Abstract
The neutralisation ability of homologous and heterologous booster vaccinations against the KP.2 variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unknown. Therefore, we aimed to evaluate the neutralisation of the Omicron variants by comparing serum samples from Chinese individuals who received heterologous boosters (from different manufacturers) with those who received homologous boosters (from the same manufacturer) against SARS-CoV-2. We collected serum samples from participants in the homologous (n = 38) and heterologous booster groups (n = 38) over 690 days. Serum pseudo virus neutralisation was tested against the prototype, XBB.1, JN.1, and KP.2 variants to detect neutralisation titres. An enzyme-linked immunosorbent assay was used to measure the total concentration of neutralising antibodies against the receptor binding ___domain of SARS-CoV-2. Neutralisation assays revealed 12.3-, 12.3-, and 11.4-fold reductions against JN.1, KP.2, and XBB.1 variants, respectively, compared with that against the prototype. However, no significant difference was observed in neutralising antibody titres among the JN.1, KP.2, and XBB.1 Omicron variants. Additionally, homologous boosters and men produced fewer neutralising antibodies compared to heterologous boosters and women. Thus, our results demonstrate that the Omicron variant KP.2 exhibits similar evasion properties to those observed in other variants.
Similar content being viewed by others
Introduction
The KP.2 variant, a subbranch of the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in samples collected in India on 2 January 20241,2. KP.2, known for its high transmissibility, is sometimes referred to as the ‘FLiRT’ variant due to two mutations in the spike protein, which increase its infectivity1. The FLiRT variant was initially discovered in the sewer system in the United States; however, its exact source remains unknown3. The rapid global spread of the KP.2 strain since February 2024, resulted in the World Health Organisation listing KP.2 as a ‘variant to be monitored’ on 3 May 20243. Subsequently, KP.2 began to spread globally between 7 and 13 May 20244. In April 2024, medical experts warned that the FLiRT variant had a significant transmission advantage and could lead to a new wave of coronavirus disease 2019 (COVID-19)5. Infectious disease experts have also reported that the latest mutation may be better at evading host immunity, predicting an imminent surge in COVID-19 cases6. Additionally, the proportion of KP.2 subbranches in global circulating strains gradually increased from 0.16% in early January 2024 to approximately 14% in early May 2024. The prevalence of KP.2 subbranches was relatively high in some countries, accounting for 10–30% of the infections3. As of 28 April 2024, Canadian national data showed that KP.2 was the most prevalent subvariant of JN.1, accounting for 26.6% of all COVID-19 cases in Canada (https://gisaid.org/). Similarly, in the United States, although other FLiRT variants, including KP.1.1, were detected as of May 2024, they were not as prevalent as KP.25. Notably, data from the Center for Disease Control and Prevention in the United States showed that KP.2 accounted for an estimated 28.2% cases in the United States in the 2 weeks ending 11 May 2024, increasing from approximately 6% in mid-April and 1% in mid-March7. Additionally, on 14 May 2024, the Chinese Center for Disease Control and Prevention reported that, as of 12 May 2024, 25 KP.2 sequences had been detected in local cases in China. However, the proportion of KP.2 in sequences reported weekly was between 0.05% and 0.3%, indicating an extremely low prevalence (https://www.chinacdc.cn).
According to a population survey conducted by the Chinese Center for Disease Control and Prevention in January 2023, the COVID-19 infection rate among the Chinese population reached 87.54% between 9 December 2022 and 30 January 20238, with most individuals being infected by the XBB.1 Omicron variant despite receiving the first booster shot of the COVID-19 vaccine5. Conversely, based on the latest epidemic data from the Chinese Center for Disease Control and Prevention in August 2024, the detection rate of COVID-19 infection in fever clinics in China was approximately 5%, with the primary circulating mutant strain being JN.1 and XDV.1 (https://www.chinacdc.cn); the XDV.1 variant accounted for 43.4% prevalence in August 2024 (https://www.chinacdc.cn). However, the mechanism of breakthrough infection among individuals receiving a booster shot of the COVID-19 vaccine remains unknown. Moreover, no systematic descriptions are available regarding the neutralisation capacity of breakthrough infections in booster sera samples after different homologous and heterologous booster vaccinations against the Omicron KP.2 variant. Therefore, we aimed to evaluate the neutralisation of the Omicron variants XBB.1, JN.1, and KP.2 by comparing serum samples of Chinese individuals who received heterologous boosters (from different manufacturers) with those who received homologous boosters (from the same manufacturer) against SARS-CoV-2.
Results
In total, 36 men and 40 women participants were enrolled in this study. Among them, 37 (48.7%, 37/76) had breakthrough SARS-CoV-2 infections, with no difference in the rate of breakthrough infection between the heterologous-type (22/38, 57.9%) and homologous-type (15/38, 39.5%) booster groups (p = 0.108) (Table 1). However, a significant difference (p < 0.001) was observed in the rate of breakthrough infection before 13 December 2022 (0/30, 0%), when dynamic zero measures were strictly implemented, compared with that after 13 December 2022 (37/46, 80.4%), when dynamic zero measures were replaced by routine epidemic control measures (Table 1). The median (quartile) age of the participants was 34.0 (23.3–53.0) years in the total group and, 27.0 (21–57.0) years and 37.5 (31–50) years in the heterologous-type and homologous-type booster groups, respectively (Mann–Whitney U test, p = 0.084). Seven samples (7/38, 18.4%) in the heterologous-type booster group and 23 samples (23/38, 60.5%) in the homologous-type booster group were collected before 13 December 2022 (p = 0.001). Additionally, 18 (18/38, 47.4%) and 22 (22/38, 52.6%) participants in the heterologous- and homologous-type booster groups, respectively, were men (p = 0.358). BMI and blood type were similar between the two groups (p = 0.702 and p = 0.719, respectively). No significant difference was observed in the duration of booster vaccination between the groups (p = 0.054). The interval between the primary and booster vaccinations was also similar between the two groups (p = 0.238). In the homologous group, 35 participants were vaccinated using the Chinese inactive COVID-19 vaccine (produced by eight manufacturers including Beijing Kexing Zhongwei, Beijing Biologics, Changchun Biologics, Beijing Kexing, Wuhan Biologics, Lanzhou Biologics) whereas three participants were vaccinated by the Chinese protein vaccine (produced by Anhui Zhifei Biologics) as primary and booster vaccinations. Whereas in the heterologous group, 38 participants received the inactive vaccine as the primary vaccination, while 31 participants received the attenuated live vaccine (produced by Tianjin CanSino Biologics), and seven participants received the protein vaccine (the product manufacturers were the same as above) for booster vaccination. Additional basic characteristics are listed in Table 1.
We evaluated the neutralisation activity of sera from the 76 booster vaccinations against the prototype, total, XBB.1, JN.1, and KP.2 Omicron variants, which showed the same reduced neutralisation as the prototype. The geometric mean neutralising titres (GMTs) against the prototype, total, XBB.1, JN.1, and KP.2 Omicron variants were 488.3 (95% CI: 293.31–812.9), 54.5 (42.9–69.3), 42.9 (35.0–52.4), 39.7 (32.9–47.9), and 39.8 (32.6–48.7), respectively (Fig. 1a). Additionally, neutralisation assays against JN.1, KP.2, and XBB.1 indicated 11.4-, 12.3-, and 12.3-fold reductions in neutralising antibody titres, respectively, compared with the prototype. Notably, no significant difference was observed in the neutralising antibody titres among the JN.1, KP.2, and XBB.1 Omicron variants (adjusted p = 0.97; Fig. 1a, Supplementary Table 2). However, a high correlation was observed between the GMT of JN.1 and KP.2 (r = 0.843, p < 0.001) (Fig. 1b) and among the XBB.1, JN.1, and KP.2 variants (r = 0.717, r = 0.751, and p < 0.001, respectively) (Fig. 1c and d, Supplementary Table 2). These results further confirmed that the immune escape capacity of the JN.1 and KP.2 variants did not increase compared with that of the previous XBB.1 variants (Supplementary Table 2). However, no correlation was observed between the total neutralising antibodies and the prototype, XBB.1, JN.1, and KP.2 variants (p = 0.240 to 0.932, r= − 0.01 to 0.136, respectively) (Supplementary Table 2).
Serum neutralisation titres and correlations among different variants for participants who received the first booster Chinese COVID-19 vaccine. (a) Serum neutralisation titres against the prototype, total, XBB.1, JN.1, and KP.2 variants. Correlation of serum neutralisation titres between (b) JN.1 and KP.2. (c) XBB.1 and KP.2. and (d) XBB.1 and JN.1 variants. The serum samples were collected between 20 October 2021 and 16 September 2023.
Subsequently, we analysed the dynamics of antibody neutralisation titres of sera against the three SARS-CoV-2 variants, XBB.1, JN.1, and KP.2, and the concentration of the total neutralising antibodies. Additionally, we analysed the influencing factors at different durations after the first booster dose of the COVID-19 vaccine within 690 days (Figs. 2 and 3). Sex, age, blood type, breakthrough infection, epidemic prevention and control measures, vaccination type, and booster vaccination interval affected the antibody titre and total antibody concentration of participants after the first booster vaccination (Figs. 2 and 3). Initially, the total antibody serum concentration was highest between 4 and 6 months after the first booster vaccine and gradually decreased with time (days 121–180 vs. before booster, p = 0.001) (Fig. 2a and Supplementary Table 3). However, antibody titres against the XBB.1, JN.1, and KP.2 variants were very low after the first booster vaccine and remained unchanged during the 690-day period (Fig. 2a and Supplementary Tables 3–7). Moreover, participants with breakthrough infection after booster vaccination had higher total antibody concentration in the serum than those without breakthrough infection (Fig. 2b, p = 0.044). Whereas the antibody titres against the XBB.1, JN.1 and KP.2 variants did not differ between populations with and without breakthrough infection (p = 0.748, 0.103 and 0.499, respectively) (Fig. 2b). Women had a higher antibody titre against the prototype and KP.2 variant after vaccination than men (p = 0.012, p = 0.023, respectively); however, no difference was observed in antibody titres against the XBB.1 and JN.1 variants (p = 0.845, p = 0.943, respectively) (Fig. 2c). Moreover, no difference was observed in antibody titres against the XBB.1, JN.1 and KP.2 variants among different blood type booster vaccinators (p = 0.674, p = 0.565, P = 0.556, respectively) (Fig. 2d, Supplementary Tables 3 and 7).
Comparison of neutralisation titres against prototype, total, XBB.1, JN.1, and KP.2 variants in booster participants. (a) Dynamics of neutralisation titres against the prototype, total, XBB.1, JN.1, and KP.2 variants among participants in the 690 days after vaccination with the first booster Chinese COVID-19 vaccine. Comparison of neutralisation titres (b) in those with and without breakthrough infection, (c) in men and women, and (d) in those with different blood types.
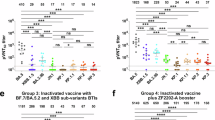
Serum collected after the liberation of the dynamic zero measures (after 13 December 2022) produced higher antibody titres against the prototype than that collected before 13 December 2022 (p = 0.009) (Fig. 3a). This trend was consistent, as shown in Fig. 2b, where participants with breakthrough infection had a higher antibody titre than those without infection. Conversely, more participants (37/46, 80.4%) had breakthrough SARS-CoV-2 Omicron variant XBB.1 infections after the liberation of the dynamic zero measures (after 13 December 2022) compared with the period before 13 December 2022 (0/30, 0%) (Table 1). In addition, booster vaccine recipients with a BMI between 18.5 and 23.9 had a higher antibody titre against JN.1 than those with BMI > 23.9 or < 18.5 (p = 0.014, Fig. 3b). Whereas booster vaccine recipients aged > 35 years old produced similar total antibody concentrations or titres compared to those aged < 35 years (Fig. 3c). Notably, participants who received heterologous booster vaccines produced an increased amount of total neutralising antibodies compared with those who received the homologous booster type vaccine (p = 0.041, Fig. 3d, Supplementary Table 4). However, no differences were observed in neutralising antibody titres against XBB.1, JN.1, and KP.2 between the heterologous- and homologous-type booster groups (p = 0.199, p = 0.855, and p = 0.224, respectively) (Fig. 3d, Supplementary Tables 5–7).
Comparison of neutralisation titres against prototype, total, XBB.1, JN.1, and KP.2 variants among booster participants. Comparison of neutralisation titres (a) before and after dynamic zero control measures, (c) in those aged < 35 years and > 35 years, (b) in those with BMI 18.5–23.9 and BMI < 18.5 or > 23.9, and (d) in those who received homologous-type and heterologous-type boosters.
Discussion
In this study, we evaluated the neutralisation of the Omicron variants XBB.1, JN.1, and KP.2 by analysing sera from individuals who received the Chinese booster vaccine to determine if these variants could evade vaccine-elicited immunity previously established by the initial dose of the booster vaccine. To the best of our knowledge, this is the first study on the serum neutralisation capacity following the first booster vaccination against the newly circulating SARS-CoV-2 variants JN.1 and KP.2 in the Chinese population.
Our findings elucidated the mechanism of breakthrough infections among the Chinese population vaccinated with the first booster COVID-19 vaccine. Most of the Chinese population received the first booster COVID-19 vaccine (inactivated vaccine, attenuated live vaccine, or protein subunit vaccine) before 13 December 2022 and before the complete liberation of epidemic control measures; however, the vaccine only contained the SARS-CoV-2 prototype strain and not the XBB.1 variant. Consequently, following the implementation of routine control measures in China, after 13 December 2022, the circulating SARS-CoV-2 strain mutated into Omicron variants, including the XBB series9. This implies that the neutralising antibodies generated by the COVID-19 vaccines, including the messenger RNA or inactivated, attenuated live, or protein subunit vaccines, were designed to contain only the prototype strain of SARS-CoV-2; they would not have cross-protection ability against other variant strains, including XBB.1, JN.1, KP.2, and XDV.14. Furthermore, our results showed that women who received the booster dose produced higher total antibodies and neutralising antibodies against KP.2 variants than men. This finding partially explained the pneumonia contributing to the susceptibility of men to SARS-CoV-2 variants6,7. Notably, this finding has not yet been reported.
Several recent publications from the USA and other regions have reported that the latest Omicron variants, JN.1 and KP.2, exhibit a markedly reduced neutralisation capacity compared with the previous Omicron variant, XBB.1, which diminishes the binding affinity of most antibody drugs8. In other regions, antibodies produced by boosters on the BA.4/5 spike protein or XBB vaccine have shown a diminished capacity to neutralise the new JN.1 and KP.2 strains10,11,12,13,14. Conversely, our results showed that the neutralisation capacity against JN.1 and KP.2 variants in serum from participants who received Chinese booster vaccinees did not significantly decrease compared with the XBB.1 variant. The observed difference may primarily be attributed to the different types of booster vaccinations administered to the study participants.
Consistent with previous studies, we found that the serum of individuals who received the Chinese booster vaccine exhibited higher immune escape capacity for the XBB.1 variant than those who received the prototype strain13. Furthermore, our results showed that the neutralising antibodies from participants who received the Chinese COVID-19 vaccine booster against the prototype strain lasted only 4–6 months. Additionally, the sera could not neutralise the emerging JN.1 and KP.2 variants within 690 days after booster vaccination. Since the KP.2 variant has only one additional V1104L S gene mutation compared with the XDV.1 variant (https://gisaid.org/lineage-comparison/), we speculated that the immune escape capacity of XDV.1 should be similar to that of KP.2. However, the sera from the population that received the booster indicated that they could not neutralise the latest emerging XDV.1 variant in China. This significant difference suggests that, in the future, a novel vaccine incorporating JN.1, KP.2, and XDV.1 variants will be required to improve population immunity and prevent breakthrough infections with emerging SARS-CoV-2 variants14.
Another notable finding of this study was that participants who received the booster vaccine and developed breakthrough infections exhibited higher total neutralising antibodies than those without breakthrough infections. However, no difference was found between booster participants who produced an antibody titre against XBB.1, JN.1, and KP.2 variants and those with and without breakthrough infection. This further suggests that the JN.1 and KP.2 variants have immune escape capacities similar to XBB.1. This is because the samples from individuals with breakthrough infection were collected before October 2023, when only the XBB.1 variant was circulating in China9. Whereas the JN.1, KP.2, and XDV.1 variants only began to emerge in China in January, May, and June 2024, respectively.
Furthermore, no difference was observed in the neutralising antibody titres against the prototype strain, XBB.1, JN.1, and KP.2 variants, between populations vaccinated with the heterologous- and homologous-type boosters. However, individuals vaccinated with the heterologous-type booster had a higher total antibody concentration than those vaccinated with the homologous-type booster (Fig. 3d). These results suggest that a difference exists between total antibody concentration and pseudo virus-neutralising antibody titres against different SARS-CoV-2 variants among booster populations. This finding is consistent with our finding that no correlation exists between enzyme-linked immunosorbent assay (ELISA) and pseudo virus neutralisation test (pVNT) results. This may be due to differences in the detection methods for the serum-neutralising antibodies. Total neutralising antibodies against the receptor binding ___domain (RBD) protein are detected using the reaction principle between antigen and antibody in ELISA15. Conversely, the pVNT employs the principle of cell transfection to identify the 50% neutralisation dilution titres for different SARS-CoV-2 variants in the serum14. Additionally, the heterologous booster group showed higher total antibody levels than the homologous group. This may be due to differences between booster and primary vaccine types, such as inactive, attenuated live, protein, or mRNA vaccines, since their mechanisms for producing antibodies in vaccinators differ13,16. This finding is similar to those of studies in other countries on heterologous booster vaccines such as mRNA vaccines, protein vaccines, or vaccines containing JN.1 variants, which showed better neutralising SARS-CoV-2 activity10,17,18,19,20.
Nonetheless, this study has some limitations. First, we adopted the classic pVNT, which is commonly used in most published COVID-19 vaccination studies, to evaluate the immunogenicity of vaccination, since the live virus neutralisation tests were inaccessible11. Second, the sample size of our study was limited due to the costs of the pVNT. Moreover, some participants may have had biased recollections of whether they had been ever infected with SARS-CoV-2, although throat swabs were used to confirm SARS-CoV-2 infection. Therefore, larger-scale studies are required to evaluate the immunogenicity and effectiveness of different vaccines against Omicron variants, including KP.3, LB.1, and XDV.11,20.
In conclusion, our results demonstrate that vaccine-induced immune protection may be more likely to be evaded by the Omicron variants, JN.1 and KP.2, compared with the prototypes and XBB.1 variants in Chinese individuals vaccinated with a first booster COVID-19 vaccine. Therefore, following the first booster, either homologous or heterologous, a subsequent JN.1, KP.2, or XDV.1 and a broad-spectrum COVID-19 vaccine booster are recommended to improve neutralisation against these new SARS-CoV-2 variants. Future research should focus on increasing the sample size to determine whether new vaccines neutralise emerging COVID-19 variants, including KP.3, LB.1, and XDV.1, after booster vaccination.
Methods
Participants and study flow
This cross-sectional study enrolled 442 participants between 24 June 2021 and 16 September 2023 from Henan Provincial People’s Hospital, Zhengzhou Municipal Chinese Medicine Hospital, and Henan Electric Power Survey and Design Institute in central China via announcements and WeChat groups. The inclusion criteria were healthy or clinically stable adults (aged 18–80 years) who received the first dose of the booster COVID-19 vaccine between 6 and 8 months (180–240 days) prior to the study. Participants vaccinated with other vaccines, such as influenza or booster, were excluded. Additionally, participants with a clinically notable acute illness or a body temperature ≥ 38 °C within 24 h before receiving the planned booster dose of the study vaccine were excluded. In total, seven participants were excluded because they did not meet the inclusion criteria. The remaining 435 participants were enrolled and divided into a homologous-type group (n = 273), consisting of participants who received a booster from the homologous manufacturer as their previous booster, and a heterologous group (n = 162), comprising those who received a booster from a different manufacturer compared to their previous booster. Participants were monitored for breakthrough infection for 690 days. Sera were collected every 2 months to detect the titre and total concentration of neutralising antibodies. Additionally, throat swab samples were collected to verify whether the participants were infected with SARS-CoV-2 using a specific reverse transcription polymerase chain reaction (RT-PCR). Notably, 269 and 160 of the 429 participants completed the 690-day period in the homologous and heterologous groups, respectively, with four and two participants in each group withdrawing consent.
We used the cluster-stratified random sampling method to ensure a better overall representation of our samples21. We classified the 429 participants into groups of 11 average sections at every two monthly intervals, based on the time between booster and primary vaccinations. Since the longest interval between booster and primary vaccinations for some participants was 690 days, the duration was divided into a total of 11 periods. Furthermore, seven study participants were randomly selected from every period group, and a similar number was required by sex and age to minimise control bias. Finally, 76 samples were randomly selected to detect titres against the prototype, XBB.1, JN.1, and KP.2 variants and the total concentration of neutralising antibodies against the RBD of SARS-CoV-2 (Fig. 4). These 76 samples included 36 men and 40 women, with 38 samples each from the homologous and heterologous groups.
Assessment of samples
Overall, 76 samples, 38 from the homologous and heterologous groups each, collected between 20 October 2021 and 16 September 2023, were selected for further analysis. The sample collection dates coincided with a change in epidemic measures from the dynamic zero clearance policy to full liberalisation before and after 13 December 2022. Under the dynamic zero clearance policy, when a resident tested positive for COVID-19 in a residential community, they were immediately sent to a centralised isolation hospital for free isolation, treatment, and observation. Meanwhile, other residents in the community were required to undergo throat swab nucleic acid testing and isolation at home for seven consecutive days. Residents who tested positive during the next seven days were also sent to a government isolation hospital. The isolation measures were only lifted if all community residents had negative throat swabs for seven consecutive days. Basic participant information, including sex, age, vaccination date, vaccine type, blood type, body mass index (BMI), vaccination interval between primary and booster doses, and duration after booster dose calculated from the booster date to the sample collection date, was collected. An ELISA was used to detect total neutralising antibodies against the SARS-CoV-2 RBD in December 2023. The 76 samples also underwent simultaneous pseudo virus-neutralising antibody testing against the prototype, XBB.1, JN.1, and KP.2 variants in June 2024.
Serum pseudo virus neutralisation test
Pseudo typed viruses were produced using 293T cells, which were transfected with an S protein expression plasmid (prototype virus, XBB.1, JN.1, and KP.2 variants) and infected with vesicular stomatitis virus glycoprotein (VSV-G) pseudo typed virus (G*ΔG-VSV)23. Subsequently, a serum pVNT was performed to detect neutralisation titres. The pseudo typed viruses used for neutralisation titre detection and the mutation sites of the S genes are listed in Supplementary Table 1. The initial dilution was 1:30 or 1:10, followed by three-fold serial dilutions. The final dilution of the sample was 1:7290. The 50% neutralisation dilution (ND50) was calculated using the Reed–Muench method, and the limit of detection (LOD) was 1:10. Results below the LOD were set to 0.5 times that of the LOD, and an ND50 titre > 1:30 was considered positive.
Measurement of total neutralising antibodies and ABO blood typing
Total neutralising antibodies were detected using a commercial ELISA kit23 (anti-SARS-CoV-2 S Kit; Shanghai GeneoDx Biotechnology Co., Ltd., Shanghai, China), which detects neutralising immunoglobulin antibodies against the SARS-CoV-2 spike protein RBD, using a universal microplate reader (DNM-9602; Beijing Pulang Co., Ltd., Beijing, China). A value > 6.5 IU/mL was considered positive. Values > 100 IU/mL were capped at 100 IU/mL as per the manufacturer’s protocols and instructions The ABO blood group was determined using the test tube method based on the manufacturer’s instructions (Chengdu United Co.,Ltd., Chengdu, China).
Throat swab samples test
Throat swab samples collected for SARS-CoV-2 analysis were analysed using RT-PCR (Shanghai Zhejiang Biotechnology Ltd., Shanghai, China). Cycle threshold values ≤ 44 on RT-PCR were considered positive.
Ethics
The study was approved by the Institutional Review Board of the Ethics Committee of Henan Provincial People’s Hospital (approval number: 20210051, approval date: 24 May 2021) and was conducted in compliance with the principles of the Declaration of Helsinki. All participants enrolled in this study provided written informed consent to participate.
Statistics
Summary statistics for the geometric means with 95% confidence intervals (CIs) are presented. Statistical significance between groups and subgroups was assessed using the Mann–Whitney U test, Student’s t-test, and one-way analysis of variance for continuous variables, and Pearson’s χ2 or Fisher’s exact test for categorical variables. Spearman’s rank correlation and r values were used to evaluate the correlation between two continuous variables. Since the total neutralising antibodies and four different neutralising antibodies against the prototype strain, XBB.1, JN.1, and KP.2 were measured for each specimen, our data were repeated measures. Therefore, a mixed linear model was used to identify the factors affecting neutralising antibodies after booster vaccination21. The log2-transformed neutralising antibody titre or total antibody concentration was considered the dependent variable in this model. While age, sex, BMI, ABO blood type, vaccination mode, the interval between primary and booster vaccination doses, SARS-CoV-2 breakthrough infection, and epidemic measures were considered independent variables. Hypothesis testing was two-sided, with P < 0.05 considered statistically significant. All statistical analyses and plotting were performed using GraphPad Prism (version 8.0; La Jolla, CA, USA) and SPSS (version 2.0; IBM Corp., Armonk, NY, USA).
Data availability
Data is provided within the manuscript or supplementary information files.
References
Kaku, Y. et al. Genotype to Phenotype Japan (G2P-Japan) Consortium, et al., Virological characteristics of the SARS-CoV-2 KP.3, LB.1, and KP.2.3 variants. Lancet Infect. Dis. 24, e482–e483. https://doi.org/10.1016/S1473-3099(24)00415-8 (2024).
Rajput, V. et al. Early detection of KP.2 SARS-CoV-2 variant using wastewater-based genomic surveillance in Pune, Maharashtra, India. J. Travel Med. 2024, taae097. https://doi.org/10.1093/jtm/taae097 (2024).
Kumar, P. et al. The emerging challenge of FLiRT variants: KP.1.1 and KP.2 in the global pandemic landscape. QJM 117, 485–487. https://doi.org/10.1093/qjmed/hcae102 (2024).
Branda, F., Ciccozzi, A., Romano, C., Ciccozzi, M. & Scarpa, F. Another variant another history: description of the SARS-CoV-2 KP.2 (JN.1.11.1.2) mutations. Infect. Dis. (Lond). 56, 581–585. https://doi.org/10.1080/23744235.2024.2358383 (2024).
Li, P. et al. Neutralization escape, infectivity, and membrane fusion of JN.1-derived SARS-CoV-2 slip, flirt, and KP.2 variants. Cell. Rep. 43, 114520. https://doi.org/10.1016/j.celrep.2024.114520 (2024).
Haitao, T. et al. COVID-19 and sex differences: Mechanisms and biomarkers. Mayo Clin. Proc. 95, 2189–2203. https://doi.org/10.1016/j.mayocp.2020.07.024 (2020).
Kitselman, A., Bédard-Matteau, K., Rousseau, J., Tabrizchi, S. & Daneshtalab, N. Sex differences in vascular endothelial function related to acute and long COVID-19. Vascul Pharmacol. 154, 107250. https://doi.org/10.1016/j.vph.2023.107250 (2024).
Benlarbi, M. et al. Temperature-dependent Spike-ACE2 interaction of Omicron subvariants is associated with viral transmission. mBio. 15, e0090724 (2024). https://doi.org/10.1128/mbio.00907-24
Zhang, L. et al. The significant immune escape of pseudo typed SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 11, 1–5. https://doi.org/10.1080/22221751.2021.2017757 (2022).
Jeworowski, L. M. et al. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro. Surveill. 29, 2300740. https://doi.org/10.2807/1560-7917.ES.2024.29.2.2300740 (2024).
Kaku, Y. et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 24, e82. https://doi.org/10.1016/S1473-3099(23)00813-7 (2024).
Planas, D. et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat. Commun. 15, 2254. https://doi.org/10.1038/s41467-024-46490-7 (2024).
Wang, Q. et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell. Host Microbe. 32, 315–321e3. https://doi.org/10.1016/j.chom.2024.01.014 (2024).
Yang, S. et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 24, e70–e72. https://doi.org/10.1016/S1473-3099(23)00744-2 (2024).
Yuan, Y. et al. Characteristics of humoral and cellular responses to coronavirus disease 2019 (COVID-19) inactivated vaccine in central China: a prospective, multicenter, longitudinal study. Front. Immunol. 14, 1107866. https://doi.org/10.3389/fimmu.2023.1107866 (2023).
Raisinghani, N., Alshahrani, M., Gupta, G. & Verkhivker, G. Atomistic prediction of structures, conformational ensembles and binding energetics for the SARS-CoV-2 Spike JN.1, KP.2 and KP.3 variants using AlphaFold2 and molecular dynamics simulations: mutational profiling and binding free energy analysis reveal epistatic hotspots of the ACE2 affinity and immune escape. BioRxiv https://doi.org/10.1101/2024.07.09.602810 (2024).
Itamochi, M. et al. COVID-19 mRNA booster vaccination induces robust antibody responses but few adverse events among SARS-CoV-2 Naive nursing home residents. Sci. Rep. 14, 23295. https://doi.org/10.1038/s41598-024-73004-8 (2024).
Savulescu, C. et al. Incidence of SARS-CoV-2 infection among European healthcare workers and effectiveness of the first booster COVID-19 vaccine, VEBIS HCW observational cohort study, May 2021–May 2023. Vaccines (Basel). 12, 1295. https://doi.org/10.3390/vaccines12111295 (2024).
Talmy, T. & Nitzan, I. Rapid rollout and initial uptake of a booster COVID-19 vaccine among Israel defense forces soldiers. J. Prev. 44, 1–14. https://doi.org/10.1007/s10935-022-00702-2 (2023).
Favresse, J. et al. Neutralizing antibody response to XBB.1.5, BA.2.86, FL.1.5.1, and JN.1 six months after the BNT162b2 bivalent booster. Int. J. Infect. Dis. 143, 107028. https://doi.org/10.1016/j.ijid.2024.107028 (2024).
Lei, J. et al. Respiratory benefits of multisetting air purification in children: a cluster randomized crossover trial. JAMA Pediatr. 179, e245049. https://doi.org/10.1001/jamapediatrics.2024.5049 (2024).
Bai, Y. et al. Study on the COVID-19 epidemic in Mainland China between November 2022 and January 2023, with prediction of its tendency. J. Biosaf. Biosecur. 5, 39–44. https://doi.org/10.1016/j.jobb.2023.03.001 (2023).
Xu, N. et al. Study of efficacy and antibody duration to fourth-dose booster of Ad5-nCoV or inactivated SARS-CoV-2 vaccine in Chinese adults: a prospective cohort study. Front. Immunol. 14, 1244373. https://doi.org/10.3389/fimmu.2023.1244373 (2023).
Acknowledgements
We thank all study participants, volunteers, partners, the Henan Provincial People’s Hospital Data Safety Monitoring Board, and the study steering committee. In addition, we are grateful to our colleagues, Wenning Yang, Yafei Chu, Nan Jing, and Wenbo Xu, who assisted with sample collection and some of the experiments.
Funding
This work was funded by the Natural Science Foundation of China (recipient: R. Wang, grant number: 82002210); the Henan Provincial Key Programs in Science and Technology (recipient: B. Wang, grant number: 242102310116); the Joint Program of Medical Science and Technology Research of Henan Province (recipient: Y. Li, grant number: LHGJ 20230021); the Henan Natural Science Foundation (grant numbers: 222300420355 and 242300421502) from the Henan Provincial Department of Education; and the Henan Province Science and Technology Research Program Project from the Henan Provincial Health Commission (recipients: H. Wang and X. Zhang, grant number: SBGJ202103016). The funder played no role in study design, data collection, data analysis, and interpretation, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
YY, JX, GC, and LO conceptualised and designed the study and drafted the manuscript; BW, LO, WY, YG, and QM performed statistical analyses. GC, XM, XZ, and BM collected serum samples from volunteers; YL, PZ, QZ, and JZ helped conduct the clinical study and acquire, analyse, and interpret the data; Yan L performed the pseudovirus neutralisation tests; RW, XZ, Yi L, XM, PZ, and HW provided material support and supervised the study. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Written informed consent for publication of their clinical details was obtained from the participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, Y., Xu, J., Chen, G. et al. Comparable immune escape capacity between KP.2 and other SARS-CoV-2 variants in the central Chinese population after the first COVID-19 booster. Sci Rep 15, 17762 (2025). https://doi.org/10.1038/s41598-025-02927-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02927-7