoracic aortic disease. Nat. Rev. Cardiol. 20(3), 168–180. https://doi.org/10.1038/s41569-022-00763-0 (2023).
Shih, C. C. et al. Obstructive sleep apnea and aortic aneurysm: A nationwide population-based retrospective study. J. Vasc. Res. 55(4), 235–243. https://doi.org/10.1159/000491928 (2018).
Grewal, N. et al. Impact of obstructive sleep apnea treatment on cardiovascular disease associated mortality and morbidity: A systematic review. Curr. Probl. Cardiol. 49(1), 102139. https://doi.org/10.1016/j.cpcardiol.2023.102139 (2024).
Mitra, A. K., Bhuiyan, A. R. & Jones, E. A. Association and risk factors for obstructive sleep apnea and cardiovascular diseases: A systematic review. Diseases. 9(4), 88. https://doi.org/10.3390/diseases9040088 (2021).
Tanriverdi, H. et al. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: Non-invasive indicators of atherosclerosis. Respiration 73, 741–750. https://doi.org/10.1159/000093531 (2006).
Lee, L. C. et al. The relative impact of obstructive sleep apnea and hypertension on the structural and functional changes of the thoracic aorta. Sleep 33, 1173–1176. https://doi.org/10.1093/sleep/33.9.1173 (2010).
Meuleman, C. et al. Is the aortic root dilated in obstructive sleep apnoea syndrome?. Arch. Cardiovasc. Dis. 101, 391–397. https://doi.org/10.1016/j.acvd.2008.06.007 (2008).
Giampá, S. Q. C., Lorenzi-Filho, G. & Drager, L. F. Obstructive sleep apnea and metabolic syndrome. Obesity 31(4), 900–911. https://doi.org/10.1002/oby.23679 (2023).
Rey, S. & Semenza, G. L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 86(2), 236–242. https://doi.org/10.1093/cvr/cvq045 (2010).
Gileles-Hillel, A. et al. Early intermittent hypoxia induces proatherogenic changes in aortic wall macrophages in a murine model of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 190(8), 958–961. https://doi.org/10.1164/rccm.201406-1149LE (2014).
Makarem, N. et al. Redefining cardiovascular health to include sleep: Prospective associations with cardiovascular disease in the MESA sleep study. J. Am. Heart Assoc. 11(21), e025252. https://doi.org/10.1161/JAHA.122.025252 (2022).
Tobaldini, E. et al. Short sleep duration and cardiometabolic risk: From pathophysiology to clinical evidence. Nat. Rev. Cardiol. 16(4), 213–224. https://doi.org/10.1038/s41569-018-0109-6 (2019).
Han, H. et al. Sleep duration and risks of incident cardiovascular disease and mortality among people with type 2 diabetes. Diabetes Care 46(1), 101–110. https://doi.org/10.2337/dc22-1127 (2023).
Grimaldi, D., Carter, J. R., Van Cauter, E. & Lerroult, E. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension 68(1), 243–250. https://doi.org/10.1161/HYPERTENSIONAHA.115.06847 (2016).
Sun, M. et al. Metaanalysis on shift work and risks of specific obesity types. Obesity Rev. 19(1), 28–40. https://doi.org/10.1111/obr.12621 (2018).
Morris, C. J., Purvis, T. E., Hu, K. & Scheer, F. A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA. 113(10), E1402–E1411. https://doi.org/10.1073/pnas.1516953113 (2016).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry. 80(1), 40–52. https://doi.org/10.1016/j.biopsych.2015.05.014 (2016).
Hall, M. H. et al. Objective sleep duration is prospectively associated with endothelial health. Sleep 40(1), 003. https://doi.org/10.1093/sleep/zsw003 (2017).
Nienaber, C. A. et al. Aortic dissection. Nat. Rev. Dis. Primers. 2, 16053. https://doi.org/10.1038/nrdp.2016.53 (2016).
Ban, E., Cavinato, C. & Humphrey, J. D. Critical pressure of intramural delamination in aortic dissection. Ann. Biomed. Eng. 50(2), 183–194. https://doi.org/10.1007/s10439-022-02906-3 (2022).
Haslach, H. W. Jr., Gipple, J., Harwerth, J. & Rabin, J. Interstitial fluid-solid interaction within aneurysmal and non-pathological human ascending aortic tissue under translational sinusoidal shear deformation. Acta Biomater. 113, 452–463. https://doi.org/10.1016/j.actbio.2020.06.045 (2020).
Bock, J., Covassin, N. & Somers, V. Excessive daytime sleepiness: an emerging marker of cardiovascular risk. Heart 108(22), 1761–1766. https://doi.org/10.1136/heartjnl-2021-319596 (2022).
Baguet, J. P. et al. Snoring but not sleepiness is associated with increased aortic root diameter in hypertensive patients: The SLEEPART study. Int. J. Cardiol. 202, 131–132. https://doi.org/10.1016/j.ijcard.2015.03.319 (2016).
Balcan, B., Akdeniz, B. & Peker, Y. Obstructive sleep apnea and pulmonary hypertension: A chicken-and-egg relationship. J. Clin. Med. 13(10), 2961. https://doi.org/10.3390/jcm13102961 (2024).
Marin-Oto, M. et al. Nocturnal hypoxemia and CT determined pulmonary artery enlargement in smokers. J. Clin. Med. 10(3), 489. https://doi.org/10.3390/jcm10030489 (2021).
Stark, P. & Chang, E. Y. Sleep apnea combined with pulmonary hypertension in a veteran patient population. J. Clin. Med. 12(14), 4634. https://doi.org/10.3390/jcm12144634 (2023).
Acknowledgements
This work was supported by National Natural Science Foundation of China (82470091).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, and preparation of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qu, X.R., Cheng, Z.Q., Li, Y.M. et al. Association of sleep disturbance and sleep apnea with the size of the thoracic aorta and the main pulmonary artery. Sci Rep 15, 16050 (2025). https://doi.org/10.1038/s41598-025-00385-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00385-9