Abstract
Remediation of heavy metal-contaminated soils is critical due to their persistent toxicity and threat to food security and ecosystem health. Therefore, a study was conducted during September to November 2024 to assess the phytoextraction potential of Madagascar Periwinkle (Catharanthus roseus) for cadmium (Cd) and lead (Pb) in soils spiked with varying concentrations of battery scrap waste (BSW: 0, 1, 2, 3, and 4% w/w). A hybrid mechanistic-kinetic (HMK) model was developed by the integration of the Freundlich isotherm and Michaelis–Menten equations to predict bioavailable heavy metal concentrations and uptake by plants. The results showed that C. roseus had higher Cd and Pb accumulation in roots compared to shoots, with maximum concentrations of 21.504 mg/kg Cd and 43.123 mg/kg Pb observed at 4% BSW. However, increasing contamination levels significantly reduced metal removal efficiency (%), as evidenced by bioconcentration factor (BCF) and translocation factor (TF) values. Also, increasing the BSW dose significantly (p < 0.05) reduced C. roseus growth, reducing plant height, root length, biomass, chlorophyll, and carotenoid content due to heavy metal toxicity. On the other hand, Freundlich coefficients (Kf) and exponent (n) were useful in predicting bioavailable heavy metals. The Michaelis–Menten-based HMK model analysis showed higher maximum uptake rates (Vmax) for Pb in roots and shoots compared to Cd, while half-saturation constant (Km) values were lower in shoots compared to roots. The developed models had high R2 and low mean absolute error (MAE), indicating goodness of fit. This study showed that C. roseus can be used for Cd and Pb phytoextraction from contaminated soils, thereby contributing to the restoration of agro-ecosystems.
Similar content being viewed by others
Introduction
Agricultural soil contamination with toxic heavy metals is a pressing global environmental issue that severely affects agricultural productivity and human health1. Anthropogenic activities such as industrial emissions, improper waste disposal, and agricultural runoff significantly contribute to the accumulation of toxic metals and metalloids such as arsenic (As), cadmium (Cd), lead (Pb), nickel (Ni), mercury (Hg), etc. in soils2. These metals persist in the soil and bring severe human and ecological health issues through bioaccumulation and biomagnification in food chains due to their non-biodegradable nature3. In the twenty-first century, the battery manufacturing and recycling sectors are particularly among the top sources of heavy metal pollution of soils4. According to recent estimates, the global production of Li-ion batteries exceeded 550 GWh per year in 2022, with electric-vehicle batteries dominating the market5,6. However, improper handling, disposal, and recycling of battery waste result in the release of several toxic heavy metals such as Ni, Cd, and Pb into the soil, intensifying their pollution7,8. Battery scrap waste primarily originates from used lead-acid batteries and is commonly generated by automotive, industrial, and informal recycling sectors. These wastes are often released into the environment through unregulated dumping and open recycling practices, particularly in low- and middle-income countries9.
Remediation of heavy metals from contaminated soil is one of the suitable methods to safeguard the agri-food chain system10. However, conventional remediation techniques, such as soil washing, immobilization, and excavation, are often expensive, energy-intensive, and not environmentally friendly11. Therefore, there is an increasing interest in sustainable and cost-effective techniques, among which phytoremediation has emerged as a promising solution12. Phytoremediation is a plant-based remediation approach that encompasses various mechanisms, including phytostabilization, phytodegradation, and phytoextraction13. Among these, phytoextraction specifically involves the uptake and translocation of heavy metals from soil to harvestable plant parts, making it a key strategy for reducing soil metal loads14. Phytoextraction utilizes plants capable of accumulating heavy metals in their tissues, thereby reducing soil contamination15. This green technology is not only eco-friendly but also holds the potential for restoring degraded lands while generating plant biomass for secondary applications, such as bioenergy16.
Madagascar Periwinkle (Catharanthus roseus [L.] G. Don), commonly known as Sadabahar (an evergreen flowering plant), is an herbaceous plant belonging to the family Dogbanes. It is widely cultivated for its floral, ornamental, and medicinal properties17,18 and has received attention for its potential role in environmental restoration17. C. roseus is used for alkaloid production for anticancer drugs19 and has high adaptability to diverse soil conditions and resilience against stressors, including heavy metals20. The root system of C. roseus is tolerant to toxic metals and has the potential to bioaccumulate them, making it a suitable species for phytoextraction. However, despite its well-documented pharmacological applications21, the use of C. roseus in soil remediation remains underexplored, indicating a significant gap in current research.
The efficacy of the phytoextraction process depends on several critical factors such as heavy metal availability in the soil and the plant’s uptake capacity22. Mathematical modeling of plant-soil interaction is a useful approach to effectively understanding these dynamics, thereby identifying mechanisms governing metal adsorption in soils and subsequent uptake by plant tissues23. Among the models available, adsorption isotherms such as the Freundlich isotherm are widely used to describe the distribution of heavy metals in the soil phases24. Similarly, plant uptake kinetics, often modeled using Michaelis–Menten equations, capture the rate-limiting steps in heavy metal absorption by plants25. However, existing models typically treat soil adsorption and plant uptake as independent processes, limiting their applicability in complex soil–plant systems. In order to address these limitations, integrating soil adsorption and plant uptake processes into a unified framework is necessary. In this, mechanistic and kinetic models that couple adsorption isotherms with plant uptake kinetics could help in enhancing phytoextraction efficiency. Such models could enable the identification of key factors influencing heavy metal availability and uptake for better design and optimized remediation strategies.
Despite the advancements in modeling approaches, no study to date has employed C. roseus to develop a hybrid mechanistic-kinetic (HMK) model for heavy metal phytoextraction. Therefore, the present study aims to develop a hybrid model that combines the Freundlich adsorption isotherm with Michaelis–Menten uptake kinetics to predict the Cd and Pb phytoextraction potential of C. roseus from BSW-contaminated soil. For this purpose, pot experiments were conducted using soils spiked with varying concentrations of battery scrap waste and assessing C. roseus growth, metal accumulation, and physiological responses. These outcomes were then used to develop and validate the proposed HMK model for predicting bioavailable metal concentrations and uptake dynamics. C. roseus was selected due to its demonstrated tolerance to heavy metals, high biomass yield, and adaptive physiological traits, making it a promising candidate for phytoremediation. Also, its use in developing a hybrid mechanistic-kinetic model for Cd and Pb uptake represents a novel approach not previously reported in the literature.
Materials and methods
Collection of experimental materials
Madagascar Periwinkle (Catharanthus roseus var. Rosea) seeds were procured from a local vendor in Haridwar City, Uttarakhand, India. Soil and battery scrap waste (BSW) were separately collected from a garden and scraping facility located in Nakur, Saharanpur, India (29°55′38.6″ N and 77°18′58.4″ E). Only BSW from Cd and Pb-based batteries were selected to focus on the targeted heavy metal contamination. The collected BSW was carefully segregated and processed to a fine powder using a mechanical grinder to ensure uniformity of particle size for experimental application. Also, C. roseus seedlings were raised in plastic bags using garden soil for 15 days for further experimentation.
Experimental design and operation
In the present study, lab-scale pot experiments were conducted from September to November 2024 to assess the uptake rates of Cd and Pb by C. roseus (Fig. 1). For this, soil was spiked with BSW at concentrations of 0 (control with no spiking), 1, 2, 3, and 4% (w/w) in order to simulate Cd and Pb contamination. BSW was uniformly mixed into the soil using batch mixing to ensure homogeneity. Prior to and following spiking, batch mixing experiments were conducted to measure plant-available concentrations of Cd and Pb using chemical extraction methods. A total of 15 plastic pots (3 replicates for each treatment), each of 10 kg capacity, were filled with 7 kg of garden soil with no prior history of BSW application. One plant seedling (age of 15 days) was planted in each pot, and experiments were conducted under controlled greenhouse conditions. Irrigation was provided using a borewell water supply regularly to maintain optimal soil moisture levels, and a photoperiod of 12 h light and 12 h dark was maintained. Cultivation was carried out for 90 days during the September to November months of optimal growth for C. roseus, ensuring suitable temperature (15–30 °C) and humidity (70%). Soil and plant samples were collected before (first day) and after (last day) of experimentation for analysis to monitor metal dynamics.
Analytical, plant growth, and physiological measurement methods
In this study, soil pH was measured using a digital pH meter (1611, ESICO International, Parwanoo, IN), with samples prepared in a 1:5 soil-to-water suspension. Organic matter (OM: %) content was determined using the Walkley–Black method26, which involves the dichromate (K2Cr2O7) oxidation of soil organic carbon followed by titration with ferrous ammonium sulfate ((NH4)2Fe(SO4)2·6H2O). Total and bioavailable concentrations of cadmium (Cd) and lead (Pb) in soil were assessed using acid digestion and chemical extraction methods, respectively. Total metal concentrations were determined by digesting soil samples with a mixture of concentrated nitric acid (HNO₃) and hydrochloric acid (HCl) in a microwave digestion system (Model: MARS 6, CEM Corporation, Matthews, North Carolina, USA). Bioavailable metal concentrations were extracted using a diethylenetriaminepentaacetic acid (DTPA) solution27. On the other hand, oven-dried plant root and shoot samples were digested using a combination of HNO3 and hydrogen peroxide (H2O2) for metal analysis28. All metal concentrations in digested soil and plant extracts were quantified using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES: 7300 DV, Perkin Elmer, Waltham, MA, USA) with high sensitivity and accuracy29. The limits of detection for Cd and Pb were 0.01 and 0.10 µg/kg, respectively, and quality control measures, such as replicate analysis, cross-validation, and certified reference materials, were ensured for the accuracy and precision of the results. All reagents of analytical grade were procured from Merck India Ltd. (Mumbai, Maharashtra, India).
Plant height and root length (cm) were measured using a calibrated ruler, while fresh and dry weights (g) of plants were recorded using a high-precision electronic balance (Johnson 1000G, JewelTech International, Mumbai, IN). Dry weight was determined after oven-drying plant samples at 70 °C to a constant weight. The number of leaves per plant was counted manually. Also, total chlorophyll and carotenoid contents were extracted from fresh leaf samples using acetone and quantified spectrophotometrically (60 Cary, Agilent Technology, USA) as described by Kumar et al.30.
Model development and validation
Mathematical models are useful tools for understanding heavy metal contamination in soil by assessing adsorption capacity and their subsequent uptake by plants23. In this study, a hybrid mechanistic-kinetic (HMK) model incorporating Freundlich isotherms and Michaelis–Menten kinetics was developed to describe heavy metal adsorption in soil and their desorption through plant uptake processes, respectively. The Freundlich isotherm is an empirical model describing the non-linear adsorption of solutes onto heterogeneous soil surfaces31. While the Michaelis–Menten model is used to describe the kinetics of nutrient uptake by plants as influenced by soil properties, correlating uptake rates to substrate availability and soil characteristics through parameters such as maximum uptake rate and affinity constant32. In this study, the following steps were adopted for the development of a hybrid mechanistic-kinetic (HMK) model for predicting bioavailable heavy metals in soil and their uptake concentration by plants:
Step 1: Freundlich isotherm for bioavailable heavy metal in soil
Considering BSW treatment doses (0–4%), the total and bioavailable Cd and Pb concentration in soil was used for fitting the Freundlich isotherm. This isolation describes the relationship between heavy metal concentration in soil and the plant-available fraction as shown in Eq. (1):
where: Cavail refers to available heavy metal concentration (mg/kg dry weight basis; DW), Cs is the total concentration of heavy metals in soil (mg/kg DW), Kf is the Freundlich adsorption coefficient (dimensionless), and n is the Freundlich exponent of adsorption intensity (dimensionless). After linearizing and taking logarithms of both sides of Eq. (1), we got Eq. (2):
By using the above equation, a plot log(Cavail) vs. log(Cs) was drawn, and linear regression analysis was performed to find the slope (1/n) and the intercept log(Kf). Then, Kf and n were calculated, and Cavail values were predicted using Eq. (1). A plot of observed vs. predicted values was drawn to verify the fitness of the model.
Step 2: Integration of the Freundlich isotherm into the Michaelis–Menten equation for plant uptake
Integration of the Michaelis–Menten kinetic model with the Freundlich isotherm can facilitate concurrent assessment of heavy metal adsorption to soil and subsequent plant uptake, thereby establishing a link between soil adsorption dynamics and bioavailability, as well as uptake kinetics. Incorporating substrate concentration (Cavail) values from the Freundlich equation into the Michaelis–Menten equation can be described in Eq. (3) as an HMK model:
where U refers to the reaction velocity as the total uptake of heavy metals by the plant (mg/kg), Vmax is the maximum uptake velocity, and Km is the half-saturation constant (mg/kg), respectively. After linearizing Eq. (3), we get the reciprocal form as the Lineweaver–Burk equation as shown in Eq. (4):
From this equation, a plot (1/U) vs. (1/Cavail) was drawn to find the slope (Km/Vmax) and intercept (1/Vmax). By using this, values of Vmax (1/intercept) and Km (slope·Vmax) were calculated to fit the experimental data into Eq. (3).
Step 3: Model validation
The mean absolute error (MAE) quantifies the average absolute deviation between observed and predicted values as a measure of the prediction accuracy of mathematical models. While the coefficient of determination (R2) refers to the proportion of variance in observed data to understand the model’s goodness-of-fit. In this study, MAE and R2 tools were used to validate the reliability of the integrated model by comparing predictions with observed results. The following Eqs. (5 and 6) were used for the calculation of MAE and R2:
Data analysis and software
Data analysis was performed using OriginPro (2024, OriginLab Corp., USA) and Microsoft Excel (Version 365, Microsoft Corp., USA) software. The significant differences among different treatment groups were tested using analysis of variance and Tukey’s post-hoc test (p < 0.05). The removal efficiency (R: %) index33 was used to calculate the percentage of heavy metal removed from the soil as given in the following formula (Eq. 7):
The bioconcentration factor (BCF) indicates the plant’s ability to accumulate metals in its vegetative tissues34. BCF was determined using the following index (Eq. 8):
On the other hand, the translocation factor (TF) indicates the mobility of heavy metal from the root to the shoot tissues of the plant35. TF was calculated using the following equation (Eq. 9):
The relative growth rate (RGR) is a measure used to evaluate the rate of growth of a plant relative to its size over a specific period36. RGR was expressed in units of growth per unit biomass per unit time (g/g/day) and was calculated using the formula (Eq. 10):
where W1 and W2 are the initial and final C. roseus plant weights (g) at t1 (0 day) and t2 (90 day) experimental time, respectively.
Results and discussion
Properties of soil spiked with battery scrap waste
The results depicted in Table 1 showed an increase in both total (Cs) and bioavailable (Cavail) concentrations of Cd and Pb in soil with higher percentages of BSW mixing for batch adsorption experiments. Thus, it was observed that heavy metal spiking increased the soil contamination levels. For Cd, total concentrations ranged from 0.03 in control soil to 154.042 mg/kg in 4% BW treatment, while Pb ranged from 0.050 to 472.091 mg/kg. On the other hand, bioavailable Cd and Pb also increased significantly under BSW spiking from 36.972 mg/kg in 1% BSW to 113.301 mg/kg in 4% BSW treatment, respectively. The increase in Cavail showed enhanced metal mobility proportional to the mixing rate, likely due to metal complexation and retention in soil matrices. Furthermore, a significant decrease in soil pH (from 7.213 to 8.142) and OM content (from 2.21% to 1.951%) was observed after BSW mixing. This decline indicates a shift in the soil reaction toward the alkaline range, which may be attributed to a loss of OM content. Additionally, increased pH may enhance the desorption of heavy metals, given their amphoteric behavior and, therefore, higher retention in soil during spiking37.
The levels of Cd remained below the World Health Organization (WHO)38 threshold of 0.8 mg/kg in control soil but exceeded it in all spiked treatments. While Pb levels in all treatments exceeded the permissible limit of 85 mg/kg, indicating potential ecological risks posed by even negligible additions of BSW. Similar results were reported by AL-Huqail et al.29 for BSW application on soil under phytoextraction using Rotundus spp. They found a significant reduction in soil pH and OM while increasing heavy metals in treated soils. Similarly, Kriti et al.39 also found that soil heavy metal fraction was proportional to the rate of BSW mixing, thereby increasing both total and bioavailable contents under treatment with lemongrass (Cymbopogon citratus) and vetiver (Chrysopogon zizanioides). Thus, these results are in line with those reported by other studies on BSW-based soil spiking and are useful for targeted phytoextraction strategies.
Uptake and bioaccumulation of Cd and Pb by C. roseus plant
Table 2 shows the concentrations of Cd and Pb in roots and shoots of C. roseus grown under BSW treatments, along with BAF and TF values. The results indicated that heavy metal accumulation in roots was higher compared to shoots, with maximum Cd (21.504 mg/kg) and Pb (43.123 mg/kg) observed in root tissues at 4% BSW, respectively. A significant reduction in the removal efficiency of Cd and Pb by C. roseus with increasing levels of BSW was observed, which may be due to higher contamination levels (Fig. 2). In particular, Cd removal decreased from 33.33% in the control to 11.02% at 4% BSW, while Pb removal reduced from 20.00 to 7.61%. The declining trend aligns with the increasing total and bioavailable metal concentrations in soil, which likely exceeded the plant’s uptake capacity. This decline can be attributed to metal toxicity effects, saturation of binding sites, and reduced plant growth due to competitive ion interactions40. On the other hand, BAF values of Cd decreased from 0.342 in the control to 0.141 at 4% BSW and Pb from 0.201 to 0.092, indicating reduced uptake efficiency under higher contamination stress. While TF values for Cd and Pb increased up to 1.7333 and 1.500 at 4% BSW treatment referring to efficient mobilization from roots to shoots despite increasing contamination. Herein, differential translocation might be due to distinct physiological responses to Cd and Pb, with Cd exhibiting higher systemic transport, possibly via metal chelation and transporters, while Pb accumulation is restricted by adsorption and immobilization in root apoplasts41.
Removal efficiency (%) of Cd and Pb concentrations in roots and shoots of C. roseus grown under varying battery solid waste treatments (bar values are average followed by the standard deviation of three replicates; different letters (a–e) indicate significant (p < 0.05) differences among treatment groups based on Tukey’s post-hoc test).
In a study, González-López et al.31 reported that the Ricinus communis plant was capable of accumulating Pb from soils contaminated with Pb-acid battery assisted with selected mycorrhizal fungi. Similarly, Kriti et al.42 also investigated the potential of Helianthus annuus grown on Ni–Cd battery’s electrolyte (eW) contaminated soil and found greater accumulation in root parts than in the shoot. A study by Adejumo et al.43 found that Gomphrena celosoides could accumulate significant levels of Cd and Cr from battery waste-contaminated sites, with higher concentrations in roots than shoots, which is in line with those observed in the present study. Therefore, these results indicate that optimization of soil contamination levels and managing stress responses are necessary for enhanced phytoextraction efficiency of plants.
Effects on C. roseus plant growth
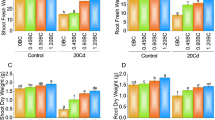
As depicted in Table 3, a significant (p < 0.05) declining trend in C. roseus growth parameters was recorded with increasing concentrations of BSW in soil due to induced effects of heavy metal toxicity. Specifically, plant height was found to be decreased from 61.403 cm in the control to 43.023 cm at 4% BSW, while root length declined from 24.832 to 11.582 cm, exhibiting impaired root elongation under elevated heavy metal stress. Similarly, fresh and dry biomass followed a similar trend, with reductions of 56.87% and 64.32%, respectively, at the highest BSW concentration, which might be due to inhibited photosynthesis and nutrient uptake due to heavy metal toxicity44. Chlorophyll and carotenoid contents are vital for photosynthetic efficiency, which also showed significant decreases, with total chlorophyll reducing from 2.404 mg/g in the control to 1.165 mg/g at 4% BSW, and carotenoid levels declining from 1.184 to 0.457 mg/g. These reductions might be associated with oxidative stress induced by Cd and Pb, which disrupt pigment biosynthesis and degrade chlorophyll via reactive oxygen species (ROS) accumulation45. The RGR also decreased from 0.014 g/g/day in control to 0.010 g/g/day at 4% BSW, depicting reduced growth, which might be caused by metabolic and enzymatic disruptions.
Plant growth could be altered by heavy metal toxicity through several mechanisms, such as impaired cellular respiration, disrupted enzymatic activity, and ion imbalance46. However, C. roseus showed moderate tolerance and growth under 1–2% BSW, which indicates its potential for phytoextraction at lower Cd and Pb contamination levels. However, the declined growth at higher BSW concentrations indicates that contamination levels should be optimized to avoid phytotoxicity. Previous studies have supported the fact that chemically stressed soils could alter plant growth and biochemical response. Out of them, Jaleel et al.47 found that two varieties of C. roseus (var. Rosea and Alba) grown under soil water deficit conditions resulted in reduced plant growth and biochemical traits, including plant height, chlorophyll, and carotenoid contents. Also, Kumar et al.48 observed that soils irrigated with battery industry effluent reduced the growth of radish (Raphanus sativus) plants due to heavy metal toxicity.
Results and validation of the hybrid model
In the present study, the Freundlich adsorption model described the relationship between Cavail and Cs as depicted by high R2 values (Cd: 0.998; Pb: 0.996). The regression plots of log(Cavail) and log(Cs) (Fig. 3a, c) exhibited a well-fitting linear trend indicating consistent adsorption behavior. Also, Freundlich parameters (Table 4), including adsorption intensity (1/n) and adsorption capacity (Kf), showed no significant variability for Cd and Pb. The Kf values (0.240 for Cd and Pb) suggest relatively similar sorption affinity of these metals in soil amended with BSW, while the n values (1.010 for Cd and Pb) also showed the fitness of the linear adsorption trend. On the other hand, observed vs. predicted bioavailable concentrations plots (Fig. 3b, d) showed the model’s goodness of fit in predicting metal mobility.
In the present experiment, the HMK model efficiently characterized the phytoextraction potential of C. roseus for Cd and Pb across soil treatments with varying levels of BSW. As shown in Figs. 4a, c, and 5a, c, the plot of 1/U vs. 1/Cavail yielded a well-fitting linear trend having R2 > 0.992 for both Cd and Pb contents in root and shoot tissues of C. roseus. By using intercept and slope values in this plot, the Michaelis–Menten model parameter values, i.e., Vmax and Km, were calculated, which further helped in the prediction of Cd and Pb uptake by C. roseus root and shoots as given in Table 5. For Cd, the Vmax was higher in roots (31.940 mg/kg) than shoots (15.16 mg/kg), indicating root-dominated Cd accumulation. The Km values (roots: 23.950 mg/kg; shoots: 11.366 mg/kg) suggest a higher binding affinity in shoots, likely due to differential transport and storage mechanisms. However, Pb exhibited a significantly higher Vmax in roots (94.756 mg/kg) and shoots (64.584 mg/kg), indicating greater Pb mobility and uptake compared to Cd, potentially due to Pb’s affinity for complexation with organic and inorganic ligands in soil. A higher uptake rate constant may reflect active transport processes, increased root surface reactivity, or enhanced expression of metal transporter proteins in C. roseus. These physiological traits are likely to contribute to its effective phytoextraction capacity.
On the other hand, the prediction of U values using the developed HMK model yielded high R2 values for both roots (Cd: 0.987, Pb: 0.997) and shoots (Cd: 0.985, Pb: 0.998), validating the model’s predictive capacity (Figs. 4b, d and 5b, d). Also, the residual values between observed and predicted uptake were minimal for Cd (< 2.029) and Pb (< 3.357), indicating good model performance. However, deviations were higher at elevated BSW concentrations (e.g., Pb root uptake at 4% BSW: observed 43.12 mg/kg, predicted 45.74 mg/kg). Moreover, Pb exhibited a higher but acceptable MAE compared to Cd, suggesting greater variability in uptake. Therefore, the results corroborate with the physiological and growth response of C. roseus, supporting the fact that Pb uptake is driven by its mobility and interaction with plant cell walls49, while Cd uptake is modulated by root-soil interactions and cellular detoxification mechanisms50. These results are supported by a previous study conducted by Kriti et al.42, who found that the Michaelis–Menten model can be successfully used to predict critical values, i.e., Vmax and Km, for phytoextraction of Ni and Cd using H. annuus from battery waste contaminated soil. Similarly, Pedron et al.51 reported that the Freundlich equation can be successfully used to predict heavy metal uptake behaviors of three crop plants, i.e., Brassica juncea, Lupinus albus, and H. annuus.
Conclusion
In the present study, the integrated application of the Freundlich isotherm and Michaelis–Menten kinetic models showed that C. roseus can be used as an effective phytoextraction of Pb and Cd in soils contaminated with BSW. Freundlich isotherm was successfully used to predict the bioavailable heavy metal concentration in BSW-spiked soil. It was also observed that Pb exhibited higher uptake rates and mobility, with Vmax values as compared to those of Cd. The lower Km values observed for Cd in shoots suggest strong binding affinity but limited translocation beyond roots, likely due to intracellular sequestration mechanisms. The developed models showed minimal residuals and high R2 values, indicating the good predictive accuracy of the models. This study showed the suitability of C. roseus for remediating BSW-contaminated soil and provides a sustainable solution for mitigating heavy metal pollution. Further research is highly suggested on assessing the impact of heavy metals on plant biochemical and enzymatic responses, along with identifying potential genes regulating the uptake mechanisms.
Data availability
Data will be made available on reasonable request to the corresponding author.
Change history
20 June 2025
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 1, which was incorrectly given as ‘Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, P.O. Box 84428, 11671, Riyadh, Saudi Arabia’. The correct affiliation is listed here: ‘Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia’. The original Article has been corrected.
References
Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R. & Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 9, 42 (2021).
Zhang, Q. & Wang, C. Natural and human factors affect the distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 231, 350 (2020).
Ray, S. & Vashishth, R. From water to plate: Reviewing the bioaccumulation of heavy metals in fish and unraveling human health risks in the food chain. Emerg Contam. 10, 100358 (2024).
Yang, Z., Huang, H. & Lin, F. Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy. Adv Energy Mater 12, (2022).
IEA. IEA Analysis Based on Mineral Commodity Summary 2022 by USGS (2022) Lithium Global Supply-Demand Balance (January 2023) from S&P Global (2023) and World Metal Statistics Yearbook by WBMS. https://pubs.er.usgs.gov/publication/mcs2022 (2023).
Shen, L., Sun, K., Xi, F., Jiang, Z., Li, S., Wang, Y., Hao, X. Conversion of photovoltaic waste silicon into amorphous silicon nanowire anodes. Energy & Environ. Sci., 18(9), 4348–4361. https://doi.org/10.1039/D5EE00020C (2025).
Jadaa, W. & Mohammed, H. Heavy metals – Definition, natural and anthropogenic sources of releasing into ecosystems, toxicity, and removal methods – An overview study. J. Ecol. Eng. 24, 249–271 (2023).
Zhang, Y., Liu, Z., Wang, J., Du, H., Sun, Q., Gao, R., Xu, Z. Efficient and high-selective lithium extraction from waste LiMn2O4 batteries by synergetic pyrolysis with polyvinyl chloride. Waste Management, 198, 95–105. https://doi.org/10.1016/j.wasman.2025.02.049 (2025).
Lin, N., Luo, X., Wen, J., Fu, J., Zhang, H., Siddique, K. H. M., Zhao, Y. Black biodegradable mulching increases grain yield and net return while decreasing carbon footprint in rain-fed conditions of the Loess Plateau. Field Crops Research, 318, 109590. https://doi.org/10.1016/j.fcr.2024.109590 (2024).
Vasilachi, I. C., Stoleru, V. & Gavrilescu, M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture 13, 1983 (2023).
Sharma, S., Tiwari, S., Hasan, A., Saxena, V. & Pandey, L. M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 8, 216 (2018).
Lavanya, M. B., Viswanath, D. S. & Sivapullaiah, P. V. Phytoremediation: An eco-friendly approach for remediation of heavy metal-contaminated soils-A comprehensive review. Environ. Nanotechnol. Monit. Manag. 22, 100975 (2024).
Peng, Y., & Yu, G. I. Prediction of the impact of ecological restoration technology on the restoration of heavy metal pollution in agricultural soil. Geology, Ecology, and Landscapes, 1–17. https://doi.org/10.1080/24749508.2024.2328900 (2024).
Wu, X., & Zhao, Y. A Novel Heat Pulse Method in Determining “Effective” Thermal Properties in Frozen Soil. Water Resources Research, 60(12), e2024WR037537. https://doi.org/10.1029/2024WR037537 (2024).
Ghori, Z. et al. Phytoextraction: The Use of Plants to Remove Heavy Metals from Soil. in Plant Metal Interaction: Emerging Remediation Techniques 361–384 (2015). https://doi.org/10.1016/B978-0-12-803158-2.00015-1.
Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 74, 21–31 (2022).
Nejat, N. et al. Ornamental Exterior versus Therapeutic Interior of Madagascar Periwinkle ( Catharanthus roseus ): The Two Faces of a Versatile Herb. The Scientific World Journal 2015, (2015).
Naeem, M. et al. Plant Efficacy and Alkaloids Production in Sadabahar (Catharanthus roseus L.): Role of Potent PGRs and Mineral Nutrients. in Catharanthus roseus 35–57 (Springer International Publishing, Cham, 2017). https://doi.org/10.1007/978-3-319-51620-2_3.
Taher, Z. M. et al. Anticancer molecules from catharanthus roseus. Indon. J. Pharmacy 30, 147 (2019).
Soumya, V., Kiranmayi, P. & Siva Kumar, K. Morpho-anatomical responses of Catharanthus roseus due to combined heavy metal stress observed under Scanning Electron Microscope. Plant Sci. Today 9, 623–631 (2022).
Kumar, S., Singh, B. & Singh, R. Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J. Ethnopharmacol. 284, 114647 (2022).
Asgari Lajayer, B., Khadem Moghadam, N., Maghsoodi, M. R., Ghorbanpour, M. & Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Poll. Res. 26, 8468–8484 (2019).
Cârdei, P. et al. Mathematical model to simulate the transfer of heavy metals from soil to plant. Sustainability 13, 6157 (2021).
Mishra, S. R., Chandra, R., Kaila, A. J. & Darshi, B. S. Kinetics and isotherm studies for the adsorption of metal ions onto two soil types. Environ. Technol. Innov. 7, 87–101 (2017).
Schimel, J. Modeling ecosystem-scale carbon dynamics in soil: The microbial dimension. Soil Biol. Biochem. 178, 108948 (2023).
Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934).
Xiao, L. et al. Assessment of earthworm activity on Cu, Cd, Pb and Zn bioavailability in contaminated soils using biota to soil accumulation factor and DTPA extraction. Ecotoxicol. Environ. Saf. 195, 110513 (2020).
Alfadul, S. M. S. & Al-Fredan, M. A. A. Effects of Cd, Cu, Pb, and Zn combinations on phragmites australis metabolism, metal accumulation and distribution. Arab. J. Sci. Eng. 38, 11–19 (2013).
AL-Huqail, A. A. et al. Bioremediation of battery scrap waste contaminated soils using coco grass (Cyperus rotundus L.): A prediction modeling study for cadmium and lead phytoextraction. Agriculture 13, 1411 (2023).
Kumar, V., Kumar, P. & Khan, A. Optimization of PGPR and silicon fertilization using response surface methodology for enhanced growth, yield and biochemical parameters of French bean (Phaseolus vulgaris L.) under saline stress. Biocatal. Agric. Biotechnol. 23, 3 (2020).
González-López, M. E., Laureano-Anzaldo, C. M., Pérez-Fonseca, A. A., Arellano, M. & Robledo-Ortíz, J. R. A critical overview of adsorption models linearization: Methodological and statistical inconsistencies. Sep. Purif. Rev. 51, 358–372 (2022).
Reid, R. J. Kinetics of nutrient uptake by plants cells. in Mineral Nutrition of Crops 41–66 (CRC Press, 2024).
Cao, Y. et al. Feasibility of nanoscale zero-valent iron to enhance the removal efficiencies of heavy metals from polluted soils by organic acids. Ecotoxicol. Environ. Saf. 162, 464–473 (2018).
Aveiga, A. M., Banchón, C., Sabando, R. & Delgado, M. Exploring the phytoremediation capability of Athyrium filix-femina, Ludwigia peruviana and Sphagneticola trilobata for Heavy Metal contamination. J. Ecol. Eng. 24, 165–174 (2023).
Yan, X. et al. Heavy metals uptake and translocation of typical wetland plants and their ecological effects on the coastal soil of a contaminated bay in Northeast China. Sci. Total Environ. 803, 149871 (2022).
Lamont, B. B., Williams, M. R. & He, T. Relative growth rate (RGR) and other confounded variables: Mathematical problems and biological solutions. Ann. Bot. 131, 555–568 (2023).
Hamid, Y. et al. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 660, 80–96 (2019).
WHO. World Health Organization. Permissible Limits of Heavy Metals in Soil and Plants; World Health Organization: Geneva, Switzerland. (1996).
Kriti, et al. Nickel and cadmium phytoextraction efficiencies of vetiver and lemongrass grown on Ni–Cd battery waste contaminated soil: A comparative study of linear and nonlinear models. J. Environ. Manage 295, 113144 (2021).
Wang, Y.-M., Zhou, D.-M., Yuan, X.-Y., Zhang, X.-H. & Li, Y. Modeling the interaction and toxicity of Cu-Cd mixture to wheat roots affected by humic acids, in terms of cell membrane surface characteristics. Chemosphere 199, 76–83 (2018).
Asare, M. O., Száková, J. & Tlustoš, P. The fate of secondary metabolites in plants growing on Cd-, As-, and Pb-contaminated soils—a comprehensive review. Environ. Sci. Pollut. Res. 30, 11378–11398 (2022).
Kriti, et al. Enhancement in Ni–Cd phytoremediation efficiency of Helianthus annuus L. from battery waste contaminated soil by bacterial augmentation, Isolated from E-Waste contaminated sites. Int. J. Environ. Res. 17, 18 (2023).
Adejumo, S. A., Tiwari, S., Thul, S. & Sarangi, B. K. Evaluation of lead and chromium tolerance and accumulation level in Gomphrena celosoides : A novel metal accumulator from lead acid battery waste contaminated site in Nigeria. Int. J. Phytoremed. 21, 1341–1355 (2019).
Riyazuddin, R. et al. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 12, 43 (2021).
Huihui, Z. et al. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 195, 110469 (2020).
Ahanger, M. Abass. Improving Stress Resilience in Plants : Physiological and Biochemical Basis and Utilization in Breeding. (Elsevier, 2024).
Jaleel, C. A., Manivannan, P., Lakshmanan, G. M. A., Gomathinayagam, M. & Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf B Biointerfaces 61, 298–303 (2008).
Kumar, N. et al. Toxicity assessment and accumulation of metals in radish irrigated with battery manufacturing industry effluent. Int. J. Vegetable Sci. 21, 373–385 (2015).
Kumar, A. & Prasad, M. N. V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 166, 401–418 (2018).
Zhang, X. et al. The uptake, transfer, and detoxification of cadmium in plants and its exogenous effects. Cells 13, 907 (2024).
Pedron, F. et al. Applicability of a freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 4, 67 (2017).
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R93), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R93), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Arwa A. AL-Huqail: Funding acquisition, Formal analysis, Validation, Writing—review & editing; Madhumita Goala: Data curation, Software, Validation, Writing—original draft; Ashish Kumar Arya: Data curation, Software, Validation, Writing –review & editing; Pankaj Kumar: Conceptualization, Methodology, Data curation, Software, Validation, Project administration, Supervision, Writing—original draft; Deep Gupta: Data curation, Software, Validation, Writing—original draft; Sudhir Kumar Gaur: Methodology, Data curation, Formal analysis, Validation, Writing –review & editing; Ivan Širić: Methodology, Data curation, Validation, Supervision, Writing –review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
AL-Huqail, A.A., Goala, M., Arya, A.K. et al. Mechanistic and kinetic modeling of cadmium and lead phytoextraction by Madagascar Periwinkle [Catharanthus roseus (L.) G.Don] in battery waste contaminated soil. Sci Rep 15, 18291 (2025). https://doi.org/10.1038/s41598-025-03080-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03080-x