Abstract
Oxidative stress and inflammation are crucial in dry eye pathogenesis. The antioxidant and anti-inflammatory activities of chlorogenic acid (CGA) have been studied in various fields, including ophthalmology. However, its therapeutic effects on dry eye remain unclear. This study aims to predict and validate the mechanisms of CGA in treating dry eye through data mining and in vitro experiments. Data mining integrated multiple online databases to predict potential therapeutic targets of CGA for dry eye and performed target enrichment analysis. In vitro experiments involved dividing rat lacrimal gland epithelial cells (LGECs) into seven groups: (1) control, (2) H2O2 model (200µM), (3) H2O2 + AKT inhibitor (MK-2206, 2.5µM), (4) H2O2 + AKT activator (SC79, 10ng/ml), (5) H2O2 + CGA (500nM), (6) H2O2 + AKT inhibitor + CGA, (7) H2O2 + AKT activator + CGA. We measured cell viability, mitochondrial ROS, PI3K/AKT/FoxO3 pathway proteins, and key indicators like Caspase3, TRAIL, Bcl-2, and SOD2. Results showed that CGA treatment and AKT activation could activate the PI3K/AKT/FoxO3 pathway, reduce ROS in LGECs, increase SOD2 production, and inhibit apoptosis. These findings suggest CGA as a promising candidate for further research in dry eye prevention and treatment.
Similar content being viewed by others
Introduction
Dry eye is one of the primary diagnoses in ophthalmology clinics, with a global prevalence ranging from 5 to 50%1. The persistent discomfort caused by dry eye significantly affects patients’ quality of life and mental health and imposes a substantial economic burden on society2. Current treatments for dry eye primarily include artificial tears, topical anti-inflammatory drugs, and immunosuppressants such as cyclosporine A. However, artificial tears have limited efficacy and require frequent administration, while long-term use of anti-inflammatory drugs or immunosuppressants may lead to local or systemic complications3. Therefore, developing safer and more effective treatments for dry eye is of paramount importance.
Oxidative stress is considered a key pathological process in many chronic ocular symptoms and inflammations4,5. Studies have shown a significant increase in oxidative stress markers in the tears and conjunctival cells of dry eye patients, indicating an imbalance with antioxidant enzymes6. Under oxidative stress, continuous generation of reactive oxygen species (ROS) by mitochondria disrupts the oxidative stress balance when ROS accumulate excessively or the body’s antioxidant defense function is reduced. This disruption leads to the accumulation of free radicals, causing damage to cellular components such as proteins, lipids, and DNA, and inducing inflammatory apoptosis of cells. In the eye, this manifests as decreased tear film stability, reduced tear volume, and inflammation of ocular surface tissues, all of which are pathological changes associated with dry eye7. Therefore, the use of antioxidants in the treatment of dry eye may be a potential breakthrough for maintaining ocular redox homeostasis and interrupting the vicious cycle between oxidative stress and inflammation on the ocular surface8,9.
Chlorogenic acid (CGA) is a polyphenolic substance composed of caffeic acid and quinic acid, which is widely found in natural plants such as Coffea, Lonicera japonica Thunb., and Chrysanthemum morifolium Ramat. The content of CGA has been used as a measure of the antioxidant capacity in many Traditional Chinese medicine formulations for the treatment of dry eye, such as JuHuang Oral Liquid and JinZhu Eye Drops10,11. CGA has strong biological activity that can not only reduce cell ROS production in ocular tissues and exert anti-inflammatory effects, but also promote the production of antioxidant proteins12,13. Numerous in vitro and in vivo studies have demonstrated CGA’s antioxidant effectiveness in age-related eye diseases such as cataracts, glaucoma, and diabetic retinopathy12,14,15. However, there is still a research gap in the field of dry eye treatment with CGA, and whether it has a therapeutic effect on dry eye and its possible mechanism of action remains to be explored16.
Modern open-source databases provide rich information sources for drug development. Data mining is an example of the successful integration of computer science and medicine. By collecting disease- and drug-related targets and pathways from various databases and combining them with corresponding bioinformatics tools, we can predict the potential therapeutic targets and mechanisms of compounds acting on diseases, thereby accelerating the subsequent experimental exploration process17. This study utilized data mining techniques to predict the potential mechanism of CGA in treating dry eye and verified it by related in vitro experiments.
Materials and methods
Mechanism prediction
Target collection and visualization
CGA target data were collected using the Pharmmapper database (http://www.lilab-ecust.cn/pharmmapper/)18 and the SwissTargetPrediction (STP) platform (http://www.swisstargetprediction.ch)19. Relevant targets for dry eye were obtained from the GeneCards (https://www.genecards.org) [20], Therapeutic Target Database (TTD)(https://db.idrblab.net/ttd/)21, and OMIM (https://omim.org) [22] databases. After removing duplicates, target names were standardized using the Uniprot database (https://www.uniprot.org)23. The intersection of dry eye and CGA targets was taken as potential therapeutic targets, and a Venn diagram of drug-disease targets was drawn. Additionally, some experimentally validated but not database-recorded potential targets were supplemented through literature retrieval. The intersected target data were imported into Cytoscape 3.8.2 software for analysis and visualization of therapeutic targets.
PPI network construction and molecular docking
The potential therapeutic targets were imported into the STRING (https://cn.string-db.org)24 network platform, with the protein species set as “Homo sapiens”, and a protein-protein interaction (PPI) network was drawn. The network was further visualized using Cytoscape 3.8.2 software. A histogram was drawn for the top 20 target proteins by degree value. The 135 predicted therapeutic targets were subjected to enrichment analysis using the Metascape database (https://metascape.org), with logP values used as the sorting criterion to predict the primary pathways through which CGA treats dry eye. This included KEGG pathway enrichment analysis25 along with GO functional enrichment analysis, encompassing BP (biological process), MF (molecular function), and CC (cellular component). The results were imported into the online bioinformatics platform (http://www.bioinformatics.com.cn) to create the corresponding enrichment bubble charts. Core proteins were used as receptors for molecular docking validation with CGA. The 3D structure of AKT was downloaded from the PDB database (https://www.rcsb.org) and PyMOL 2.5.2 was used to remove organic compounds and water molecules. The three-dimensional structure of CGA was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). Finally, molecular docking was performed using Autodock-vina. The docking results were visualized and analyzed using PyMOL 2.5.2.
In vitro experimental protocol
Key reagents and apparatus
Name | Company | identifier | |
|---|---|---|---|
reagents | Rabbit Anti PI3K | Affinity | AF6241 |
Mouse Anti AKT | Bioss | bs-0115 M | |
Rabbit Anti p-AKT | Affinity | AF0016 | |
Rabbit Anti FOXO3 | Affinity | AF6020 | |
Rabbit Anti p-FOXO3 | Affinity | AF3020 | |
Rabbit Anti Caspase-3 | Affinity | AF6311 | |
Rabbit Anti Cleaved Caspase-3 | Abcam | AB179517 | |
HRP conjugated Goat Anti-Rabbit IgG (H + L) | Servicebio | GB23303 | |
Pan Cytokeratin | Bioss | bs-1712R | |
Cy3-Goat Anti-Rabbit IgG | ABclonal | AS007 | |
Rabbit Anti Bcl-2 | Bioss | bs-34012R | |
Rabbit Anti TRAIL | Bioss | bs-1214R | |
BCA Protein Quantification Kit | Elabscience | E-BC-K318-M | |
Superoxide Dismutase 2 (SOD2) ELISA Kit | MEIMIAN | MM-50282R1 | |
TUNEL Detection Kit Beyotime | Beyotime | C1088 | |
MitoSOX | YESEN | 40778ES50 | |
DAPI Staining Solution KeyGEN | KeyGEN | KGA215-50 | |
Hoechst 33342 | Beyotime | C1026 | |
ROS Reactive Oxygen Species Detection Kit | KeyGEN | KGT010-1 | |
RIPA Cell Lysis Buffer | APPLYGEN | C1053 | |
apparatus | Electrophoresis apparatus | LIUYI | DYY-6C |
Ultra-high sensitivity chemiluminescence imaging system | Bio-Rad | Chemi DocTM XRS+ | |
Chemiluminescent imaging system | Tanon | Tanon-5200 | |
Automatic Microplate Reader | LIUYI | WD-2012B | |
Low-Temperature High-Speed Centrifuge | Eppendorf | 5424R | |
Electric Thermostatic Incubator | YIHENG | DHP-9082B | |
Fluorescence Microscope | OLYMPUS | CKX53 | |
Electric Thermostatic Water Bath | BLUE POWER | HH-11-2 | |
Thermostatic Shaker | Tocan | TC-100B | |
Refrigerator-Freezer | Midea | BD/BC-415DKEM |
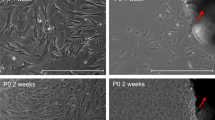
Cell isolation and culture
Four to six-week-old SD rats (SPF grade, male, License No.: SCXK (Xiang) 2019 − 2004, by Hunan Slaughter Kingda Laboratory Animal Co. Ltd.) were euthanized by cervical dislocation and immersed in 75% ethanol for 3–5 min. The lacrimal glands were excised using ophthalmic scissors under a sterile hood and washed three times with PBS buffer. The lacrimal gland tissues were then isolated under a dissecting microscope and cut into 1–2 mm pieces with ophthalmic scissors after rinsing in the original solution. The tissues were digested with 0.2% type II collagenase at 37 °C for approximately 25 min, and the digestion was terminated by adding culture medium. The gland cell clusters were gently dissociated into smaller clusters. The cell suspension was filtered through a 40 μm cell strainer and centrifuged at 300 g for 5 min. The pellet was resuspended in complete culture medium and triturated into a single-cell suspension, which was then seeded into culture plates. Differential adhesion was performed twice, each for 30 min, to purify the cells. The cell suspension was collected and seeded onto culture plates pre-coated with 1/100 Matrigel and incubated. When the cell density reached 80-90%, the cells were passaged. During passaging, the cell suspension was divided at a 1:3 ratio into prepared culture dishes, labeled, and incubated. According to experimental requirements, cells were plated in 6-well plates.
Immunofluorescence identification of isolated LGECs
Cultured LGECs were fixed and blocked. For primary antibody immunoreaction: the blocking solution was aspirated, and rabbit polyclonal anti-Pan Cytokeratin antibody (1:100; Cat# bs-1712R, Bioss, China) was added to the culture dish, followed by incubation at 4 °C overnight. Fluorescent secondary antibody reaction: The culture dish was washed with PBS three times, each for 3 min. After aspirating the excess liquid, Cy3-conjugated goat anti-rabbit IgG (1:200; Cat# AS007, ABclonal, China) was added, followed by incubation at 37 °C for 30 min. Nuclear staining and localization: DAPI solution (Cat# KGA215-50, KeyGEN, China) was added and incubated in the dark for 5 min to stain the nuclei, followed by washing with PBS to remove excess DAPI. Mounting: the culture dish was sealed with 50% glycerol, and images were observed and collected under a fluorescence microscope.
Cell grouping and treatment
This study includes seven experimental groups: (1) Control group, (2) H2O2 model group (200 µM), (3) Model + AKT inhibitor group (MK-2206, 2.5 µM), (4) Model + AKT activator group (SC79, 10 ng/ml), (5) Model + CGA group (500 nM), (6) Model + AKT inhibitor + CGA group, (7) Model + AKT activator + CGA group.
MK-2206 is a non-ATP-competitive AKT inhibitor that directly binds to AKT, inhibiting its serine/threonine phosphorylation and thereby blocking AKT-mediated signaling pathways [26]. In contrast, SC79 promotes AKT phosphorylation and enhances downstream signaling pathways, such as the upregulation of Bcl-2 and Bcl-xL, thereby improving cell survival27.
The concentrations of H2O2 (200 µM), MK-2206 (2.5 µM), SC79 (10 ng/ml), and CGA (500 nM) were determined through preliminary CCK-8 assays (see Results 3.2.2 and Fig. 5A-C) and supported by previous studies12,13,28,29. Cells were treated with 200 µM H2O2 for 2 h. After modeling, the medium was replaced with low-glucose DMEM containing 10% fetal bovine serum, and the respective CGA and pathway modulators were added. The cells were then cultured for an additional 24 h.
CCK-8 cell viability assay
Cell viability was measured using the CCK-8 assay. First, preliminary dose-response curves were established by treating LGECs with CGA (0-2000 nM), MK-2206 (0–10 µM), and SC79 (0-100 ng/ml) for 24 h to determine non-toxic concentrations. Based on these results, cells were subsequently treated with the selected drug concentrations for 24 h according to the groupings, and cell viability was measured again. The treated cells were incubated with 1/10 diluted CCK-8 reagent at 37 °C for 2 h in a thermostatic incubator; the absorbance of each well was then measured at 450 nm using a microplate reader.
MitoSOX staining for mitochondrial ROS detection
Mitochondrial ROS was detected using MitoSOX staining. The experimental procedure was as follows: 13 µl of DMSO was added to 50 µg of MitoSOX to prepare a 5 mM stock solution; this stock solution was diluted 1:1000 with HBSS and added to the culture dish to cover the cells. The cells were incubated at 37 °C in the dark for 10 min and then gently washed three times with pre-warmed PBS. Hoechst 33342 was added and incubated in the dark for 3 min for nuclear staining, followed by washing with PBS to remove excess Hoechst 33342. The cells were then observed under a microscope. Fluorescence intensity was quantified using ImageJ.
Western blot analysis
Lacrimal gland epithelial cells were collected, and total protein was extracted using RIPA lysis buffer. The lysate was centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was collected. Total protein concentration was quantified using a BCA protein assay kit. Protein samples were denatured and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to a PVDF membrane (Millipore) using a constant current of 300 mA. The PVDF membrane was blocked with non-fat milk and incubated with primary antibody at 4 °C overnight. The next day, the membrane was incubated with secondary antibody at room temperature, followed by incubation with chemiluminescent substrate. Imaging was performed using a highly sensitive chemiluminescence imaging system (Chemi Doc XRS+, Bio-Rad Laboratories, Shanghai). To clearly delineate the experimental structure and reveal the roles of the AKT pathway and CGA in counteracting oxidative damage in LGECs, the Western blot experiments were divided into two groups. Corresponding to the cell group numbers, the first four groups were 1, 2, 3, and 4; the latter four groups were 2, 5, 6, and 7.
ELISA detection of SOD2
The intracellular content of SOD2 was measured using an ELISA kit. Cell lysates were centrifuged at 1,000 g for 20 min, and the supernatant was collected. The ELISA was performed according to the manufacturer’s instructions to determine the SOD2 content.
TUNEL apoptosis detection
Cells cultured on coverslips in culture plates were washed three times with PBS and fixed with paraformaldehyde. The cells were then permeabilized at room temperature for 20 min, followed by thorough washing with PBS three times, each for 5 min. Excess PBS around the coverslip was removed with absorbent paper, and a sufficient amount of TUNEL detection solution was added to each coverslip. The cells were incubated at 37 °C in the dark for 2 h. Nuclear counterstaining was performed by washing away the excess detection solution with PBS, adding DAPI, and incubating in the dark for 5 min. The specimens were then washed with PBS to remove excess DAPI. Mounting was performed by drying the coverslips with absorbent paper and sealing them with an antifade mounting medium. Images were observed and collected under a fluorescence microscope. The results were expressed as the percentage of TUNEL-positive cells.
Data analysis
Quantitative data were expressed as mean ± SD. For normally distributed data, t-tests or ANOVA were used; for non-normally distributed data, Mann-Whitney U or Kruskal-Wallis H tests were used. Data were analyzed with IBM SPSS Statistics 19. One-way ANOVA, with LSD and S-N-K for pairwise comparisons, was used for multiple group comparisons. A significance level of α = 0.05 was set, with P < 0.05 considered significant.
Results
Mechanism prediction
Potential therapeutic targets
A total of 377 CGA targets were collected. Pharmmapper identified 367 targets, and SwissTargetPrediction identified 24 targets. A total of 2,283 dry eye targets were collected. GeneCards identified 2,122 targets (relevance score ≥ 2.47), TTD identified 6 targets, and OMIM identified 181 targets.
The integration of dry eye and CGA targets resulted in 133 intersecting genes, as shown in the Venn diagram (Fig. 1A). Additionally, two potential targets related to the mechanism of CGA in treating dry eye were supplemented through literature review30. Visualization of the CGA targets for dry eye treatment is shown in Fig. 1B.
Enrichment analysis results and molecular docking validation
The PPI network enrichment results showed 134 nodes and 1,601 edges, with an average node degree of 23.9. The node degree indicates the number of connections a target has; a higher node degree suggests that the target has close interactions within the disease network, potentially indicating it as a key active component target. The size and color gradient of the nodes represent the degree value of the targets (see Fig. 2A). The results identified potential targets for CGA in treating dry eye, including ALB, AKT1, EGFR, IGF1R, HRAS, and ESR1. Figure 2B shows the top 20 targets ranked by degree value. Enrichment analysis revealed 279 pathways and 6,515 GO terms, including 397 CC, 758 MF, and 4,964 BP terms. The top 20 significantly enriched items, arranged in ascending order of logP (Fig. 2C), are shown in the GO and KEGG pathway enrichment bubble chart in Fig. 2C. In the chart, the horizontal axis represents the ratio of enriched genes to the total number of pathway genes, the bubble size represents the number of enriched genes, and the bubble color indicates the P value. The KEGG pathway enrichment analysis revealed significant pathways, including Phosphatidylinositol 3-kinase (PI3K)/AKT (also known as protein kinase B, PKB) signaling pathway, Ras signaling pathway, MAPK signaling pathway, Rap1 signaling pathway, and FoxO signaling pathway. The enriched GO terms for the intersecting targets focused on biological processes and molecular functions such as kinase activity, positive regulation of protein phosphorylation, and cellular response to stress. In the PPI analysis, proteins like AKT1, IGF1, IGF1R, apoptosis-related protein Caspase 3, and Bcl-2 were active in the target network. Additionally, oxidoreductase activity in molecular functions and the antioxidant enzyme SOD2 appeared prominently in the enrichment results. During the molecular docking simulation, the ligand and receptor exhibited a tight "pocket-like" docking conformation (Fig. 3), with a docking score of -8.1 kcal/mol. CGA formed eight hydrogen bonds with the AKT residues LYS-41, LYS-138, ARG-4, ASN-141, and SER-7, indicating a strong affinity between CGA and AKT.
(A) Network diagram of protein-protein interaction (PPI). Nodes represent targets and the lines indicate the relationships between them. The higher the degree value, the larger the node. (B) Top 20 sorted target degree values. The horizontal axis represents the target degree values, and the vertical axis represents the target names. (C) Enrichment bubble plots of GO and KEGG pathway analysis. Subfigures (a)-(d) show the enrichment analysis results for KEGG pathways, cellular component (CC), molecular function (MF), and biological process (BP), respectively. Note: The size and color of the dots represent the degree of enrichment analysis.
Molecular docking simulation of CGA with AKT (PDB ID: 3CQW). The docking model shows good shape complementarity between the contact area of the receptor and the ligand in the docking image. CGA forms eight hydrogen bonds with residues LYS-41, LYS-138, ARG-4, ASN-141, and SER-7 of AKT, showing good affinity (-8.1 kcal/mol).
Experimental validation
Immunofluorescence identification of isolated LGECs
As shown in Fig. 4, immunofluorescence detected the expression of the lacrimal gland epithelial cell marker protein pan-cytokeratin (red fluorescence) on the cell membrane, indicating the successful isolation of lacrimal gland epithelial cells.
CCK-8 cell viability assay
Compared to the control group, cell viability in each group showed a concentration-dependent decrease (Fig. 5A, 5B, and 5C). Among the tested concentrations, 500 nM CGA demonstrated a moderate protective effect, improving cell viability compared to other CGA-treated groups. To validate these findings, further analyses were conducted. Cell viability in the SC79-treated group (10 ng/ml) was significantly higher than that of the model group (P < 0.05, Fig. 5B). Similarly, treatment with 500 nM CGA after H2O2 exposure significantly improved cell viability compared to the model group (P < 0.05, Fig. 5C). To ensure efficacy and maintain certain cell viability, the experimental concentration of MK-2206 was chosen as 2.5 µM (cell viability 87.1%); SC79 at 10 ng/ml (cell viability 93.18%); and CGA at 500 nM, based on comprehensive literature review results12,13,28,29.
Overall, data indicated that cell viability significantly decreased after 24 h of treatment with the respective drugs compared to the control group (P < 0.0001). Compared to the model group, cell viability in the MK-2206 group significantly decreased (P < 0.0001), whereas no significant difference was observed in the SC79 group (P > 0.05). Compared to the model group, CGA significantly improved cell viability (P < 0.05); however, cell viability in the combined CGA and MK-2206 group was significantly reduced compared to the CGA group (P < 0.0001), while no significant change was observed in the combined CGA and SC79 group (P > 0.05). Cell viability in each group is shown in Fig. 5D.
CCK-8 assay validation of the effects of various drugs on LGEC viability. (A) Cell viability decreased to 87.1% with 2.5 µM MK-2206. (B) SC79 (10 ng/ml) maintained high cell viability, significantly improving viability compared to the model group. (C) CGA exhibited a biphasic effect: 500 nM partially rescues H2O2-induced viability loss (vs. model group, P < 0.05), while higher doses (≥ 2000 nM) exacerbate toxicity. (D) Effects of individual and combined drug treatments on cell viability. Note: * Compared to the control group, *P < 0.05, ****P < 0.0001; # Compared to the model group, P < 0.05.
MitoSOX staining for mitochondrial ROS detection
The MitoSOX staining results (Fig. 6A and B) showed that ROS production in the model group was significantly higher than in the control group (P < 0.05). Compared to the model group, ROS levels significantly increased in the MK-2206 group (P < 0.001), while the average fluorescence intensity of ROS in the AKT activator group was not significantly different from the model group (P > 0.05). The ROS levels in the CGA-treated group were significantly lower than in the model group (P < 0.05). However, in the group treated with both CGA and the AKT inhibitor, ROS levels significantly increased compared to the model group (P < 0.0001). Additionally, no significant difference was observed between the CGA-treated group and the group treated with both CGA and SC79 (P > 0.05).
Western blot analysis
PI3K/Akt signaling pathway and anti-apoptotic protein Bcl-2
Western blot analysis revealed significant changes in the PI3K/Akt signaling pathway and anti-apoptotic protein Bcl-2 across different treatment groups. Compared to the control group, the expression levels of PI3K, phosphorylated AKT (p-AKT/AKT), phosphorylated FoxO3 (p-FoxO3/FoxO3), and Bcl-2 were significantly reduced in the model group (P < 0.05, Fig. 7A and B). Treatment with MK-2206 further decreased these protein levels (P < 0.05), except for p-FoxO3/FoxO3, which did not show a significant reductionP. Conversely, SC79 treatment led to an overall increase in these proteins, with PI3K and p-FoxO3/FoxO3 showing significant upregulation (P < 0.05, Fig. 7A and B).
CGA treatment significantly upregulated the expression of PI3K, p-AKT/AKT, p-FoxO3/FoxO3, and Bcl-2 compared to the model group (P < 0.05, Fig. 7C and D). When combined with MK-2206, CGA-treated cells exhibited a downward trend in these protein levels, with all markers showing significant reductions except for p-FoxO3/FoxO3 (P < 0.05). In contrast, co-treatment with SC79 and CGA further enhanced their expression (P < 0.05, Fig. 7C and D).
Cleaved-caspase3, caspase3, and pro-apoptotic protein TRAIL
The pro-apoptotic markers TRAIL and Caspase3 were significantly elevated in the model group compared to the control (P < 0.0001, Fig. 7B). MK-2206 treatment further increased their expression, while SC79 treatment significantly reduced them (P < 0.0001, Fig. 7B). Similarly, CGA treatment significantly downregulated TRAIL and Caspase3 compared to the model group (P < 0.0001, Fig. 7D).
Compared to the CGA-treated group, TRAIL and Caspase3 expression increased significantly in the MK-2206 + CGA group (P < 0.0001), while SC79 + CGA co-treatment further reduced their levels (P < 0.05, Fig. 7D). The expression trends of cleaved-Caspase3 and Caspase3 were generally consistent. However, while total Caspase3 levels significantly increased in the H2O2 model group and the MK-2206 + CGA group (P < 0.05), cleaved-Caspase3 levels showed a decreasing trend (Fig. 7B and D).
(A) Expression levels of PI3K, p-AKT/AKT, and p-FoxO3/FoxO3 in groups 1–4 (B) Expression levels of Caspase3/cleaved-Caspase3, TRAIL, and Bcl-2 in groups 2, 5–7 (C) Expression levels of PI3K, p-AKT/AKT, and p-FoxO3/FoxO3 in groups 5–8 (D) Expression levels of Caspase3/cleaved-Caspase3, TRAIL, and Bcl-2 in groups 5–8. Note: For Figures A and B: Compared to the control group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Compared to the model group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001. For Figures C and D: Compared to the model group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Compared to the CGA-treated group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
ELISA detection of SOD2
As shown in Fig. 8, the SOD2 content in the oxidative damage model group was significantly lower than that in the control group (P < 0.001). Compared to the model group, the SOD2 content in the SC79-treated group significantly increased (P < 0.01), whereas there was no significant difference in the MK-2206 group. The SOD2 content in the CGA-treated group was significantly higher than in the model group (P < 0.05). Compared to the CGA-treated group, the SOD2 content significantly increased in the SC79 and CGA co-treated group (P < 0.0001), while there was no statistically significant change in the SOD2 content in the CGA and MK-2206 co-treated group.
TUNEL apoptosis detection
TUNEL analysis (Fig. 9) showed that, compared to the control group, the fluorescence signal in H2O2-treated cells was significantly increased (P < 0.01). Compared to the model group, the number of apoptotic cells was significantly reduced in both the SC79 and CGA-treated groups (P < 0.001, P < 0.05), while no significant difference was observed in the MK-2206 group. On the other hand, compared to the CGA-treated group, there was an increasing trend in the apoptosis signal in the CGA and MK-2206 co-treated group, and a decreasing trend in the CGA and SC79 co-treated group, but neither reached statistical significance (P > 0.05).
Discussion
The antioxidant and pro-oxidant systems within human cells maintain a certain balance. When additional oxidative stress occurs or antioxidant levels are insufficient, cellular aerobic metabolic balance is disrupted, leading to oxidative damage31. Environmental stressors, such as ultraviolet (UV) radiation and air pollutants, along with aging-related metabolic changes, can induce excessive accumulation of reactive oxygen species (ROS), causing lipid, protein, and DNA damage7. This oxidative damage cascade further activates pro-inflammatory pathways, including NF-κB, promoting the release of inflammatory cytokines such as IL-6 and TNF-α, thereby establishing a vicious cycle of “oxidative stress-inflammation”32. Common endogenous antioxidants include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione. When these antioxidants are deficient in ocular surface tissues, the clearance of ROS is impaired, leading to the accumulation of ROS and subsequent dry eye9. Antioxidants act as scavengers of pro-oxidants to maintain the stability of biological redox states. This evidence suggests that maintaining oxidative balance in ocular surface tissues can be a key point for the prevention and treatment of dry eye.
Through data mining, this study identified enriched intersecting targets between CGA and dry eye, emphasizing the PI3K/AKT signaling pathway, FoxO signaling pathway, and oxidative stress processes. Additionally, upstream molecules of PI3K/AKT such as IGF1 and its receptor IGF1R, as well as apoptosis-related markers Caspase3, Bcl-2, and antioxidant enzyme SOD2, were found to be central in the target network. These findings suggest the importance of the PI3K/AKT/FoxO signaling pathway and oxidative stress in the treatment of dry eye with CGA. Molecular docking analysis revealed that CGA forms stable hydrogen bonds with the AKT kinase ___domain (binding energy < -8.1 kcal/mol), suggesting that it may allosterically activate AKT and subsequently initiate downstream cascade reactions.
Previous studies have shown that IGF-1 levels are significantly reduced in the tears of elderly individuals, and this reduction is closely related to dry eye symptoms [33]. Additionally, the hyperosmolar state of the tear film in dry eye can inhibit PI3K/AKT signaling, promoting inflammation and apoptosis of corneal epithelial cells34. IGF-1 plays a critical role in maintaining corneal epithelial homeostasis by binding to IGF1R and activating the PI3K/AKT pathway [35]36. Downstream of IGF1R, the PI3K/AKT pathway is a classic regulatory axis for cell survival and oxidative stress responses. AKT activation phosphorylates FoxO transcription factors, promoting their nuclear export and inhibiting apoptosis37, with FoxO3 playing a crucial role in antioxidant defense, stress response, and anti-aging38. SOD is a major antioxidant enzyme in tissues, responsible for removing ROS from cells, and its deficiency in ocular surface tissues can lead to ROS accumulation and dry eye9. FoxO3 has been shown to reduce oxidative stress by directly increasing the protein and mRNA levels of SOD2 in the mitochondrial matrix39. These findings suggest that reactivation of the PI3K/AKT signaling pathway may be a crucial therapeutic target for dry eye treatment. Based on this background, this study utilized data mining to elucidate the regulatory role of CGA in dry eye and focused on the PI3K/AKT/FoxO pathway and oxidative stress-related mechanisms.
TRAIL and its receptor system play a crucial role in inflammation and cell apoptosis associated with dry eye. In a dry eye mouse model, TRAIL expression was significantly upregulated in lacrimal gland acinar cells, accompanied by extensive infiltration of inflammatory cells40. As an anti-apoptotic protein, Bcl-2 expression is often downregulated in parallel with increased cell apoptosis. This study found that CGA significantly inhibited TRAIL expression while upregulating Bcl-2, suggesting that it may exert a protective effect by blocking the inflammation-apoptosis axis. Additionally, Caspase3 is a terminal effector protease in the mitochondria-dependent apoptotic pathway. Studies have shown that the levels of Bcl-2 and Caspase3 are closely related to inflammation and apoptosis in dry eye41,42. SOD mimetics have been reported to upregulate Bcl-2 via FoxO3 activation43. In this study, the upregulation of Bcl-2 and the inhibition of Caspase3 by CGA were synchronized with an increased p-FoxO3/FoxO3 ratio (Fig. 7C), further supporting the pivotal role of FoxO3 phosphorylation in anti-apoptotic mechanisms (Fig. 7D).
The structural changes of the lacrimal gland are highly sensitive to aging and oxidative stress accumulation, leading to reduced tear secretion and dry eye44. The lacrimal gland not only secretes tears but also produces peroxidases, growth factors, and antimicrobial peptides essential for maintaining ocular physiological functions45[46]. H2O2 can simulate oxidative environments, rapidly causing cellular damage, and has been widely used in models of oxidative damage to corneal, lens, and retinal cells13,14,47.
The experimental results of this study clearly support the role of CGA in the antioxidative and anti-apoptotic mechanisms of lacrimal gland cells. Specifically, CGA enhanced PI3K/AKT signaling by increasing the p-AKT/AKT ratio and further activated downstream effectors, including p-FoxO3/FoxO3 and Bcl-2. Meanwhile, CGA treatment significantly suppressed the expression of TRAIL and Caspase3, an effect accompanied by an upregulation of SOD2 levels and a reduction in ROS levels. These findings suggest that CGA may mediate cellular oxidative stress defense through the PI3K/AKT/FoxO3 axis (Fig. 10). Notably, the AKT inhibitor MK-2206 completely abolished the protective effects induced by CGA, whereas the AKT activator SC79 further enhanced these effects, providing additional evidence for the critical role of this pathway.
There are conflicting findings regarding the roles of p-FoxO3 and FoxO3 in cellular oxidation and apoptosis. Some studies suggest that increased phosphorylation of FoxO3 enhances its activity, inhibiting oxidative damage and apoptosis48,49, while other studies propose that reduced p-FoxO3 increases FoxO3 nuclear localization, protecting cells from oxidative damage-induced apoptosis43. In one study, FoxO3 promoted ROS generation and apoptosis in undifferentiated 3T3-L1 cells but inhibited apoptosis in differentiated 3T3-L1 cells by promoting the expression of ROS scavenging enzymes50. These differences highlight the need for further research on the phosphorylation status of FoxO3 and its pathway-specific effects, influenced by cell type and experimental conditions. In this study, the upregulation of p-FoxO3 was positively correlated with SOD2 expression (Figs. 7C and 8), suggesting that AKT-mediated FoxO3 phosphorylation may inhibit its nuclear translocation, thereby promoting the mitochondrial localization of SOD2 and enhancing ROS clearance capacity.
The combination treatment experiments with the AKT inhibitor (MK-2206) and activator (SC79) provided strong evidence for the central role of the AKT signaling pathway in the mechanism of CGA action. MK-2206 reversed the protective effects of CGA—manifested as decreased cell viability, increased ROS levels, and suppressed Bcl-2 expression—demonstrating the indispensability of AKT activation in CGA-mediated effects. Conversely, SC79 acted synergistically with CGA, enhancing pro-survival signaling, further confirming the involvement of this pathway. Notably, there was no significant difference between the CGA + SC79 group and the CGA-alone group, suggesting that at the tested concentration, CGA may have already achieved maximal AKT activation. This hypothesis requires further validation through dose-response studies in future research.
The CCK-8 assay revealed a biphasic response to CGA treatment. At a concentration of 500 nM, CGA enhanced cell viability, whereas higher concentrations (≥ 2,000 nM) exacerbated H₂O₂-induced cytotoxicity. This phenomenon is consistent with previous reports on other polyphenolic compounds. The observed toxicity at high concentrations may be attributed to the iron-chelating properties of CGA, which could promote the Fenton reaction under oxidative conditions [51]. This finding underscores the importance of dose optimization in CGA-based therapeutic applications. Further studies should explore CGA’s corneal permeability and tear fluid pharmacokinetics to determine its optimal ocular surface concentration and minimize high-dose toxicity.
This study is the first to elucidate the regulatory mechanism of CGA on the PI3K/AKT/FoxO3 pathway in a lacrimal gland epithelial model. However, several questions remain to be addressed. For instance, although the H₂O₂-induced in vitro model is widely used, it does not fully recapitulate the chronic inflammatory microenvironment of clinical dry eye. Additionally, challenges in primary lacrimal gland epithelial cell culture and the limited sample size may have impacted the statistical power of some analyses. Furthermore, as indicated by pathway enrichment results, CGA may exert its therapeutic effects on dry eye through multiple targets, which were not explored in this study. It is worth noting that excessive activation of the PI3K/AKT signaling pathway may play an important role in corneal cell apoptosis and damage36. Therefore, we hypothesize that moderate activation of this pathway is crucial in dry eye treatment. Future studies should focus on how to modulate the PI3K/AKT signaling pathway to achieve balanced and beneficial effects. Future studies should refine experimental models and methodologies to enhance statistical robustness and translational potential.
In summary, this study integrated data mining and molecular docking to predict the targets of CGA and, for the first time, validated its protective effects against oxidative damage in a lacrimal gland cell model, elucidating its specific molecular mechanisms. These findings position CGA as a promising natural compound, particularly due to its dual antioxidative and anti-apoptotic properties, making it a potential candidate for dry eye treatment. Further preclinical and clinical studies are expected to translate these findings into effective therapeutic strategies for dry eye.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- CGA:
-
chlorogenic acid
- LGECs:
-
lacrimal gland epithelial cells
- ROS:
-
reactive oxygen species
- SOD2:
-
superoxide dismutase 2
- PPI:
-
protein-protein interaction
- GO:
-
Gene Ontology
- BP:
-
biological process
- MF:
-
molecular function
- CC:
-
cellular component
- PI3K:
-
phosphatidylinositol 3-kinase
- AKT:
-
protein kinase B
References
Stapleton, F. et al. TFOS DEWS II epidemiology report. Ocul Surf. 15 (3), 334–365 (2017).
Partridge, L., Deelen, J. & Slagboom, P. E. Facing up to the global challenges of ageing. Nature 561 (7721), 45–56 (2018).
Kate, A. et al. Role of topical and systemic immunosuppression in aqueous-deficient dry eye disease. Indian J. Ophthalmol. 71 (4), 1176–1189 (2023).
Lemos, C. N. et al. Oxidative stress in the protection and injury of the lacrimal gland and the ocular surface: Are there perspectives for therapeutics?? Front. Cell. Dev. Biol. 10, 824726 (2022).
Ahmad, A. & Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 41 (3), 257–271 (2020).
Navel, V. et al. Oxidative and antioxidative stress markers in dry eye disease: A systematic review and meta-analysis. Acta Ophthalmol. 100 (1), 45–57 (2022).
Ung, L. et al. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. (Lond). 131 (24), 2865–2883 (2017).
Chen, Y., Mehta, G. & Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul Surf. 7 (4), 176–185 (2009).
Seen, S. & Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 96 (4), e412–e20 (2018).
Li, C., Zhang, C., Wang, C., Zeng, R. & Huo, Y. Research on quality control method for Juhuang oral liquid. Chin. Tradit Herb. Drugs. 46, 1779–1784 (2015). (Chinese).
Ying, A. & Ping, Q. Preparation and stability of JINZHU eye drops. Prog Pharm. Sci. 30, 224–227 (2006). (Chinese).
Ouyang, H. et al. Chlorogenic acid improves diabetic retinopathy by alleviating blood-retinal-barrier dysfunction via inducing Nrf2 activation. Phytother Res. 36 (3), 1386–1401 (2022).
Gong, W. et al. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell. Cycle. 18 (14), 1549–1559 (2019).
Song, J., Guo, D. & Bi, H. Chlorogenic acid attenuates hydrogen peroxide–induced oxidative stress in lens epithelial cells. Int. J. Mol. Med. 41 (2), 765–772 (2018).
Jang, H. et al. Chlorogenic acid and coffee prevent hypoxia-induced retinal degeneration. J. Agric. Food Chem. 62 (1), 182–191 (2014).
Tang, Y. et al. Antioxidant potential of chlorogenic acid in age-related eye diseases. Pharmacol. Res. Perspect. 12, e1162 (2024).
Noor, F., Asif, M., Ashfaq, U. A. & Qasim, M. Tahir Ul Qamar M. Machine learning for synergistic network pharmacology: a comprehensive overview. Brief. Bioinform 6. (2023).
Wang, X. et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45 (W1), W356–w60 (2017).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47 (W1), W357–w64 (2019).
Rebhan, M., Chalifa-Caspi, V., Prilusky, J. & Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. 13 (4), 163 (1997).
Zhou, Y. et al. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 50 (D1), D1398–d407 (2022).
Hamosh, A., Amberger, J. S., Bocchini, C., Scott, A. F. & Rasmussen, S. A. Online Mendelian inheritance in man (OMIM®): Victor McKusick’s magnum opus. Am. J. Med. Genet. A. 185 (11), 3259–3265 (2021).
UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 49(D1):D480-d9. (2021).
Szklarczyk, D. et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47 (D1), D607–d13 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Rehan, M., Beg, M. A., Parveen, S., Damanhouri, G. A. & Zaher, G. F. Computational insights into the inhibitory mechanism of human AKT1 by an orally active inhibitor, MK-2206. PLoS One. 9 (10), e109705 (2014).
Zhou, H. et al. Protective effects the Akt activator SC79 in hepatic Ischemia-Reperfusion injury. Med. Sci. Monit. 24, 4346–4354 (2018).
Han, J. et al. EphA2 inhibits SRA01/04 cells apoptosis by suppressing autophagy via activating PI3K/Akt/mTOR pathway. Arch. Biochem. Biophys. 711, 109024 (2021).
Gong, Y. Q. et al. SC79 protects retinal pigment epithelium cells from UV radiation via activating Akt-Nrf2 signaling. Oncotarget 7 (37), 60123–60132 (2016).
Kang, W. S. et al. Peucedanum Japonicum Thunberg and its active components mitigate oxidative stress, inflammation and apoptosis after urban particulate Matter-Induced ocular surface Damage. Antioxid. (Basel), 10(11). (2021).
Luo, J., Mills, K., le Cessie, S., Noordam, R. & van Heemst, D. Ageing, age-related diseases and oxidative stress: what to do next? Ageing Res. Rev. 57, 100982 (2020).
Dogru, M., Kojima, T., Simsek, C. & Tsubota, K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Invest. Ophthalmol. Vis. Sci. 59 (14), Des163–des8 (2018).
Patel, R., Zhu, M. & Robertson, D. M. Shifting the IGF-axis: an age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm. IGF Res. 40, 69–73 (2018).
Li, H. et al. The effect of Astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 197, 108113 (2020).
Wu, Y. C., Zhu, M. & Robertson, D. M. Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells. PLoS One. 7 (8), e42483 (2012).
Chen, K. et al. The role of the PI3K/AKT signalling pathway in the corneal epithelium: recent updates. Cell. Death Dis. 13 (5), 513 (2022).
Gong, C. et al. NCAPG promotes the proliferation of hepatocellular carcinoma through PI3K/AKT signaling. Onco Targets Ther. 12, 8537–8552 (2019).
Klotz, L. O. et al. Redox regulation of FoxO transcription factors. Redox Biol. 6, 51–72 (2015).
Liu, H. et al. Expression of SIRT1 and oxidative stress in diabetic dry eye. Int. J. Clin. Exp. Pathol. 8 (6), 7644–7653 (2015).
Ji, Y. W. et al. HIF1α-mediated TRAIL expression regulates lacrimal gland inflammation in dry eye Disease. Invest. Ophthalmol. Vis. Sci. 61 (1), 3 (2020).
Zhang, W. et al. Effects of vitamin A on expressions of apoptosis genes Bax and Bcl-2 in epithelial cells of corneal tissues induced by Benzalkonium chloride in mice with dry eye. Med. Sci. Monit. 25, 4583–4589 (2019).
Chen, X. M. et al. A novel rabbit dry eye model induced by a controlled drying system. Transl Vis. Sci. Technol. 10 (4), 32 (2021).
Yoon, H. E., Kim, S. J., Kim, S. J., Chung, S. & Shin, S. J. Tempol attenuates renal fibrosis in mice with unilateral ureteral obstruction: the role of PI3K-Akt-FoxO3a signaling. J. Korean Med. Sci. 29 (2), 230–237 (2014).
Rocha, E. M., Alves, M., Rios, J. D. & Dartt, D. A. The aging lacrimal gland: Changes in structure and function. Ocul Surf. 6 (4), 162–174 (2008).
Herzog, V. & Miller, F. The localization of endogenous peroxidase in the lacrimal gland of the rat during postnatal development. Electron microscope cytochemical and biochemical studies. J. Cell. Biol. 53 (3), 662–680 (1972).
de Souza, R. G., de Paiva, C. S. & Alves, M. R. Age-related autoimmune changes in lacrimal glands. Immune Netw. 19 (1), e3 (2019).
Yin, Y. et al. Oxidative stress suppresses cellular autophagy in corneal epithelium. Invest. Ophthalmol. Vis. Sci. 59 (8), 3286–3293 (2018).
Tan, M. et al. LNK promotes granulosa cell apoptosis in PCOS via negatively regulating insulin-stimulated AKT-FOXO3 pathway. Aging (Albany NY). 13 (3), 4617–4633 (2021).
Chen, Z. et al. Anti-Apoptosis and autophagy effects of melatonin protect rat chondrocytes against oxidative stress via regulation of AMPK/Foxo3 pathways. Cartilage 13 (2_suppl), 1041s–53s (2021).
Kojima, T., Norose, T., Tsuchiya, K. & Sakamoto, K. Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor FoxO. Apoptosis 15 (1), 83–93 (2010).
Babich, H., Schuck, A. G., Weisburg, J. H. & Zuckerbraun, H. L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 467305 (2011).
Acknowledgements
The graphical abstract and Figure 10 are modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License (https://creativecommons.org/licenses/by/3.0/).
Funding
This work is supported by National Natural Science Foundation of China (82174443); Natural Science Foundation of Hunan Province of China (2021JJ30527); Key Program of Administration of Chinese Medicine of Hunan Province of China (2021023); Key Project of Educational Commission of Hunan Province of China (20A370) and Open Fund Project of Hunan Provincial Engineering Technology Research Center for the Prevention and Treatment of Ophthalmology and Otolaryngology Diseases and Visual Function Protection with Chinese Medicine (2018YZD02).
Author information
Authors and Affiliations
Contributions
Yu Tang was responsible for conceiving and designing the study, analyzing the data, and writing the manuscript. Yuan Zhong, Jian Shi, Huimei Chen and Lihao Chen conducted experiments and contributed to data analysis. Xiaolei Yao and Qinghua Peng designed and supervised the entire study, contributed to data interpretation, and participated in manuscript preparation. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study protocol was approved by the Institutional Review Board of Hunan University of Chinese Medicine (protocol code LL2021092301, 2023-09-23). The experimental procedures complied with the International Guiding Principles for Biomedical Research Involving Animals and the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Y., Zhong, Y., Shi, J. et al. Chlorogenic acid attenuates oxidative damage in rat lacrimal gland epithelial cells via PI3K/AKT/FoxO3 signaling: network pharmacology and experimental evidence. Sci Rep 15, 19058 (2025). https://doi.org/10.1038/s41598-025-02788-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02788-0