Abstract
Fear of disease progression (FoP) is a multidimensional concept that refers to the fear or worry about disease progress. Little is known about the distinct FoP profiles and their determinants in culturally specific contexts, especially among hematologic malignancies (HM) patients in China. This study aimed to identify heterogeneous profiles of FoP and their associated predictors among Chinese patients with HM. A convenience sample of patients suffering from HM were enrolled from March 2023 to February 2024. To gather multidimensional data from the Fear of Progression Questionnaire-Short Form (FoP-Q-SF), the Brief Illness Perception Questionnaire (BIPQ), the Hospital Anxiety and Depression Scale (HADS), the Family Hardiness Index (FHI), and the EuroQol-Visual Analogue Scale (EQ-VAS), we performed a questionnaire-based cross-sectional study on 455 survivors with HM. The statistical method included latent profile analysis (LPA) and multivariate logistic regression. Three latent profiles of FoP were found: the low-risk fear group (20.88%), the moderate-risk fear group (54.73%), and the high-risk fear group (24.49%). Patients with higher levels of illness perception, anxiety, and depression were more likely to report higher levels of FoP. The study revealed that female gender (OR 2.295–2.577), age > 65 years (OR 4.140–9.363), lower education (OR 0.270–0.365), and lymphoma diagnosis (OR 2.95) significantly predicted higher FoP risk (all P < 0.05), while higher income (OR 0.390–0.477, P < 0.05) and greater family resilience showed protective effects. The findings underscore the need for risk-stratified interventions targeting psychosocial vulnerabilities, particularly in elderly and female adults with HM. This study provides empirical evidence supporting the application of precision psycho-oncology approaches in HM survivorship management. It also contributes to the broader comprehension of FoP and highlights the importance of family-centered interventions .
Similar content being viewed by others
Introduction
Hematologic malignancies (HM) are primary cancers originating from cells of the bone marrow and lymphatic system. They consist of four main categories: leukemia, lymphoma, multiple myeloma (MM), and myelodysplastic syndrome (MDS)1. These malignant blood disorders comprised nearly 4% of new cancer diagnoses in China in 20222. Moreover, the number of blood-related cancer cases would increase about 48% in Asia by 2027 as compared to 20223. Although overall survival rates of patients with HM have steadily improved due to advancements in chemotherapy and bone marrow transplantation4, the follow-up examinations, invasive therapies, and symptom burden can contribute to lower psychosocial health and health state utility values5. Given that these life-threatening cancers are associated with high mortality, patients often face long, aggressive treatments that require lengthy hospital stays. As a result of theses prolonged and intensive treatments, patients frequently experience a myriad of physical challenges and psychological distress6.
A significant concern among patients with HM is the fear of diseases progression (FoP)7, which is defined as the fear of cancer recurrence or progression. Key features of FoP include high levels of preoccupation, intrusive thoughts and catastrophic thinking, all of which can lead to mental distress7,8. FoP not only negatively affects patients’ emotional well-being but can also lead to poor interpersonal relationship and dysfunctional families. These effects are characterized by unpredictable and intermittent periods of remission and relapse9. Thus, FoP is recognized as a significant psychological burden that begins during the disease course and continues through the patient’s life10. To a certain extent, FoP is a common psychological reaction that is clinically relevant; nonetheless, it can lead to excessive distress and substantial functional impairments11. The prevalence of FoP in HM survivors has been estimated to range between 4.2% and 69%12,13,14. Clinically, the FoP has been shown to be negatively correlated with resilience and sleep quality scores in patients with HM (r = − 0.560, − 0.537, P < 0.01)15.
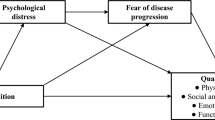
Meanwhile, many research investigations in the field of psycho-oncology have focused on the relationship between patients and their family members16,17. To date, the most comprehensive family-based model to analyze factors influencing FoP in patients and their families was proposed by Mellon and colleagues18. In this model, personal factors, concurrent family stressors, and appraisals of the meaning of illness were found to be associated with an individual’s degree of FoP.
Family-related factors (e.g., patient-reported quality of life, living situation, family history of cancer, family’s perception of the illness, and family characteristics), partner-related factors (e.g., disclosure to partner, cognition of partner, and partner’s sources of support), and parenthood-related factors (e.g., having children and parenting stress) have been widely investigated in various cancer types to determine their influence on the level of FoP during different stages of the cancer trajectory19. These studies have identified both negative associations (e.g., family hardiness, social support, and financial factor)14 and positive associations (e.g., disease course20, social constrains19, mediator of perceived stress, and living situation21 with FoP in cancer survivors. These factors, in turn, impact self-management and psychological health18. However, most of them cannot be generalized to all cancer survivors.
Variable-centered statistical analysis may fail to reflect heterogeneity and overlook individual differences22. Notably, none of these studies have tested the family-based model with the varied profiles of FoP in HM survivors. Such research could shed light on how significant others of HM patients can affect and be affected by disease-related concerns. To further explore the trajectory of FoP in patients with HM, the present study adopted latent profile analysis (LPA) to examine FoP status based on a family model among HM patients. LPA is a person-centered approach used to identify individuals according to similar features and classify similar individuals into latent discrete groups23. The LPA results should reveal homogeneity within the same latent group and heterogeneity between different groups. Additionally, a study by Yang et al.24 also claimed that LPA is an appropriate approach for exploring patterns of FoP among breast cancer patients. By integrating Mellon’s framework with LPA, we aimed to: (1) identify distinct FoP profiles incorporating individual, physical, psychological, and family-related dimensions; and (2) establish profile-specific predictors for FoP profiles.
Therefore, the current cross-sectional analysis of 455 HM patients further examined how sociodemographic characteristics, physical and psychological adaptability (illness perception, anxiety and depression), psychosocial health outcomes (quality of life), and family-related factors (family resilience) affect FoP trajectories. This multidimensional focus on individual psychosocial factors and family ecology enhances precision psycho-oncology in the nursing care of patients with HM.
Methods
Study design, setting, and participants
This study adopted a descriptive and individual-centred cross-sectional survey design. All methods were performed in accordance with the STROBE guidelines and regulations. Given the clinical accessibility needs of the target population, the questionnaire survey for HM patients was conducted during rest periods of hospitalization to maximize patient compliance. To enhance the representativeness of the sample, patients were continuously enrolled over an 11-month period using a convenience sampling method.
From March 2023 to February 2024, patients who were hospitalized in the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (IHCAMS) were selected as the survey subjects. Inclusion criteria for participants were as follows: (1) aged 18 years or older; (2) diagnosed with hematologic malignancies (including leukemia, lymphoma, multiple myeloma, and myelodysplastic syndromes); (3) informed of their hematologic diagnosis; and (4) proficient in speaking and reading Chinese. Exclusion criteria were as follows: (1) having end-stage disease (i.e., expected to live less than 6 months); (2) having moderate to severe risk of myelosuppression; (3) complicated by severe cardiovascular or cerebrovascular diseases, liver or renal dysfunctions, systemic immune disorders, or other malignant tumors.
Researchers screened the electronic medical records of hospitalized patients daily to identify patients with HM who met the inclusion and exclusion criteria. Subsequently, the attending physician reviewed the patients’ diagnosis, staging, treatment plan, and physical condition. Certified hematology nurses then conducted face-to-face interviews with the patients, which lasted approximately 15 min, in the activity room during non-treatment periods. This approach not only assessed the patients’ cognitive and communication abilities but also identified their unmet needs. Lastly, after being informed about the study’s purpose, patients who agreed to participate in the survey signed an informed consent form before receiving the self-reported questionnaires. All procedures were performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Ministry of Medicine of IHCAMS (No. FRFCU2023042-EC-2).
Sample size
The sample size was calculated by employing the following formula:
This calculation was performed to determine the sample size required to explore the influencing factors of specific variables, based on a cross-sectional survey design. In this study, a 95% confidence level (two-sided) was applied, with a corresponding Z1−α/2 value of 1.96. Referring to our pilot study, the estimated prevalence of fear of disease progression among HM patients was 62%, and a margin of error of 5% was deemed acceptable. Consequently, a minimum sample size of 362 was determined. Meanwhile, a 20% anticipated missing rate was accounted for in the study, resulting a total of 452 (362/0.8) being identified as the minimum sample size. This sample size also meets the requirements for achieving statistical power in LPA, as previous methodological research has shown that N ≈ 300–1000 is commonly used25. Therefore, a total of 500 participants were screened, and 45 were excluded due to the following reasons: lack of time (n = 17); refusal to talk about the topic (n = 14); concerns about the personal information (n = 8); being evaluated in a frailty status (n = 4); and diagnosis with a psychiatric disease (n = 2). (Fig. 1).
Data collection
Prior to the formal investigation, convenience sampling was used to select 20 patients with HM from Leukemia Treatment Center, Lymphoma Treatment Center, and Myelodysplastic Syndrome Treatment Center in the hospital, according to the inclusion and exclusion criteria. The sequence of the survey questionnaires was adjusted to determine the feasibility and duration of the survey procedure. Researchers conducted training and guidance sessions for a total of three surveyors from the three departments, covering standardized instructions, informed consent procedures, questionnaire completion methods, and relevant precautions. A questionnaire package was collected via an online survey platform called Questionnaire Star. Surveyors guided patients to scan the QR code to access the questionnaire on the Questionnaire Star platform. When it was difficult for the patients to understand specific items on the questionnaire, surveyors explained the meaning to them and confirmed the on-site collection of the questionnaires. Data were double-entered and verified by two individuals, and any discrepancies were promptly addressed.
Measures
Sociodemographic and clinical characteristics
The general information survey form is designed by the researchers and include sociodemographic data such as age, gender, education level, marital status, employment status, monthly per capita household income, and medical insurance status. It also included clinical characteristics, including diagnosis of hematologic malignancies as well as disease and treatment related data.
Fear of disease progression
The simplified Fear of Progression Questionnaire-Short Form (FoP-Q-SF) was developed by Mehnert et al.26 with 12 items selected from the original 43-item questionnaire (FOP-Q)27. The FoP-Q-SF is a self-report questionnaire that can be used to measure the fear of disease progression in cancer and chronic disease patients. The Chinese version was adapted by Wu28, and it includes two dimensions: physiological health (6 items) and social family (6 items). Items 1 to 3, 5, 9 to 10 represent the physiological health dimensions, while items 4, 6 to 8, 11 to 12 represent the social family dimension. Each item uses a Likert 5-point (1–5 points) scoring method, with a total score ranging from 12 to 60 points. Higher scores indicate more severe fear of disease progression. A score of 34 points or higher indicates a dysfunctional level of FoP. The Cronbach’s α was 0.926 for the total scale and 0.877 and 0.875 for the two subscales, respectively.
Illness perception
Illness perception was assessed using the Brief Illness Perception Questionnaire (BIPQ)29, a validated 9-item questionnaire measuring patients’ cognitive and emotional representations of any illness or health condition on an 11-point scale (0 = never, 10 = very often) from item 1 to 8. The last item is an open-ended question asking patients:“ What are the most 3 possible reasons of your illness?”. The higher total score (range: 0–80) indicates a severe negative perception of illness. The Chinese translation of the BIPQ has been proved to have good reliability and validity in HM patients30, while Cronbach’s α was 0.801 in the present study.
Anxiety and depression
Patient’s anxiety and depression were measured using the Hospital Anxiety and Depression Scale (HADS)31. This is a 14-item self-report questionnaire that can be used to assess psychological distress, which seven items assessing anxiety and seven items assessing depression. Items were scored on a four-point Likert scale (0–3), and the total score was calculated by summing the item score. Patients were classified as having present (> 11), mild (8–10), or no symptoms (0–7) for both subscales of anxiety and depression31. Cronbach’s α was 0.900 for the total scale, 0.825 for the anxiety subscale, and 0.822 for the depression subscale.
Family resilience
Family resilience was evaluated using the Family Hardiness Index (FHI)32, which consists of three domains, namely commitment, challenge, and control. The Chinese version was translated by Liu and has been tested in parents of hospitalized Chinese children, with a Cronbach’s α of 0.80333. The FHI contains 20 items, and responses are answered on a 4-point Likert scale (0 = totally disagree, 1 = disagree, 2 = agree, 3 = totally agree), with higher scores indicating a higher level of family resilience. Cronbach’s α for HM patients in the current study was 0.885.
Health outcome
The EuroQol-Visual Analogue Scale (EQ-VAS) was used to reflect the patients’ self-rated heath on a straight line with two endpoints, where the 0 is labelled “The worst health you can imagine” and 100 is labeled “The best health you can imagine.” Regarding the outcome of EQ-VAS, which ranges from 0 to 100, it can be seen as a quantitative assessment of patient-reported health status34.
Data analysis
This study used TidyLPA package in R version 4.3.2 to analyze the potential profile model of fear of disease progression. Using mean values of EQ-VAS, BIPQ, HADS-anxiety, HADS-depression, and FHI as external indicator, 1–5 profiles were selected for analysis stepwise. The model fit indices included the Akaike information criteria (AIC), Bayesian information criteria (BIC), adjusted Bayesian information criteria (aBIC), information entropy, Lo–Mendell–Rubin likelihood ratio test (LMRT), and bootstrap likelihood ratio test (BLRT)25. Smaller AIC, BIC, and aBIC values indicate better model fit. The information entropy ranges from 0 to 1, with values closer to 1 indicating more accurate classification. LMRT and BLRT are used to compare the fit differences between k class and k−1 class models. When these differences are both statistically significant (P ≤ 0.05), the k class profile model is considered superior to the k−1 class profile model35.
After determining the optimal potential profile model, statistical analysis was conducted using SPSS 24.0. The count data were described using frequency and percentage, and the normality of measurement data was tested using the Shapiro–Wilk test. The normal data were described using the mean and standard deviation, while the skewed data were expressed as median and interquartile range. Group comparisons were conducted using the identified profiles as grouping variables. Chi-square tests were used for count data, and the non-parametric Kruskal–Wallis H test was used for measurement data. Then, multivariate logistic regression analysis was used to identify the influencing factors across different profiles. All statistical analyses were bilateral tests with P ≤ 0.05 and considered statistically significant.
Results
General characteristics of survey subjects
There were 500 patients invited to participate in the cross-sectional study, and data from 455 (91%) were available. The general sociodemographic characteristics of the sample are presented in Table 1. The majority were male (n = 247; 54.29%) and lived in urban areas (n = 221; 48.57%). The median and interquartile range of age when surveyed was 46.00 (32.00, 58.00) years, and the duration of diagnosis was 0 (0, 1) years. Furthermore, to ensure consistency for subsequent univariate analysis, both age and time since diagnosis were divided into three groups. Additionally, 247 of 455 (54.29%) participants had been diagnosed with leukemia, 145 with lymphoma (31.87%), 52 with MDS (11.43%), and 11 with multiple myeloma (2.41%), respectively. More than half of the participants were unemployed or retired (n = 291; 63.96%).
Potential profile analysis of fear of disease progression
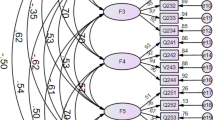
The scores of FoP-Q-SF, EQ-VAS, BIPQ, HADS-anxiety, HADS-depression, and FHI exhibited non-normal distribution. Therefore, the median and interquartile range for each variable were as follows: FoP-Q-SF, 35.5 (28,43); EQ-VAS, 70 (50,86.5); BIPQ, 41 (35,49); HADS-anxiety, 8 (6,11); HADS-depression, 7 (4,10); and FHI, 55 (47,59). Starting from the initial model, four potential profile models were fitted, as shown in Table 2. As the number of categories increased, the information entropy values were all greater than 0.8, and AIC and BIC values gradually decreased, reducing to 0.79 when the number of categories reached 5. Further comparison of the fit indices of various potential profile models, considering the P values of LMRT and BLRT, as well as the clinical significance of FoP, led to the selection of a 3-category potential profile model as the optimal model for FoP in the study. The patients with HM were divided into three subgroups based on their risk levels of fear: low-risk fear group (LRF), moderate-risk fear group (MRF), and high-risk fear group (HRF), as illustrated in Fig. 2.
The total scores of each variable in the three potential profile categories, as well as the results of pairwise comparison between them, are presented in Table 3. The total score of the low-risk fear group (LRF) was 21 (20, 24), with BIPQ scores being low and EQ-VAS and FHI scores being high. The total score of the moderate-risk fear group (MRF) was 34 (30, 39), with BIPQ, EQ-VAS and FHI scores being moderate. Moreover, the results reveled that the total score of the high-risk fear group (HFR) was 45 (39.5,50.5), with EQ-VAS and FHI scores being low and BIPQ scores being high level. However, only the FHI scores showed no significant differences in pairwise comparisons between LRF, MRF and HRF.
Multivariate logistic analysis results on influencing factors of profiles of fear of diseases progression
Univariate analysis was conducted to identify the significant differences between the three profiles in terms of sociodemographic and clinical factors, and the results are presented in Table 4. Thus, gender (χ2 = 14.64, P < 0.01), age (χ2 = 33.43, P < 0.01), marital status (χ2 = 25.13, P < 0.01), education background (χ2 = 39.46, P < 0.01), monthly per capita household income (χ2 = 17.36, P < 0.01), employment status (χ2 = 23.25, P < 0.01), and hematologic diagnosis (χ2 = 23.94, P < 0.01) were identified in univariate analysis. The variable selection followed a hierarchical strategy: (1) initial univariate screening (variables with P < 0.05) and (2) rigorous control for collinearity (VIF < 2.5 for all retained variables). Based on these results, multivariate logistic regression models were used for analysis, with the potential profile categories as the dependent variable (LRF = 1, MRF = 2, HRF = 3).
Table 5 presents the results of the multivariate logistic regression analysis of the variables that identified different patterns of fear of disease progression. McFadden’s pseudo-R2 values was 0.462, demonstrating reasonable explanatory power of the model. Compared with the low-risk fear group, female HM patients were more likely to report a moderate-risk fear (OR 2.577, 95% CI 1.511–4.396 ) or high-risk fear (OR 2.295, 95% CI 1.217–4.323 ); elderly patients were more likely to report a moderate-risk fear (OR 4.140, 95% CI 0.996–17.187) or high-risk fear (OR 9.363, 95% CI 1.981–44.252) than younger patients; HM patients with a higher academic degree were less likely to report a moderate-risk fear (OR 0.365, 95% CI 0.162–0.818) or high-risk fear (OR 0.270, 95% CI 0.104–0.693) than those with junior high school and lower education; HM patients with a moderate family income (3000 CNY ~ 5000 CNY) were less likely to report a moderate-risk fear (OR 0.477, 95% CI 0.104–0.693) or high-risk fear (OR 0.409, 95% CI 0.190–0.879) than those with a lower income (< 3000 CNY); patients with lymphoma were more likely to report a high-risk fear than those with leukemia (OR 2.295, 95% CI 1.120–4.797).
Discussion
Adults who receive a life-threatening diagnosis of hematologic malignancy at a significant stage or transition to later life are particularly vulnerable to psychosocial problems. The aim of this cross-sectional study was to analyze the differences in patterns of fear of disease progression based on a family model, considering personal factors, physical and psychological adaptability, psychosocial health outcomes, and family-related factors. This study scientifically and reliably utilized the latent profile analysis (LPA) model to identify the influencing factors of the FoP latent profiles among patients with hematologic malignancies .
Profiles of fear of disease progression
In our study, three latent patterns of FoP were identified: class 1-the low-risk fear group (20.88%), class 2-the moderate-risk group (54.73%), and class 3-the high-risk group (24.39%). These results suggested that hematologic malignancy patients’ FoP varied and reflected individual differences. The latent profile analysis is widely used to identify potential characteristics in varied populations. In a related study by Li et al.36, three latent patterns of fear of cancer recurrence (FCR) were identified among breast cancer (BC) patients. Social support, monthly family income, employment status, and medical coping modes were found to impact FCR among newly diagnosed BC patients. Another cross-sectional study37 identified two groups of FCR among family caregivers of patients with HM, suggesting that healthcare professionals can tailor personalized support and intervention measures to help patients and caregivers cope with emotional burden. For children with leukemia, researchers used the LPA model to categorize them based on the chemotherapy-related symptoms. They indicated that identifying heterogeneity in symptom severity is important to facilitate symptom-oriented care and maximize intervention effectiveness38. Thus, these findings highlight the importance of understanding the diverse profiles of FoP in patients with HM and suggest that targeted interventions should be developed to address the specific needs of each group, ultimately improving their psychological well-being and quality of life.
Predictors of the potential profile group of FoP
The impact of illness perception, anxiety, and depression on the FoP
This study found that HM survivors in the high-risk fear group were more likely to present a high level of illness perception, as well as anxiety and depression. These results were consistent with the findings of several previous studies focusing on the relationship between illness perception and FoP among hematopoietic stem cell transplantation survivors39, lung cancer patients40 and chronic heart failure patients41. HM patients who felt higher emotional distress exhibited stronger negative illness perception, were more likely to rely on passive coping strategies, and had lower quality of life. The results of Shen et al.’s study showed that social constraints can enhance negative illness perception, and the latter can promote patients’ FoP39. Mellon18 noted that the concurrent stress of devastating events may drain survivors’ and family caregivers’ mental energy. Illness-related stressors may further exacerbate psychological distress and concerns about FoP. In addition, according to the theory of FoP, the cognitive process by which patients experience internal (physical symptoms and side effects) or external ( social concerns, medical follow-up, etc.) trigger is affected by factors associated with the surrounding environment. Thus, negative factors increase survivors’ negative perception of illness and psychological distress8.
Furthermore, we found that the levels of anxiety and depression were the lowest among the low-risk fear group and the highest among the high-risk group. Considering that most of the HM patients in our study were young and middle-aged adults, with more than 70% of them having been diagnosed less than one year, we assumed that the reasons for anxiety and depression were not only the consequences of sudden diagnosis or unprepared treatment, but also the cognitive processes of concerns and worries about whether the illness would be cured, progressed, or temporally controlled. Throughout the treatment process, cancer patients may experience physical symptoms that cause pain, uncomfortable waiting times, and compromised self-image and self-esteem42. In addition, physical symptoms and functional problems can degrade functioning in daily life and increase vulnerability to anxiety, depression, and fear of illness progression or recurrence43. Collectively, illness perception and emotional burden are the key elements of the cognitive process, reflecting the patients’ physical and psychological burden of experiences. Empowering patients with appropriate perception and effective coping strategies could be beneficial in managing fear and uncertainty related to the disease, thus improving mental well-being and reducing FoP.
Family resilience’s protective role on the FoP
The results of this study also showed that family resilience played a protective role in the patterns of FoP subgroup, with the highest level among the low-risk fear group and lowest level among the high-risk group, consistent with findings in studies of cancer survivors18 and breast cancer patients44. Family resilience encompasses multiple dimensions used to measure family members’ hardiness to stress, including family co-oriented commitment, confidence in dealing with problems, attitudes towards current challenge, and sense of control over family life45. These dimensions have been demonstrated to have a significant impact on the FoP experienced by patients18. The median FHI score was 55 (1st quartile 47, 3rd quartile 59), consistent with the results of a Chinese study on patients with heart failure (mean 57.95, SD 11.41)46. These assessments indicate that the level of family resilience plays an equivalent and primary protective role within traumatic events.
Especially in Chinese culture, where family bonds and care are strongly connected among family members36, the active involvement of spouses, parents, children, and siblings in the present study can further provide powerful and helpful social support, including emotional and financial aid. Family resilience, deeply rooted in Chinese culture, provides high levels of support and understanding, mitigating survivors’ isolation and anxiety, enables them to make practical decisions, empowers personal resilience, encourages positive evaluations of current situations, and reduces the FoP47,48. However, the lack of statistical significance in FHI among the three potential classes in our study might be due to the fact that most of the HM participants were married. Consequently, spouses or partners exhibited equivalent level of resilience. These results may pave the way for tailored interventions focused on dyadic coping mechanisms or strategies for spouses or partners, aimed at mitigating the FoP and improving overall health outcomes for both patients and the families.
The effect of general characteristics on the FoP
In terms of sociodemographic characteristics of the participants, several studies have reported that FoP is more prevalent among survivors who are female and younger49,50. Similarly, the study found that compared with males, female patients were more likely to report moderate- or high-risk fear, which aligns with the findings in patients with gastrointestinal stromal tumor51 and hematological cancer52. Based on recent studies in the field of neuroscience, females recognize and express emotions more easily than males53. It can also be interpreted in the qualitative evidence synthesis study conducted by Chomhaill et al.54 that most women were aware of the prognosis, which led to concerns about missing out on meaningful milestones in their lives of spouse and children. Therefore, FoP aggravated women’s worries for the future of their loved ones55.
An interesting result was found in the patients’ characteristics: Older HM patients were at moderate to high risk of FoP, which was inconsistent with the findings of other studies56,57. This discrepancy is mainly due to the fact that older HM survivors, compared with younger patients, had more social frailty, isolation and economic issues. Most elderly patients face financial burdens due to side effects and complications during treatment. For example, in addition to common side effects of chemotherapy such as fatigue, vomiting, appetite loss, and insomnia, complications like invasive fungal infections and bacterial infections present frequent adverse outcomes for HM patients, resulting in high physical and economic burden58. The increasing number of adverse outcomes may indicate an increasing risk of life-threatening events or disease severity in elderly patients, which could contribute to severe concerns about disease progression among them. Notably, the median levels of anxiety and depression was 8 and 7, respectively, indicating a relatively moderate level of psychological distress and emotional burden. This is in line with findings from Sarkar and colleagues, who presented the impact of FoP on aging populations’ mental health-related quality of life prior to the treatment59. Regarding the reasons for physical frailty, emotional distress, and economic burden, elderly HM patients tend to be more vulnerable to worsening FoP.
Moreover, concerning sociodemographic variables such as monthly per capita household income and educational background, the results showed that the HM patients with a high level of education or income were more likely to have a low risk of FoP. Gallenkamp et al.60 demonstrated that patients who were socially isolated 5 to 7 years post-diagnosis, as well as those with less education or income, were at a greater risk of experiencing moderate to high levels of FoP, which can impact their QoL. The results suggest the need to pay more attention to these vulnerable patients, especially during and after the treatment period. Clinicians should consider providing effective strategies to help them learn about the prognosis and make shared decisions with patients and family caregivers.
The differences of hematologic diagnosis on the FoP
This study found that lymphoma patients have a significantly higher FoP than leukemia patients, consistent with the findings of Borreani et al.52. This results may stem from a difference in the perception of disease outcomes. Studies have reported that the cure rate for lymphoma patients is much higher than that for leukemia patients61. However, leukemia survivors have a much lower risk of late relapse, second primary malignancies, cardiovascular complications, and debilitating long-term treatment sequelae (such as polyneuropathy) compared to lymphoma patients62. Therefore, the characteristic relapse-remitting course of lymphoma may impose a unique psychological burden63, as patients must continuously face the uncertainty of the “watchful waiting” period and the cumulative pressure of multi-line treatments. It is noteworthy that among the 145 lymphoma patients in this study, 71.7% (104/145) were not at their initial diagnosis, and 45% of these patients required multi-line therapy for relapsed/refractory cases, which may explain the higher levels of FoP. Additionally, 72% of the 145 patients received anthracycline-containing regimens, which may have exacerbated FoP due to concerns about cardiac toxicity. Thus, further research with larger samples and longitudinal data is needed to differentiate and validate the differences in FoP levels among different subtypes of malignant hematological patients.
Strengths and limitations
Our study innovatively integrates latent profile analysis with the family model to systematically reveal the heterogeneous characteristics and key predictors of fear of progression among Chinese patients with hematologic malignancies. Leveraging the resources of a national clinical research center for malignant hematology and collaborating with a team of clinicians and hematological oncology nurses, the study ensures the scientific rigor and clinical applicability of the results through multidimensional assessment and quality control of the research process. Additionally, the identification of risks associated with female, older age, lower education level, lower family income, and lymphoma subtypes holds significant practical implications, providing a theoretical basis for clinical screening and family-involved care.
However, the current study presents some limitations that need to be considered. Firstly, we chose participants by convenience sampling from a single center. Meanwhile, the homogeneous sample was selected with regional bias, which could be mitigated to some extent because patients came from almost all over the country to one of the most renowned hematology-specific hospital. Second, the study was a cross-sectional analysis investigated hospitalized patients, which does not allow for a casual interpretation of the entire treatment cycle of HM patients. In addition, the limited sample size in this study may have introduced bias in the subgroup analysis. In particular, among the different hematological cancers, the lymphoma group included a relatively large proportion of relapsed and refractory cases. Moreover, variables such as treatment cycles, chronic disease index, and sources of social support can also affect the primary outcome indicators. Further research should address these limitations by analyzing FoP and explore biomarkers of psychological distress in multi-center hospitals over the full course of treatment.
Clinical implications
Hematologic malignancy patients are at varied risk of FoP and other physical and psychological distress. In terms of these results, we suggest that medical staff should pay attention to the HM patients who are female, older, have low levels of education or income, as well as those at risk of anxiety, depression, and negative illness perception, to identify moderate to high risk of FoP at an early stage and offer effective interventions to mitigate psychological distress.
Specifically, we implemented an intervention for patients in these three wards previously mentioned. Using data from initial cross-sectional surveys to identify the incidence of FoP and the core concerns and fears of HM patients, we collaborated with mental health professionals to develop a mindfulness learning program delivered via a WeChat mini-program on mobile phone. During hospitalization, nurses provided in-person guidance to patients and their caregivers, including introducing mindfulness practices and using videos and audio materials for weekly exercises, as well as teaching them to continue online mindfulness practice and community interaction via mobile phones after discharge. After one year of project implementation, we observed significant improvement in FoP, anxiety, and depression among patients and caregivers (unpublished results from the MindFOP-HM study).
Conclusions
In this cross-sectional study of patients with hematologic malignancies, three latent profiles of fear of disease progression were identified using the LPA technique based on the family model. Taken together, our investigation supposed that these profiles of FoP were significantly associated with gender, age, education, family income, hematologic diagnosis, patient-reported health conditions, illness perception, anxiety, and depression.
To address the heterogeneous needs of survivors, healthcare professionals should adopt a multidimensional approach to mitigate FoP and enhance psychological well-being. Tailored intervention plans should be developed to targets different risk groups with specific strategies. For example, clinical teams could provide peer support for the low-risk group, focus on family resilience and social support for the moderate-risk group, and design cognitive-behavioral therapy for the high-risk group. Moreover, healthcare providers can leverage AI-driven intelligent healthcare to develop monitoring systems that capture patient self-assessment scores and complaints. These systems can track physical and psychological symptoms and offer evidence-based, personalized interventions. Importantly, policymakers should advocate for incorporating FoP screening into psycho-oncology services and developing family-centered activities in collaboration with community hospitals and professional mental health institutions.
Data availability
The data supporting this study’s findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Harris, N. L. et al. The world health organization classification of hematological malignancies report of the clinical advisory committee meeting, airlie house, Virginia, November 1997. Mod. Pathol. 13, 193–207. https://doi.org/10.1038/modpathol.3880035 (2000).
Zheng, R. S. et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 46, 221–231. https://doi.org/10.3760/cma.j.cn112152-20240119-00035 (2024).
Cancer, I. A. f. R. o. Data Visualization Tools for Exploring the Global Cancer Burden in 2022. https://gco.iarc.fr/today/en/about (2022).
Ehooman, F. et al. Long-term health-related quality of life of critically ill patients with haematological malignancies: a prospective observational multicenter study. Ann. Intensive Care. 9, 2. https://doi.org/10.1186/s13613-018-0478-3 (2019).
Osaki, K. et al. Quality of life of patients with hematological malignancies and factors affecting health state utility values. Support Care Cancer. 30, 5319–5327. https://doi.org/10.1007/s00520-022-06958-y (2022).
Albrecht, T. A., Boyiadzis, M., Elswick, R. K. Jr., Starkweather, A. & Rosenzweig, M. Symptom management and psychosocial needs of adults with acute myeloid leukemia during induction treatment: A pilot study. Cancer Nurs. 40, E31–e38. https://doi.org/10.1097/ncc.0000000000000428 (2017).
Mutsaers, B. et al. Identifying the key characteristics of clinical fear of cancer recurrence: an international Delphi study. Psychooncology 29, 430–436. https://doi.org/10.1002/pon.5283 (2020).
Simonelli, L. E., Siegel, S. D. & Duffy, N. M. Fear of cancer recurrence: a theoretical review and its relevance for clinical presentation and management. Psychooncology 26, 1444–1454. https://doi.org/10.1002/pon.4168 (2017).
Zhang, X. et al. Factors correlated with fear of cancer recurrence in cancer survivors: A meta-analysis. Cancer Nurs. 45, 406–415. https://doi.org/10.1097/ncc.0000000000001020 (2022).
Denlinger, C. S. et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 12, 34–45. https://doi.org/10.6004/jnccn.2014.0005 (2014).
Andersen, B. L., Rowland, J. H. & Somerfield, M. R. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American society of clinical oncology guideline adaptation. J. Oncol. Pract. 11, 133–134. https://doi.org/10.1200/jop.2014.002311 (2015).
Barata, A., Wood, W. A., Choi, S. W. & Jim, H. S. Unmet needs for psychosocial care in hematologic malignancies and hematopoietic cell transplant. Curr. Hematol. Malig. Rep. 11, 280–287. https://doi.org/10.1007/s11899-016-0328-z (2016).
Göbel, P. et al. Interconnectivity of fear of progression and generalized anxiety—Network analysis among a sample of hematological cancer survivors. Support Care Cancer. 31, 238. https://doi.org/10.1007/s00520-023-07701-x (2023).
Hu, X., Wang, W., Wang, Y. & Liu, K. Fear of cancer recurrence in patients with multiple myeloma: prevalence and predictors based on a family model analysis. Psychooncology 30, 176–184. https://doi.org/10.1002/pon.5546 (2021).
Tian, Y. & Wang, Y. L. Resilience provides mediating effect of resilience between fear of progression and sleep quality in patients with hematological malignancies. World J. Psychiatry. 14, 541–552. https://doi.org/10.5498/wjp.v14.i4.541 (2024).
Winter, M. A., Hoppe, R. & Albrecht, T. A. I don’t have a choice but to keep getting up and doing the things that protect her: The informal caregiver’s adaptation to the cancer diagnosis. J. Psychosoc Oncol. 1–14. https://doi.org/10.1080/07347332.2024.2310813 (2024).
Xie, M. et al. Effects of remote dignity therapy on mental health among patients with hematologic neoplasms and their significant others: A randomized controlled trial. Int. J. Nurs. Stud. 151, 104668. https://doi.org/10.1016/j.ijnurstu.2023.104668 (2024).
Mellon, S., Kershaw, T. S., Northouse, L. L. & Freeman-Gibb, L. A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psychooncology 16, 214–223. https://doi.org/10.1002/pon.1074 (2007).
Faraji, A., Dehghani, M. & Khatibi, A. Familial aspects of fear of cancer recurrence: current insights and knowledge gaps. Front. Psychol. 14, 1279098. https://doi.org/10.3389/fpsyg.2023.1279098 (2023).
Wu, L. M., McGinty, H., Amidi, A., Bovbjerg, K. & Diefenbach, M. A. Longitudinal dyadic associations of fear of cancer recurrence and the impact of treatment in prostate cancer patients and their spouses. Acta Oncol. 58, 708–714. https://doi.org/10.1080/0284186x.2018.1563714 (2019).
Tao, L. et al. Investigating fear of cancer recurrence among female breast cancer survivors and their spouses in Southwest China: a cross-sectional study. BMJ Open. 14, e077964. https://doi.org/10.1136/bmjopen-2023-077964 (2024).
Liu, X., Zhang, Q., Yu, M. & Xu, W. Patterns of posttraumatic stress disorder and posttraumatic growth among breast cancer patients in China: A latent profile analysis. Psychooncology 29, 743–750. https://doi.org/10.1002/pon.5332 (2020).
Cui, C., Wang, L. & Wang, X. Profiles of social constraints and associated factors among breast cancer patients: a latent profile analysis. BMC Psychiatry. 22, 750. https://doi.org/10.1186/s12888-022-04407-y (2022).
Li, Y. et al. Exploring fear of cancer recurrence and related factors among breast cancer patients: A cross-sectional study. J. Adv. Nurs. https://doi.org/10.1111/jan.16009 (2023).
Nylund-Gibson, K. & Choi, A. Y. Ten frequently asked questions about latent class analysis. Transl. Issues Psychol. Sci. 4, 440–461. https://doi.org/10.1037/tps0000176 (2018).
Mehnert, A., Herschbach, P., Berg, P., Henrich, G. & Koch, U. [Fear of progression in breast cancer patients–validation of the short form of the fear of progression questionnaire (FoP-Q-SF)]. Z. Psychosom. Med. Psychother. 52, 274–288. https://doi.org/10.13109/zptm.2006.52.3.274 (2006).
Herschbach, P. et al. Fear of progression in chronic diseases: psychometric properties of the fear of progression questionnaire. J. Psychosom. Res. 58, 505–511. https://doi.org/10.1016/j.jpsychores.2005.02.007 (2005).
Wu, Q., Ye, Z., Li, L. & Liu, P. Reliability and validity of Chinese version of fear of progression Questionnaire-Short form for cancer patients. Chin. J. Nurs. 50, 1515–1519 (2015).
Broadbent, E., Petrie, K. J., Main, J. & Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 60, 631–637. https://doi.org/10.1016/j.jpsychores.2005.10.020 (2006).
Li, J., Tian, R. & Li, Q. Current situation and influencing factors of social constraints in patients with hematological malignancies. Int. J. Nurs. 42, 2352–2355. https://doi.org/10.3760/cma.j.cn221370-20201230-00575-1 (2023).
Olssøn, I., Mykletun, A. & Dahl, A. A. The hospital anxiety and depression rating scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 5, 46. https://doi.org/10.1186/1471-244x-5-46 (2005).
McCubbin, H. I. & McCubbin, T. A. Family Assessment: Resilincy, Coping and Adapation-Inventories for Research and Practice, 130–189 (Universuty of Wisconsin System, 1996).
Liu, Y. et al. Reliability and validity of the Chinese version of family hardiness index. J. Nurs. Adm. 14, 770–772 (2014).
Foundation, E. R. EQ-5D-5L. https://euroqol.org/information-and-support/euroqol-instruments/eq-5d-5l/ (2024).
Nylund, K. L., Asparouhov, T. & Muthén, B. O. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct. Equ. Model. Multidiscip. J. 14, 535–569. https://doi.org/10.1080/10705510701575396 (2007).
Li, Y. et al. Exploring fear of cancer recurrence and related factors among breast cancer patients: A cross-sectional study. J. Adv. Nurs. 80, 2403–2414. https://doi.org/10.1111/jan.16009 (2024).
Sheng, L. et al. Fear of cancer recurrence and associated factors in family caregivers of patients with hematologic malignancy receiving chemotherapy: A latent profile analysis. Asia Pac. J. Oncol. Nurs. 11, 100382. https://doi.org/10.1016/j.apjon.2024.100382 (2024).
Wang, M. et al. Chemotherapy-related symptoms in children with leukemia: application of latent profile analysis and network analysis. Support Care Cancer. 32, 207. https://doi.org/10.1007/s00520-024-08410-9 (2024).
Shen, Z., Shi, S., Li, C. & Ruan, C. The influence of social constraints on the quality of life of hematopoietic stem cell transplantation survivors: the chain mediating effect of illness perceptions and the fear of cancer recurrence. Front. Psychol. 13, 1017561. https://doi.org/10.3389/fpsyg.2022.1017561 (2022).
Liu, Q. W., Qin, T., Hu, B., Zhao, Y. L. & Zhu, X. L. Relationship between illness perception, fear of progression and quality of life in interstitial lung disease patients: A cross-sectional study. J. Clin. Nurs. 30, 3493–3505. https://doi.org/10.1111/jocn.15852 (2021).
Xiong, J., Qin, J., Zheng, G., Gao, Y. & Gong, K. The relationship between symptom perception and fear of progression in patients with chronic heart failure: a multiple mediation analysis. Eur. J. Cardiovasc. Nurs. 22, 638–646. https://doi.org/10.1093/eurjcn/zvad024 (2023).
Elliott, J. et al. The health and well-being of cancer survivors in the UK: findings from a population-based survey. Br. J. Cancer. 105 (Suppl 1), 11–20. https://doi.org/10.1038/bjc.2011.418 (2011).
Dempsey, L. E., Karver, M. S., Labouliere, C., Zesiewicz, T. A. & De Nadai, A. S. Self-perceived burden as a mediator of depression symptoms amongst individuals living with a movement disorder. J. Clin. Psychol. 68, 1149–1160. https://doi.org/10.1002/jclp.21901 (2012).
Li, Y. et al. Effects of social support, family resilience, and individual resilience on fear of cancer recurrence among persons with breast cancer: A cross-sectional study. West. J. Nurs. Res. 45, 993–1000. https://doi.org/10.1177/01939459231200772 (2023).
Persson, C., Benzein, E. & Årestedt, K. Assessing family resources: validation of the Swedish version of the family hardiness index. Scand. J. Caring Sci. 30, 845–855. https://doi.org/10.1111/scs.12313 (2016).
Peng, Y., Wang, J., Sun, G. & Liu, S. Family hardiness in patients with heart failure: exploring protective factors and identifying the mediator. Psychol. Res. Behav. Manag. 14, 355–364. https://doi.org/10.2147/prbm.S301765 (2021).
Li, Y., Wang, K., Yin, Y., Li, Y. & Li, S. Relationships between family resilience, breast cancer survivors’ individual resilience, and caregiver burden: A cross-sectional study. Int. J. Nurs. Stud. 88, 79–84. https://doi.org/10.1016/j.ijnurstu.2018.08.011 (2018).
Coughlin, S. S. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 177, 537–548. https://doi.org/10.1007/s10549-019-05340-7 (2019).
Bergerot, C. D. et al. Fear of Cancer recurrence in patients with localized renal cell carcinoma. JCO Oncol. Pract. 16, e1264–e1271. https://doi.org/10.1200/op.20.00105 (2020).
Luigjes-Huizer, Y. L. et al. What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta-analysis. Psychooncology 31, 879–892. https://doi.org/10.1002/pon.5921 (2022).
van de Wal, D. et al. Fear, anxiety and depression in Gastrointestinal stromal tumor (GIST) patients in the Netherlands: data from a cross-sectional multicenter study. Int. J. Clin. Health Psychol. 24, 100434. https://doi.org/10.1016/j.ijchp.2023.100434 (2024).
Borreani, C., Alfieri, S., Farina, L., Bianchi, E. & Corradini, P. Fear of cancer recurrence in haematological cancer patients: exploring socio-demographic, psychological, existential and disease-related factors. Support Care Cancer. 28, 5973–5982. https://doi.org/10.1007/s00520-020-05434-9 (2020).
Kret, M. E. & De Gelder, B. A review on sex differences in processing emotional signals. Neuropsychologia 50, 1211–1221. https://doi.org/10.1016/j.neuropsychologia.2011.12.022 (2012).
Chomhaill, N. G., Ward, C., Dowling, M. & J. & Fear of recurrence in women with ovarian cancer: A qualitative evidence synthesis. Eur. J. Oncol. Nurs. 68, 102487. https://doi.org/10.1016/j.ejon.2023.102487 (2024).
Kyriacou, J., Black, A., Drummond, N., Power, J. & Maheu, C. Fear of cancer recurrence: A study of the experience of survivors of ovarian cancer. Can. Oncol. Nurs. J. 27, 236–242. https://doi.org/10.5737/23688076273236242 (2017).
Tsai, L. Y., Lee, S. C., Wang, K. L., Tsay, S. L. & Tsai, J. M. A correlation study of fear of cancer recurrence, illness representation, self-regulation, and quality of life among gynecologic cancer survivors in Taiwan. Taiwan. J. Obstet. Gynecol. 57, 846–852. https://doi.org/10.1016/j.tjog.2018.10.014 (2018).
Hefner, J. et al. High prevalence of distress in patients after allogeneic hematopoietic SCT: fear of progression is associated with a younger age. Bone Marrow Transpl. 49, 581–584. https://doi.org/10.1038/bmt.2013.228 (2014).
Afhami, S. et al. Risk factors, and outcomes of invasive fungal infections in patients with hematologic malignancies. Int. J. Hematol. Oncol. Stem Cell. Res. 18, 75–82. https://doi.org/10.18502/ijhoscr.v18i1.14746 (2024). Rate.
Sarkar, S. et al. Fear of recurrence and its impact on quality of life in patients with hematological cancers in the course of allogeneic hematopoietic SCT. Bone Marrow Transpl. 49, 1217–1222. https://doi.org/10.1038/bmt.2014.139 (2014).
Koch-Gallenkamp, L. et al. Fear of recurrence in long-term cancer survivors-Do cancer type, sex, time since diagnosis, and social support matter? Health Psychol. 35, 1329–1333. https://doi.org/10.1037/hea0000374 (2016).
Kornblith, A. B. et al. Comparison of psychosocial adaptation of advanced stage Hodgkin’s disease and acute leukemia survivors. Cancer and leukemia group B. Ann. Oncol. 9, 297–306. https://doi.org/10.1023/a:1008297130258 (1998).
Baum, J. et al. Impairment of vocational activities and financial problems are frequent among German blood cancer survivors. Sci. Rep. 13, 22856. https://doi.org/10.1038/s41598-023-50289-9 (2023).
Zhang, Q. et al. Evaluating the psychometric properties of the simplified Chinese version of PROMIS-29 version 2.1 in patients with hematologic malignancies. Sci. Rep. 14, 11153. https://doi.org/10.1038/s41598-024-61835-4 (2024).
Acknowledgements
All authors would like to gratitude the patients who completed our investigation in the present study.
Funding
The current research was sponsored by Special Research Fund for Central Universities, Peking Union Medical College (Grant/Award number: 3332023063) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS) (Grant/Award number2022-I2M-C&T-B-093).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The wards and appropriated research timing were offered by L.J.J., Z.J.Y., W.Z.X. and T.F. Material preparation, sample recruitment, and data collection were performed by L.Y.T., S.Z.L., L.X.B., and Z.J.Y. Data analysis were performed by Z.T.T. and Z.Q.Q. The first draft of the manuscript was written by L.Y.T. and Z.Q.Q., and the revised version was critically reviewed by X.W.J., L.J.J., Y.F., and L.Q.Y. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zhang, Q., Zhao, J. et al. Latent profile analysis of fear of progression in Chinese hematologic malignancy survivors. Sci Rep 15, 15265 (2025). https://doi.org/10.1038/s41598-025-00415-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00415-6