Abstract
Major depressive disorder (MDD) is a leading cause of disability that affects over 300 million people globally. Despite multiple antidepressant trials, approximately one-third of MDD patients remain symptomatic, progressing to treatment-resistant depression (TRD). This persistence possibly is due to the multifaceted etiology of TRD, encompassing biological, psychological, and environmental factors. Chronic stress, prevalent in modern life, significantly contributes to mental health disorders and complicates TRD treatment. This study investigated psilocybin as a potential TRD treatment using a diathesis-stress animal model. Twenty-two male Wistar-Kyoto (WKY) rats were divided into control and stress groups, with the stress group further subdivided to receive either sham treatment or psilocybin as early intervention. Behavioral assessments demonstrated a significant and sustained beneficial effect of psilocybin on behavioral despair and cognitive impairment. Biochemical analyses revealed psilocybin-induced increases in thyroid-stimulating hormone (TSH) levels without significant changes in the hypothalamic-pituitary-adrenal (HPA) axis. The ability of psilocybin to counter stress-induced TSH reductions suggested that TSH may serve as a proxy marker of therapeutic response, although its causal role in mood regulation remains unclear. Additionally, following psilocybin administration, changes in cannabinoid receptor type I (CB1R) suggest a potential modulation of psilocybin intervention on the component of the endocannabinoid system (ECS), though causal links remain unconfirmed without antagonist studies. These findings highlight the potential of psilocybin to treat TRD through the targeting of previously unexplored biological pathways.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide1, affecting over 300 million individuals2. Chronic stress is a major risk factor for MDD3, and genetic predisposition, accounting for approximately 30% of cases, further amplifies risk when coupled with life stressors4. Treatment-resistant depression (TRD), a subtype of MDD defined by inadequate response to at least two different antidepressants despite appropriate dosing and duration5, underscores the limitations of current therapies and the urgent need for novel intervention6,7,8.
Psilocybin is a serotonergic psychedelic, and recent clinical trials have demonstrated its efficacy in treating MDD and TRD9,10,11,12. Unlike conventional antidepressants13, psilocybin elicits rapid and sustained antidepressant effects, with a single dose enhancing neuroplasticity and improving mood within hours14,15. This rapid therapeutic action positions psilocybin as a promising alternative to conventional antidepressants, even though it has not been determined how psilocybin affects the stress response in the development of depression.
A prior study, using a stress-induced depression model, demonstrated that psilocybin can dampen the chronic stress-induced abnormalities of the endocannabinoid system (ECS)16. The ECS is composed of endocannabinoids (eCBs) and cannabinoid receptors (CBRs)17. Within the CNS, the primary eCBs are anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), and the predominant CBRs are cannabinoid receptor type I (CB1R)17,18. ECS is essential for regulating responses to both internal and external stimuli and contributes significantly to central nervous system development and synaptic adaptability19. Previous studies have shown that ECS plays a pivotal role in mediating the downregulation of the hypothalamic-pituitary-adrenal (HPA) axis17,20, a central endocrine system involved in stress buffering. In response to stress, ECS facilitates the inhibitory regulation of HPA-axis activation; however, chronic stress subjecting could lead to abnormal eCB expression and loss of CBRs19. In addition, prior studies have described increased depressive- and anxiety-like behaviors in transgenic mice with CBR knock-out or 2-AG dysfunction21,22. To investigate the enduring effects of psilocybin in the diathesis-stress model of TRD, this research focused primarily on 2-AG because of its stable and prolonged alterations in response to stress stimuli20,23 and its greater expression level than that of AEA24.
This study examined how psilocybin modulates chronic stress-induced behavioral changes, hormonal modulation, and neurochemical alterations in a rodent model of TRD. Our findings indicate that early intervention with psilocybin (1) mitigates cognitive impairments in a TRD model, (2) produces enduring antidepressive and antianxiety effects, and (3) may modulate endocrine expression in response to stress through enhancing the release of thyroid-stimulating hormone (TSH), which might reflect an overlooked mechanism in the MDD model triggered by psychosocial stressors. However, no existing study has specifically examined psilocybin’s enduring effects in a TRD model. Furthermore, research on ketamine, a dissociative agent with psychedelic-like properties25, suggests that ECS modulation may play a role in its mechanism of action26,27. Building on this premise, the present study also provides the first investigation into the potential interaction between psilocybin and the ECS in a TRD model.
Materials and methods
Animals
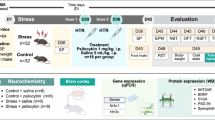
Wistar-Kyoto (WKY) rats are an animal model of TRD due to limited responsiveness to standard antidepressant drugs28,29. Under chronic social instability stress (SIS) conditions, stressed WKY rats represent a diathesis-stress model of TRD that better mimics biopsychosocial interactions. Twenty-two male WKY rats (approximately 300 g) aged 60 days were acquired from Charles River Laboratories. The rats were housed in the facility with a 12-hour light/dark cycle and were kept at a steady 22 °C, and the humidity was regulated between 50 and 55% 19. The rats were housed in pairs with ad libitum access to food and water throughout the 45-day experiment, except where noted, and all procedures were carried out during the light cycle (06:00–18:00), except for the second predator odor exposure (POE), which was conducted during the night cycle (18:00–06:00).
All animal procedures were conducted in strict compliance with the Guidelines of the Canadian Council on Animal Care and were approved by the Animal Care and Use Committees of the Health Sciences Laboratory Animal Services at the University of Alberta.
Experimental design
After seven days of acclimatization, the rats were randomly assigned to three groups: one control group (CTL, N = 6) and two stress groups (SIS, N = 16). The stress groups were further divided into a sham treatment subgroup (SIS-Sham, N = 8) and a psilocybin treatment group (SIS-PSI, N = 8). On the first day, the animals were exposed to their first POE. The stress groups then underwent 10 days of chronic social instability stress (SIS) as previously described19. On day 12, a second POE was performed to enhance the stress stimulus, followed by an additional 18 days of SIS19. In contrast, CTL animals were not exposed to SIS but did undergo both POEs with distilled water19. One hour after POE, all animals received intraperitoneal (IP) interventions, as detailed in earlier studies30,31. Between Days 33 and 39, behavioral tests were conducted, and on Day 40, the animals were euthanized for blood sample collection and brain tissue analysis (Please see Fig. S1 for more details).
Predator odor exposure (POE)
As previously described19, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT, 97% purity, SRQ Bio), a compound derived from red fox urine32, was used to simulate predator presence and induce innate fear and stress33. During the 1-hour POE, the rats were individually placed in a clean cage containing a conical centrifuge tube (14–432-22; Fisher Scientific) with a piece of filter paper moistened with 5 µL of TMT or distilled water (for the CTL group). The conical tube and its lid were perforated with small holes, allowing odor exposure without direct contact with the filter paper. The first POE occurred during the light cycle (06:00–18:00) on Day 1, and the second occurred during the night cycle (18:00–06:00) on Day 12.
Drug delivery
A 1.0 mg/kg psilocybin (99.7% purity, Psygen Labs, Inc.) solution was prepared in sterile saline (0.9%) on the day of the POE. The solution was vortexed thoroughly to ensure a consistent dose and then administered via IP injection immediately after POE experience as early intervention34. Animals in the CTL group and SIS-Sham subgroup received IP injections of sterile saline (0.9%, vehicle), while those in the SIS-PSI subgroup received psilocybin at a volume of 1 ml/kg34,35.
Social instability stress (SIS)
The SIS protocol, adapted from previous studies19,36,37, involved daily changes in the cage partners of the individuals in the SIS subgroups during the stress periods. In brief, over a 7-day rotation, each rat within the same subgroup was paired with a different rat from the same SIS subgroup for a 24-hour period between days 2–11 and days 13–32. After being placed in a new cage, the rat was allowed to stay there for an additional day with the introduction of a new partner. On the third day, the rat that had been residing in the cage was moved to a new cage, assuming the role of the new partner. The strict modulation of rotation ensured that no rat remained in the same cage for more than two consecutive days, thereby minimizing dominant behaviors36. The CTL group was not exposed to SIS and remained undisturbed.
Behavioral tests
A series of behavioral tests were performed to assess various aspects of rodent behavior, including general activity, anhedonia, recognition memory, anxiety, and behavioral despair. The animals were subsequently transported to the procedure room and acclimated for at least 30 min before the start of each test. The tests were conducted in the following order: open field test (OFT), sucrose preference test (SPT), novel object recognition (NOR), elevated plus maze (EPM), and forced swimming test (FST). Behavioral data were recorded and analyzed using ANY-maze software (version 7.20, Stoelting Co., Wood Dale, IL, USA). The testing apparatus was cleaned between animals, and only one test was conducted per day, with at least 24 h between tasks.
Open field tests (OFTs)
Following established methodologies, the OFT was used to evaluate exploratory activity and locomotion38. The testing apparatus was a black PVC box measuring 48 × 48 × 50 cm (L × W × H), and its interior was digitally divided into 16 equal squares using ANY-maze software. The layout included a central zone, composed of 4 squares, and a peripheral zone, consisting of 12 squares. During the test, the rats were placed in the center of the apparatus and allowed to explore the area for 15 min. The distance travelled (in cm) and the amount of time spent immobile (in sec) were recorded to assess the rats’ exploratory behavior and locomotion.
Sucrose preference test (SPT)
Due to capacity limitations within the animal facility, only WKY rats in the SIS-Sham and SIS-PSI underwent the SPT, as a result, CTL animals were not included in this behavioral test. The SPT was utilized to assess anhedonia-like behavior in rodents39 and involved the use of a 6-day protocol divided into four phases: acclimatization, training, deprivation, and testing38,39,40. During the acclimatization phase (days 1–2), each rat was housed individually with access to two water bottles and an ad libitum food supply, allowing them to become familiar with the presence of two bottles39. In the training phase (day 3), the rats were given two bottles filled with a 1% (w/v) sucrose solution40. On the following day (day 4), water was replaced with water in one of the bottles, while the other was filled with sucrose solution40. The bottle positions were then switched to prevent the development of conditioned place preference38. On day 5, the animals were deprived of food and water for 12 h overnight (18:00–06:00). The next morning (day 6), they were given a pre-weighed bottle of drinking water and a pre-weighed bottle with a 1% (w/v) sucrose solution, with both bottles accessible for 8 h during the light cycle. To avoid ___location-based preferences, the positions of the bottles were swapped every 2 h for a total of 8 h38. The difference in the weights of the bottles before and after the test was used to calculate the percentage of sucrose preference, defined as the amount of sucrose solution consumed (in g) divided by the total fluid intake (in g) multiplied by 100 (sucrose/total fluid * 100)38.
Novel object recognition (NOR)
The NOR test was conducted in the same arena as the OFT, as previously described19. On the training day (day 1), two identical 500 mL transparent plastic culture media bottles were placed in opposite corners of the arena, 9 cm from the walls. On the testing day (day 2), we introduced a novel object, a 125 mL transparent plastic culture flask similar in height to the familiar objects but differing in width. It was positioned in a third adjacent corner, also 9 cm from the walls. Again, each rat was placed facing away from the objects and allowed to explore for 5 min. The time spent interacting with each object was recorded to assess recognition memory, which is based on the natural tendency of rodents to favor new objects and requires the formation of memories of familiar objects41,42,43. The recognition index (RI), a measure of novelty preference and memory retention, was calculated by dividing the time spent exploring the new object (TN) by the total time spent exploring both the novel and familiar objects (TN + TF): RI = TN/(TN + TF)43.
Elevated plus maze (EPM) test
The EPM consisted of a platform with two open arms and two enclosed arms, each measuring 50 × 10 cm (L × W), as previously described19. Specifically, the closed arms were surrounded by 45 cm high walls, and the entire apparatus was elevated 55 cm above the floor19. The rats were placed at the junction of the four arms, oriented toward an open arm, and allowed to explore the maze freely for 5 min. The time spent in both the open and closed arms was recorded to evaluate anxiety-like behaviors. The time spent on risk assessment behaviors was also documented to enhance the predictive validity of the EPM test44,45. Risk assessment was defined as a stretched-attend posture, where the rat extends its head and shoulders toward either the open or closed arms, followed by cautious withdrawal to its initial position44,45.
Forced swimming test (FST)
The forced swim test (FST) was conducted using Plexiglas cylinders with internal dimensions of 20 × 50 cm (D × H) filled with water maintained at 23–25 °C. The water depth was sufficient to prevent the rats’ hind legs or tails from reaching the bottom of the container. As previously described, the FST consisted of a habituation session (day 1) and a testing session (day 2)19,46,47. Each rat was individually placed in a water-filled cylinder during the 15-minute habituation phase. Afterward, the rats were dried with towels, kept in a heated cage until fully dry, and returned to their home cages. The rats were reintroduced to the cylinder for a 10-minute test on the testing day. The first 2 min served as an acclimatization period, followed by 8 min, during which the time spent in both inactive and active behaviors was recorded. Immobility time, indicative of inactivity, was noted when the rat made minimal movements to keep its head above water, floated, or slightly turned38. Active behaviors were further categorized into swimming and climbing48,49, with climbing defined as vertical movement against the walls and swimming identified as horizontal movement across the water’s surface48.
Blood and brain sample collection
The animals were euthanized in an induction chamber using an inhalational anesthetic machine with isoflurane (5% in oxygen), before cardiac blood collection. Cardiac blood samples were collected with 20-gauge 1.5″ needles (305175, Becton Dickinson) into blood collection tubes (0268393 A, Fisher Scientific). The samples were left to clot before being centrifuged at 1000 ×g for 10 min at 4 °C to separate the serum. The serum was then aliquoted into 1.5 ml microcentrifuge tubes (05–408-129; Fisher Scientific) and stored at −80 °C. After blood collection, the rats were intracardially perfused with 1x phosphate-buffered saline (PBS, BP3994, Fisher Scientific). Brain tissues from six rats in each SIS subgroup were harvested and divided into left and right hemispheres. The right hemisphere was further dissected into four regions of interest (ROIs), namely, the prefrontal cortex (PFC), amygdala (AMG), hippocampus (HIP), and hypothalamus (HYP). The left hemisphere was postfixed in 4% paraformaldehyde (PFA; A11313.22; Thermo Fisher Scientific) for 16 h and then stored in 1x PBS for future analysis. Additionally, two rats from each subgroup were initially perfused with 4% PFA intracardially, collected whole, postfixed in 4% PFA for 16 h, and finally stored in 1x PBS for future use.
Luminex assay
Serum samples were diluted 1:2 with 1x PBS to assess the levels of circulating BDNF and stress-related hormones, including adrenocorticotropic hormone (ACTH), corticosterone (CORT), and thyroid stimulating hormone (TSH). The diluted samples were then analyzed using the Luminex assay (RSH-02–205 and RPT-04–212; Eve Technologies). Any leftover serum samples were stored at −80 °C for potential future analysis.
Enzyme-linked immunosorbent assay (ELISA)
ELISA detection kits (MBS1600322, MyBioSource, Inc.) were utilized to quantify 2-arachidonoylglycerol (2-AG) levels, prioritizing the HIP and HYP. Brain samples, including the PFC, AMG, HIP, and HYP, were first weighed and then lysed in radioimmunoprecipitation assay buffer (RIPA, 89901; Thermo Scientific) containing protease (A32963; Thermo Scientific) and phosphate inhibitors (A32957; Thermo Scientific). The tissues were homogenized using a tissue homogenizer (PRO Scientific Bio-Gen PRO200, Cole-Parmer) and incubated on ice for 20 min. The homogenates were centrifuged at 1200 ×g for 30 min at 4 °C to acquire the supernatants, which were subsequently stored at −80 °C.
Western blot (WB)
Western blot analyses were conducted only in the SIS-PSI and SIS-Sham groups. The SIS-Sham group thus served as the baseline comparator to assess psilocybin’s effects under chronic stress conditions. WB was performed to measure the expression levels of CB1R, BDNF, receptor tyrosine kinase B (TrkB), extracellular signal-regulated kinase (ERK), protein kinase B (Akt), and mammalian target of rapamycin (mTOR). Protein lysates, as previously prepared for ELISA, were quantified using the Bradford protein assay (5000002, Bio-Rad) and read on a FLUOstar Omega Microplate Reader (BMG). Equal amounts of protein (20 µg per lane) were loaded and run on 10% hand-cast sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS‒PAGE) gels and then transferred onto 0.45 μm nitrocellulose membranes (1620115, Bio-Rad). The membranes were blocked with 5% bovine serum albumin (BSA) in 1× Tris-buffered saline (TBS) before overnight incubation at 4 °C with primary antibodies in 1x TBS supplemented with 0.1% Tween-20 (BP337500, Fisher Scientific). After three washes (10 min each) with 1x TBS supplemented with 0.1% Tween-20 (1x TBST), the membranes were incubated with secondary antibodies for 1 h at room temperature, followed by three additional washes. Protein bands were detected and quantified using Image Studio Lite software (LI-COR Biosciences, Lincoln, NE Image Studio software Ver 5.2). GAPDH (1:1000; 97166 S; Cell Signaling) was used as a loading control, and all protein band intensities were normalized to that of GAPDH to ensure equal loading across samples.
The following primary antibodies were used: anti-CB1R (rabbit, 1:500; ab23703, Abcam), anti-BDNF (rabbit, 1: 1000; ab108319, Abcam), anti-TrkB (rabbit, 1:1000; 4603 S, Cell Signaling), anti-ERK1/2 (rabbit, 1:1000; 9102 S, Cell Signaling), anti-p-ERK1/2 (rabbit, 1: 1000; 4376 S, Cell Signaling), anti-Akt (mouse, 1:1000; 2920 S, Cell Signaling), anti-p-Akt (rabbit, 1:1000; 4060 S, Cell Signaling), anti-mTOR (rabbit, 1:1000; 2983 S, Cell Signaling), and anti-p-mTOR (rabbit, 1:1000; 5536 S, Cell Signaling). The secondary antibodies goat anti-rabbit-IR800 (926–32211, LI-COR Biosciences) and goat anti-mouse-IR680 (926–68070, LI-COR Biosciences) were used at a 1:1000 dilution.
Statistical analysis
The data are presented as the mean ± SEM. PRISM software (ver 10.2.3, GraphPad Software) was used to generate graphs and conduct statistical analysis. Statistical analyses were chosen based on the group comparison structure and are described in detail in the Results section. One-way ANOVA with Tukey’s post hoc test was applied for comparisons involving all three groups (CTL, SIS-Sham, and SIS-PSI). For experiments where the analysis focused solely on the effects of psilocybin within stressed animals, unpaired two-tailed t-tests or Mann-Whitney U tests were used to compare SIS-Sham and SIS-PSI subgroups. p < 0.05 indicated statistical significance.
Results
Early psilocybin intervention ameliorates chronic stress-induced behavioral despair and recognition impairment in a TRD model in Wistar-Kyoto rats.
To determine whether a 1.0 mg/kg dose of psilocybin could mitigate chronic stress-induced behavioral alterations in WKY rats, a series of behavioral tests were conducted following the stress induction protocol. In the OFT, a test that evaluates general activity, rats in the SIS subgroup showed increased travel distance (Fig. 1A, F (2,16) = 5.461, p = 0.0156) compared to CTL rats, with no notable differences between the SIS-PSI subgroup and the SIS-Sham subgroup. Additionally, no significant differences in immobility time were observed among the three conditions (Fig. 1B, F (2,16) = 2.287, p = 0.1338).
Early psilocybin intervention mitigates behavioral alterations induced by chronic stress in a diathesis-stress model of treatment-resistant depression (TRD) using Wistar-Kyoto (WKY) Rats. (A) In the open field test (OFT), rats subjected to social instability stress (SIS) traveled greater distances (cm) than the control (CTL) rats. However, there was no significant increase in the distance traveled between psilocybin-treated rats (SIS-PSI) and sham-treated rats (SIS-Sham). (B) Compared with CTL rats, SIS-Sham rats exhibited a negligible reduction in immobility time (sec), with a minor and nonsignificant difference observed between the SIS-PSI and SIS-Sham subgroups. (C, D) No significant differences were noted in the time (sec) spent in the open or closed arms of the elevated plus maze (EPM) among all groups. (E) The number of entries into the open arms of the EPM did not significantly differ between the CTL and SIS-Sham subgroups; however, the number of entries into the open arms was slightly, though not significantly, greater in the SIS-PSI rats than in the SIS-Sham rats. (F) Compared with CTL rats, SIS-Sham rats engaged in risk assessment behaviors for longer periods (sec). In contrast, compared with those in the SIS-Sham subgroup, the time spent on risk assessment behaviors was lower in the SIS-PSI group. (G) In the forced swim test (FST), the SIS-Sham rats demonstrated greater immobility than the CTL rats, whereas the SIS-PSI rats exhibited less immobility than their SIS-Sham counterparts. (H, I) Notably, compared with those in the SIS-Sham group, the number of climbing and swimming movements (sec) in the SIS-PSI group was significantly greater. (J) Additionally, though modest, the sucrose preference (%) was greater in the SIS-PSI rats relative to the SIS-Sham rats; CTL data were not collected for the SPT. (K) The novel object recognition test revealed a significantly lower recognition index (RI) for the SIS-sham rats than for the CTL rats. In contrast, the SIS-PSI rats achieved a significantly greater RI than did the SIS-Sham rats. The dotted line indicates chance performance (RI = 0.5). For A-G and K, the sample sizes were as follows: CTL, n = 5; SIS-Sham, n = 7; and SIS-PSI, n = 7. The data are presented as the mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) and were analyzed by one-way ANOVA followed by Tukey’s post hoc test. For H-J, the sample sizes were as follows: SIS-Sham, n = 7; SIS-PSI, n = 7. The data are expressed as the mean ± SEM (p < 0.05) and were analyzed by two-tailed Mann-Whitney U tests.
EPM is often used to evaluate anxiety and risk assessment behaviors44,45. Our results did not exhibit significant differences in the time spent in the open arms (Fig. 1C, F (2,16) = 0.2867, p = 0.7545) or closed arms (Fig. 1D, F (2,16) = 1.856, p = 0.1884) across the three conditions. Similarly, the number of entries into the open arms did not significantly differ among the conditions (Fig. 1E, F (2,16) = 2.513, p = 0.1099). However, one-way ANOVA revealed significant differences in the amount of time spent on risk assessment behaviors (F (2,16) = 8.273, p = 0.0034). Post hoc analysis of the risk assessment behaviors revealed that the SIS-Sham subgroup spent more time performing risk assessment behaviors than did the CTL group (Fig. 1F, p = 0.0030), while rats in the SIS-PSI subgroup exhibited less risk assessment than did rats in the SIS-Sham subgroup did (Fig. 1F, p = 0.0463).
The FST revealed significant differences in despair-like behavior across the three conditions (F (2,16) = 39.25, p < 0.0001). The movement of the rat in the SIS-Sham subgroup was lower than that of the rat in the CTL group (Fig. 1G, p < 0.0001), but early psilocybin intervention decreased immobility (Fig. 1G, p = 0.0010). To specifically evaluate whether psilocybin targets active versus adaptive coping strategies within the context of chronic stress, we restricted our analysis to the SIS-Sham and SIS-PSI subgroups, dissecting active behaviors into climbing and swimming based on established methods48. Our data demonstrated that psilocybin treatment significantly increased both climbing time (Fig. 1H, Mann-Whitney U = 5, p = 0.0111, two-tailed) and swimming time (Fig. 1I, Mann-Whitney U = 6.500, p = 0.0192, two-tailed) in the SIS-PSI rats compared to those in the SIS-Sham rats. Anhedonia-like behaviors, also associated with depression39, were tested using the SPT. Due to capacity limitations, SPT comparisons were restricted to the SIS-Sham and SIS-PSI subgroups to assess the relative effects of psilocybin under stress conditions; no significant differences were observed between these groups (Fig. 1J, Mann-Whitney U = 17.50, p = 0.6573, two-tailed).
Finally, the NOR test was conducted to assess recognition memory. Significant differences in the recognition indices (RIs) were found among the three conditions (F (2,15) = 3.841, p = 0.0434). While no significant differences were observed between the SIS-Sham subgroup and CTL group, the SIS-PSI subgroup displayed a notably greater RI than did the SIS-Sham subgroup (Fig. 1K, p = 0.0431).
Early psilocybin intervention reverses stress-induced TSH suppression in a TRD model with inherent HPA axis resilience.
Serum concentrations of stress-related hormones, including adrenocorticotropic hormone (ACTH), corticosteroids (CORT), and thyroid-stimulating hormone (TSH), were analyzed using the Luminex assay. No notable differences were found in ACTH levels (Fig. 2A, F (2,16) = 1.686, p = 0.2165) or CORT levels (Fig. 2B, F (2,16) = 1.869, p = 0.1864) across the three conditions. However, the TSH levels varied significantly (Fig. 2C, F (2,16) = 3.634, p = 0.0500), with the SIS-Sham subgroup displaying significantly lower TSH levels than did the CTL group (Tukey’s post hoc test, p = 0.0039). On the other hand, the SIS-PSI subgroup showed a marked increase in TSH compared to that in the SIS-Sham subgroup (p = 0.0091).
Early psilocybin intervention counteracts stress-induced suppression of the HPT axis in a diathesis-stress model of TRD with inherent HPA axis resilience. (A) Stress hormone concentrations in blood serum were quantified using the Luminex assay. The levels of ACTH did not significantly differ between the CTL and SIS-Sham subgroups; however, the SIS-PSI rats displayed a slight, though not significant, decrease in ACTH levels compared to the SIS-Sham rats. (B) The levels of CORT did not significantly differ between the CTL and SIS-Sham subgroups; however, compared with those in the SIS-Sham group, the levels of CORT in the SIS-PSI group were slightly, though not significantly, lower. (C) TSH levels in the SIS-Sham rats were significantly lower than those in the CTL rats, whereas TSH levels in the SIS-PSI rats were significantly elevated compared to those in the SIS-Sham rats. No significant differences were observed between the SIS-PSI and CTL rats at any of the measurements. Sample sizes were as follows: CTL, n = 5; SIS-Sham, n = 7; and SIS-PSI, n = 7. The data are presented as the mean ± SEM (**p < 0.01) and were analyzed by one-way ANOVA followed by Tukey’s post hoc test.
Early psilocybin intervention upregulates BDNF and TrkB levels
BDNF levels were assessed in blood serum using the Luminex assay and in four key brain regions via WB analysis. One-way ANOVA revealed significant differences in the serum BDNF concentrations across all three conditions (Fig. 3A, F (2, 16) = 17.19, p = 0.0001). The SIS-Sham subgroup showed a marked reduction in circulating BDNF compared to that in the CTL group (Fig. 3A, p < 0.0001). In contrast, the circulating BDNF levels in the SIS-PSI subgroup were significantly greater than those in the SIS-Sham subgroup (Fig. 3A, p = 0.0331). Nonetheless, BDNF levels in the SIS-PSI subgroup remained lower than those in the CTL subgroup (Fig. 3A, p = 0.0116).
Early psilocybin intervention modifies BDNF and TrkB levels and selectively stimulates the ERK, Akt, and mTOR signaling cascades in various brain regions. (A) Circulating levels of BDNF in blood serum were quantified using the Luminex assay. Circulating BDNF levels in the SIS-Sham rats were significantly lower than those in the CTL rats, whereas circulating BDNF levels in the SIS-PSI rats were significantly elevated compared to those in the SIS-Sham rats. The sample sizes were CTL, n = 5; SIS-Sham, n = 7; and SIS-PSI, n = 7. The data are presented as the mean ± SEM (**p < 0.01) and were analyzed by one-way ANOVA followed by Tukey’s post hoc test. (B) Representative WB images illustrating the expression of BDNF, TrkB, p-ERK/ERK, p-Akt/Akt, and p-mTOR/mTOR in the prefrontal cortex (PFC), amygdala (AMG), hippocampus (HIP), and hypothalamus (HYP) of WKY rats exposed to SIS, with GAPDH serving as a loading control. (C) Quantification of BDNF levels in all four brain regions: In the PFC, HIP, and HYP, the BDNF levels were significantly greater in the SIS-PSI rats than in the SIS-Sham rats. In the AMG, a marginal but nonsignificant increase in BDNF was observed in the SIS-PSI rats compared to the SIS-Sham rats. (D) Quantification of TrkB levels in all four brain regions: SIS-PSI rats showed notable upregulation of TrkB expression in the AMG and HIP. Increases in TrkB levels in the PFC and HYP were less pronounced. (E) Quantification of p-ERK/ERK levels in all four brain regions: Increased phosphorylation of ERK was significantly observed in the PFC and HYP of the SIS-PSI rats compared to those in the SIS-Sham rats. A minor and nonsignificant increase was noted in the AMG and HIP. (F) Quantification of p-Akt/Akt levels in all four brain regions: a significant increase in Akt phosphorylation in the HIP was observed in the SIS-PSI rats, with only slight and nonsignificant increases observed in the PFC, AMG, and HYP. (G) Quantification of p-mTOR/mTOR levels in all four brain regions: SIS-PSI rats demonstrated significantly heightened mTOR phosphorylation exclusively in the HIP, with minor and nonsignificant increases in the other three brain regions. The sample sizes for the SIS-Sham and SIS-PSI groups (C-G) were 5 for each group. The data are presented as the mean ± SEM († p < 0.05, †† p < 0.01) and were analyzed using multiple unpaired t-tests.
In the brains of the rats in the SIS subgroups, Western blot data (Fig. 3B) confirmed that psilocybin intervention led to a notable increase in BDNF levels within the PFC, HIP, and HYP relative to the sham-treated subgroup (Fig. 3C, t(8) = 4.766, p = 0.0014; t(8) = 3.270, p = 0.0114; and t(8) = 2.683, p = 0.0278), but no significant change was observed in the AMG (Fig. 3C, t(8) = 1.047, p = 0.3256). Additionally, the expression of TrkB, the receptor for BDNF, was significantly elevated in the AMG and HIP in the SIS-PSI subgroup relative to the SIS-Sham subgroup (Fig. 3D, t(8) = 3.111, p = 0.0144; t(8) = 2.381, p = 0.0445). No notable differences were found in TrkB expression in the PFC and HYP (Fig. 3D, t(8) = 1.485, p = 0.1759; t(8) = 1.170, p = 0.2757).
Early psilocybin intervention triggers varying degrees of downstream activation of the ERK, Akt, and mTOR signaling pathways across distinct brain regions in a TRD model
Within SIS-Sham and SIS-PSI subgroups, Western blotting was performed across all four brain regions to evaluate the phosphorylation levels of TrkB downstream signaling pathway components in the stress condition, including ERK, Akt, and mTOR, in the SIS group (Fig. 3E, F, G). Relative to SIS-Sham subgroup, psilocybin intervention led to a significant increase in ERK phosphorylation in both the PFC and HYP (Fig. 3E, t(8) = 2.432, p = 0.0411; t(8) = 2.923, p = 0.0192), but no significant change was observed in the AMG and HIP (Fig. 3E, t(8) = 0.4375, p = 0.6733; t(8) = 0.1545, p = 1.572). In contrast, Akt phosphorylation was significantly elevated in the HIP (Fig. 3F, t(8) = 2.309, p = 0.0498) following psilocybin administration relative to SIS-Sham subgroup, but not in the PFC, AMG, and HYP (Fig. 3F, t(8) = 0.3596, p = 0.7285; t(8) = 0.2268, p = 0.8263; t(8) = 0.0025, p = 0.9980). Notably, relative to the SIS-Sham subgroup, mTOR phosphorylation was significantly increased only in the HIP (Fig. 3G, t(8) = 3.065, p = 0.0155). No notable differences were found in mTOR expression in the PFC, AMG, and HYP (Fig. 3G, t(8) = 1.129, p = 0.2916; t(8) = 0.7466, p = 0.4767; t(8) = 1.427, p = 0.1913).
Psilocybin promotes CB1R expression in a TRD model but does not alter 2-AG levels
2-AG levels were assessed using ELISA, and the analysis revealed significant differences in the HYP across the three conditions (Fig. 4A, F (2, 10) = 14.43, p = 0.0011). Notably, rats in both the SIS-Sham and SIS-PSI subgroups have lower levels of 2-AG than those in the CTL group (Fig. 4A, p = 0.0031 and p = 0.0012) in the HYP. Early psilocybin intervention, however, did not result in any significant changes in 2-AG levels compared to those in the sham-treated group within the HYP (Fig. 4A, p = 0.7510). 2-AG levels were only measured in HIP within the SIS subgroups, revealing no significant differences (Fig. 4B, Mann-Whitney U = 9, p = 0.5476, two-tailed). The levels of CB1R, the receptor of 2-AG, as demonstrated by Western blots (Fig. 4C), were significantly greater in the PFC, AMG, and HIP in the SIS-PSI subgroup relative to the SIS-Sham group (Fig. 4D, t-test, t(8) = 3.166, p = 0.0133; t(8) = 2.725, p = 0.0260; t(8) = 3.194, p = 0.0127, respectively); however, no significant increase was observed in the HYP (Fig. 4D, t(8) = 1.627, p = 0.1423).
Psilocybin enhances CB1R expression across four regions in the TRD model compared with WKY rats without substantially diminishing 2-AG levels. (A) 2-AG levels in HYP were quantified using the Luminex assay. 2-AG levels in the SIS subgroup were significantly lower than those in the CTL subgroup. 2-AG levels did not differ significantly within the SIS subgroups. The sample sizes were CTL, n = 5; SIS-Sham, n = 7; and SIS-PSI, n = 7. The data are presented as the mean ± SEM (**p < 0.01) and were analyzed by one-way ANOVA followed by Tukey’s post hoc test. (B) As measured by ELISA, 2-AG levels did not significantly differ in the HIP between the SIS-Sham and SIS-PSI subgroups. (C) Representative WB images illustrating the expression of CB1R in the PFC, AMG, HIP, and HYP of TRD rats exposed to SIS, with GAPDH serving as a loading control. (D) Quantification of CB1Rs in all four brain regions: In the PFC, HIP, and HYP, the CB1Rs were significantly elevated in the SIS-PSI rats compared to those in the SIS-Sham rats. In the AMG, a marginal but nonsignificant increase in CB1Rs was observed in the SIS-PSI rats compared to the SIS-Sham rats. The sample sizes for the SIS-Sham and SIS-PSI groups (B, D) were 5 for each group. The data are presented as the mean ± SEM († p < 0.05, †† p < 0.01) and were analyzed using a two-tailed Mann-Whitney U test for (B) and multiple unpaired t-tests for (D). Data Availability Statement. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Discussion
The present study demonstrates that early intervention with low-dose psilocybin effectively mitigates behavioral despair and recognition impairment in a diathesis-related stress model of TRD in WKY rats. WKY rats are known for their limited responsiveness to conventional antidepressants28,29, their exposure to the chronic SIS paradigm incorporates biological, psychological, and social stressors to create a more comprehensive and accurate TRD model involving diathesis-stress interaction. Stressed WKY rats exhibit behavioral changes including increased locomotion in the OFT, increased risk assessment behavior in the EPM, and increased immobility during the FST. Early psilocybin intervention attenuates some of these behavioral responses. Namely, psilocybin intervention significantly reduces immobility in the FST, suggesting improved passive coping strategies typically observed in depressive-like states50. Furthermore, our findings corroborate previous studies that demonstrated improvements in familiarity recognition and memory retention in NOR test19, along with reduced time spent on cognitively driven risk assessment behaviors in the EPM51,52, suggesting psilocybin’s cognitive benefits.
While the serotonin 2 A (5-HT2 A) receptor is commonly believed to be the primary mediator for psilocybin’s psychoactive effects53, recent research indicates that the antidepressant effects of psilocybin may occur independently of its psychedelic properties and, therefore, may not rely on the activation of 5-HT2 A receptor54,55. Previous research has suggested that antidepressants targeting norepinephrine (NE) typically promote climbing in the FST, whereas those affecting serotonin (5-HT) enhance swimming48. In this study, psilocybin intervention decreased immobility and enhanced both swimming and climbing behaviors, suggesting that psilocybin may target NE in addition to 5-HT56,57. Although not significant, increased general activity and open-arm exploration in SIS-PSI rats within EPM support the hypothesis that psilocybin may act through non-serotonergic pathways. These behaviors are associated with NE and dopamine (DA) signaling58,59. Moreover, the modest increase in sucrose preference suggests psilocybin may alleviate anhedonia, a core symptom of reward deficits primarily driven by the DA system60,61, possibly through modulation of both DA and 5-HT. Given the absence of CTL data in the SPT, the interpretation of SPT findings is constrained to stressed conditions. Incorporating CTL comparisons in future studies will benefit the full assessment of the extent of restoration. In summary, these findings highlight that psilocybin may influence the DA and NE systems in addition to its primary action on 5-HT.
The observed restoration of thyroid-stimulating hormone (TSH) levels following psilocybin intervention highlights a previously underexplored physiological response in the context of TRD. Previous depression studies have focused mainly on investigating disruptions to the HPA axis62,63,64. Persistent elevation of CORT levels can disrupt the normal functioning of the HPA axis65,66, leading to gradual resistance to CORTs in target tissues, including the pituitary gland and HYP67,68. The hypothalamic-pituitary-thyroid (HPT) axis, like the HPA axis, originates in HYP and aids in regulating stress-related hormones69. TSH plays a crucial role in regulating the metabolism necessary for growth, and lower TSH levels have been linked to the onset of several mental health disorders70,71,72. Extensive research has demonstrated that prolonged stress and resulting hyperactivity of the HPA axis can suppress TSH production73,74, suggesting an inverse relationship between CORTs and TSH levels75. In the present study, even with heightened HPA axis resilience, psilocybin can mitigate stress-induced behavioral changes, which appear to lead to the restoration of TSH levels. This is evident from the significant reduction and subsequent restoration of TSH levels in the SIS-Sham and SIS-PSI subgroups, respectively, highlighting the potential compensatory role of TSH in mood regulation and cognitive processes, especially when HPA-axis alterations are not prominent. Future studies examining a full panel of thyroid hormones (such as triiodothyronine (T3), thyroxine (T4), and thyrotropin-releasing hormone (TRH)) will be able to provide more mechanistic insight into the direct causal modulation of psilocybin on the HPT-axis.
Furthermore, a recent study suggested that TSH may have neuroprotective effects by increasing brain-derived neurotrophic factor (BDNF) concentrations76, which aligns with our findings. Our data revealed that early psilocybin intervention restored circulating BDNF levels relative to CTL animals. In addition, Western blot analyses showed increased BDNF expression in psilocybin-treated rats compared to SIS-Sham animals within all four brain regions, although the change was not significant in the AMG. Currently, the molecular mechanism by which psilocybin acts is predominantly centered on its engagement with the 5-HT2 A receptor, which is believed to be mainly responsible for the “psychedelic experience” associated with psilocybin53,77. However, recent evidence also implicates direct interaction with receptor tyrosine kinase B (TrkB), a high-affinity BDNF receptor and mediator of antidepressant response78. In our study, early psilocybin intervention increased TrkB expression across all brain regions relative to the SIS-Sham group, though modest in the PFC and HYP, suggesting a potential promotive effect of psilocybin on neurotrophic TrkB signaling78,79.
mTOR, a serine/threonine protein kinase vital for neural development and synaptic plasticity80,81, is activated downstream of the extracellular signal-regulated kinase (ERK) and protein kinase B (Akt) cascades80, which are activated following TrkB stimulation. Increasing evidence has emphasized the importance of mTOR as a core regulator in treating neurological disorders82,83,84. Downstream analysis revealed that, relative to SIS-Sham animals, psilocybin selectively enhances ERK and Akt in a region-specific manner, with notable increases in mTOR activation in the HIP. Variations in TrkB expression and the activation of downstream pathways across different brain areas could be due to regional differences in neurotransmitter systems and neural circuitry85,86,87. These disparities influence the unique interactions between psilocybin and neuronal networks. Notably, the varying yet concurrent changes in the AMG and HIP may be explained by their roles as integral parts of the limbic system, which closely interact to regulate emotions, memory, and stress responses86,87. Previous studies have indicated that the AMG and HIP work together to consolidate contextual information when generating emotional responses88. Additionally, both structures regulate the HPA axis, with the hippocampus primarily exerting inhibitory effects and the amygdala facilitating the activation of HPA axis secretion86,87. Conversely, the PFC and HYP are key centers within brain networks and are particularly important for higher-order cognitive functions such as decision-making, as are their roles in regulating emotions and managing stress responses86,87,89. Importantly, because Western blot data were only collected for SIS-Sham and SIS-PSI animals, our comparisons reflect region-specific molecular modulation under stress conditions, rather than full restoration. Future studies incorporating CTL groups into molecular assays can determine whether these changes reflect normalization or psilocybin-induced alterations unique to stressed conditions.
Finally, this study investigated the potential involvement of the ECS in the therapeutic effects of psilocybin in this diathesis-stress model of TRD. Our results demonstrated that chronic stress leads to a notable decrease in 2-AG levels in the HYP, consistent with the findings of previous research involving chronic stress models employing multiple stress factors (e.g., chronic unpredictable stress models)20,90. This decrease in eCB levels contrasts with scenarios in which chronic stress involves fewer stressors (e.g., social instability), which generally results in elevated eCB levels91,92. Interestingly, our laboratory previously subjected Wistar rats to the chronic stress paradigm described in this study, and we observed an increase in 2-AG levels upon chronic stress19. This finding illustrates the complex and diverse effects of stress on the ECS and suggests a unique response to stress in this diathesis-stress model of TRD.
In this diathesis-stress model, psilocybin did not modulate 2-AG levels despite the observed upregulation of CB1R expression in all four brain regions. This finding suggested the potential involvement of ECS components following psilocybin intervention. Although direct evidence confirming psilocybin’s direct modulation of the ECS and the detailed molecular mechanisms involved are lacking. Several studies on ketamine, often classified as an atypical psychedelic93, have indicated that the activation of 2-AG signaling is critical for its psychostimulant and reinforcing effects26. Inhibition or genetic deletion of CB1Rs has been shown to reduce ketamine-induced neuronal remodeling and behavioral changes26,94. Furthermore, the administration of LSD, another serotonergic classical psychedelic similar to psilocybin, leads to the accumulation of eCBs95, and 3,4-methylenedioxymethamphetamine (MDMA), which elicits prosocial effects96 and induces a dissociative state similar to, though milder than, psilocybin or ketamine97, is at least partially reliant upon the ECS in modulating its reward and reinforcing properties98. This evidence supports the possibility that psilocybin may possess properties that enable it to directly modulate the ECS. However, the causal role of the ECS in psilocybin’s effects should be interpreted with caution until confirmed by studies using CB1R antagonists or agonists.
In conclusion, this study provides compelling evidence that psilocybin is a unique and potent agent for treating TRD through possible mechanisms distinct from traditional antidepressant pathways. By enhancing TSH expression and upregulating BDNF-related signaling pathways, psilocybin alleviates behavioral despair and cognitive impairment. TSH may serve as a potential proxy marker until a full panel of thyroid hormones is assessed, which could clarify whether it also functions as a therapeutic mediator. While changes in CB1R expression suggest potential involvement of the ECS, further studies are needed to establish its causal role. These findings lay the groundwork for future clinical trials investigating the efficacy of psilocybin in humans, particularly in early intervention strategies for TRD. Continued research exploring the interaction between the ECS and HPT axes may yield further insights into the full therapeutic potential of psilocybin and broaden our understanding of the underlying neurobiology of depression.
Limitation
The current study, utilizing an animal model of TRD, demonstrates the therapeutic potential of early psilocybin intervention in alleviating depression-like behaviors and cognitive impairments induced by the diathesis-stress interaction. While our biochemical results suggest that early psilocybin intervention can enhance BDNF-mTOR signaling, whether this occurs independently of 5-HT receptor activation remains unclear. Future work should include measuring head twitch responses shortly after psilocybin injection and assessing 5-HT receptor expression, particularly 5HT2 AR, within brain ROIs to evaluate the involvement of the serotonergic system. Although changes in eCBs and CB1R expressions have been recorded, future studies could employ selective CB1R antagonists (e.g., Rimonabant) to better understand CB1R’s roles in mediating psilocybin’s therapeutic effects. In addition, it would be beneficial to include other brain regions, for example, the Nucleus Accumbens (NAc) and the Bed Nucleus of the Stria Terminalis (BNST), alongside the PFC and AMG assessed in this study. Future work should also include control groups in biochemical assays to strengthen mechanistic conclusions on how psilocybin’s effects are processed in brain regions with distinct functions by providing a normalized baseline. Additionally, the present study was conducted exclusively in male rats, limiting the generalizability of findings to females. Given established sex differences in stress susceptibility, hormonal modulation (e.g., estrogen and progesterone), neuroplasticity, and serotonergic signaling, future studies should incorporate both sexes to determine whether psilocybin’s therapeutic effects and underlying mechanisms differ by sex. Finally, optimizing dosing strategies, especially microdosing (< 1 mg/kg), is also essential to maximize therapeutic benefits while minimizing psychoactive effects.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bains, N. & Abdijadid, S. In StatPearls (StatPearls Publishing Copyright © 2024 (StatPearls Publishing LLC., 2024).
Chodavadia, P., Teo, I., Poremski, D., Fung, D. S. S. & Finkelstein, E. A. Prevalence and economic burden of depression and anxiety symptoms among Singaporean adults: results from a 2022 web panel. BMC Psychiatry. 23, 104. https://doi.org/10.1186/s12888-023-04581-7 (2023).
Hidaka, B. H. Depression as a disease of modernity: explanations for increasing prevalence. J. Affect. Disord. 140, 205–214. https://doi.org/10.1016/j.jad.2011.12.036 (2012).
Su, Y. Y. et al. Specific and cumulative lifetime stressors in the aetiology of major depression: A longitudinal community-based population study. Epidemiol. Psychiatr Sci. 31, e3. https://doi.org/10.1017/s2045796021000779 (2022).
Parikh, S. V. et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: sect. 2. Psychological treatments. Can. J. Psychiatry. 61, 524–539. https://doi.org/10.1177/0706743716659418 (2016).
Gronemann, F. H. et al. Treatment patterns in patients with treatment-resistant depression in Danish patients with major depressive disorder. J. Affect. Disord. 287, 204–213. https://doi.org/10.1016/j.jad.2021.03.029 (2021).
Penn, E. & Tracy, D. K. The drugs don’t work? Antidepressants and the current and future Pharmacological management of depression. Ther. Adv. Psychopharmacol. 2, 179–188. https://doi.org/10.1177/2045125312445469 (2012).
Andrade, C. Psychotropic drugs with long Half-Lives: implications for drug discontinuation, occasional missed doses, dosing interval, and pregnancy planning. J. Clin. Psychiatry. 83 https://doi.org/10.4088/JCP.22f14593 (2022).
Gukasyan, N. et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J. Psychopharmacol. 36, 151–158. https://doi.org/10.1177/02698811211073759 (2022).
Reed, F. & Foldi, C. J. Do the therapeutic effects of psilocybin involve actions in the gut? Trends Pharmacol. Sci. 45, 107–117. https://doi.org/10.1016/j.tips.2023.12.007 (2024).
Goodwin, G. M. et al. Single-Dose psilocybin for a Treatment-Resistant episode of major depression. N Engl. J. Med. 387, 1637–1648. https://doi.org/10.1056/NEJMoa2206443 (2022).
Rosenblat, J. D. et al. Psilocybin-assisted psychotherapy for treatment resistant depression: A randomized clinical trial evaluating repeated doses of psilocybin. Med 5, 190–200e195. https://doi.org/10.1016/j.medj.2024.01.005 (2024).
Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. https://doi.org/10.1016/s0140-6736(17)32802-7 (2018).
Mans, K. et al. Multifaceted improvements in mental Well-Being following psychedelic experiences in a prospective opportunity sample. Front. Psychiatry. 12, 647909. https://doi.org/10.3389/fpsyt.2021.647909 (2021). Sustained.
Calder, A. E. & Hasler, G. Towards an Understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 48, 104–112. https://doi.org/10.1038/s41386-022-01389-z (2023).
Wang, Z. et al. Early psilocybin intervention alleviates behavioral despair and cognitive impairment in stressed Wistar rats. Prog Neuropsychopharmacol. Biol. Psychiatry. 136, 111243. https://doi.org/10.1016/j.pnpbp.2024.111243 (2025).
Lu, H. C. & Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry. 79, 516–525. https://doi.org/10.1016/j.biopsych.2015.07.028 (2016).
Reggio, P. H. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr. Med. Chem. 17, 1468–1486. https://doi.org/10.2174/092986710790980005 (2010).
Wang, Z. et al. Wistar-Kyoto rats and chronically stressed Wistar rats present similar depression- and anxiety-like behaviors but different corticosterone and endocannabinoid system modulation. Prog Neuropsychopharmacol. Biol. Psychiatry. 127, 110825. https://doi.org/10.1016/j.pnpbp.2023.110825 (2023).
Danan, D., Todder, D., Zohar, J., Cohen, H. & Is PTSD-Phenotype associated with HPA-Axis sensitivity?? The endocannabinoid system in modulating stress response in rats. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22126416 (2021).
Soriano, D., Brusco, A. & Caltana, L. Further evidence of anxiety- and depression-like behavior for total genetic ablation of cannabinoid receptor type 1. Behav. Brain Res. 400, 113007. https://doi.org/10.1016/j.bbr.2020.113007 (2021).
Imperatore, R. et al. Genetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB₁R signaling and anxiety-like behavior. J. Neurochem. 135, 799–813. https://doi.org/10.1111/jnc.13267 (2015).
Morena, M., Patel, S., Bains, J. S. & Hill, M. N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41, 80–102. https://doi.org/10.1038/npp.2015.166 (2016).
Zou, S. & Kumar, U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int. J. Mol. Sci. 19 https://doi.org/10.3390/ijms19030833 (2018).
Wolfson, P. & Vaid, G. Ketamine-assisted psychotherapy, psychedelic methodologies, and the impregnable value of the subjective-a new and evolving approach. Front. Psychiatry. 15, 1209419. https://doi.org/10.3389/fpsyt.2024.1209419 (2024).
Xu, W. et al. Endocannabinoid signaling regulates the reinforcing and psychostimulant effects of ketamine in mice. Nat. Commun. 11, 5962. https://doi.org/10.1038/s41467-020-19780-z (2020).
Pacheco, D. D. F., Romero, T. R. L. & Duarte, I. D. G. Ketamine induces central antinociception mediated by endogenous cannabinoids and activation of CB(1) receptors. Neurosci. Lett. 699, 140–144. https://doi.org/10.1016/j.neulet.2019.01.059 (2019).
Willner, P. et al. Validation of chronic mild stress in the Wistar-Kyoto rat as an animal model of treatment-resistant depression. Behav. Pharmacol. 30, 239–250. https://doi.org/10.1097/fbp.0000000000000431 (2019).
Aleksandrova, L. R., Wang, Y. T. & Phillips, A. G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci. Biobehav Rev. 105, 1–23. https://doi.org/10.1016/j.neubiorev.2019.07.007 (2019).
Ritov, G. & Richter-Levin, G. Pre-trauma methylphenidate in rats reduces PTSD-like reactions one month later. Transl Psychiatry. 7, e1000. https://doi.org/10.1038/tp.2016.277 (2017).
Xue, F. et al. Early intervention with electroacupuncture prevents PTSD-like behaviors in rats through enhancing hippocampal endocannabinoid signaling. Prog Neuropsychopharmacol. Biol. Psychiatry. 93, 171–181. https://doi.org/10.1016/j.pnpbp.2019.03.018 (2019).
Chan, C. E., Lee, Y. U. & Swoap, S. J. Physiological response to the odorant TMT in fully fed and calorically restricted laboratory mice. J. Therm. Biol. 95, 102819. https://doi.org/10.1016/j.jtherbio.2020.102819 (2021).
Tyler, R. E., Weinberg, B. Z. S., Lovelock, D. F., Ornelas, L. C. & Besheer, J. Exposure to the predator odor TMT induces early and late differential gene expression related to stress and excitatory synaptic function throughout the brain in male rats. Genes Brain Behav. 19, e12684. https://doi.org/10.1111/gbb.12684 (2020).
Huang, J., Pham, M., Panenka, W. J., Honer, W. G. & Barr, A. M. Chronic treatment with psilocybin decreases changes in body weight in a rodent model of obesity. Front. Psychiatry. 13, 891512. https://doi.org/10.3389/fpsyt.2022.891512 (2022).
Malikowska-Racia, N., Koniewski, M., Golebiowska, J. & Popik, P. Acute but not long-lasting antidepressant-like effect of psilocybin in differential reinforcement of low-rate 72 schedule in rats. J. Psychopharmacol. 37, 1149–1156. https://doi.org/10.1177/02698811231205692 (2023).
Koert, A. et al. The social instability stress paradigm in rat and mouse: A systematic review of protocols, limitations, and recommendations. Neurobiol. Stress. 15, 100410. https://doi.org/10.1016/j.ynstr.2021.100410 (2021).
Planchez, B., Surget, A. & Belzung, C. Animal models of major depression: drawbacks and challenges. J. Neural Transm (Vienna). 126, 1383–1408. https://doi.org/10.1007/s00702-019-02084-y (2019).
De Gregorio, D. et al. Repeated lysergic acid diethylamide (LSD) reverses stress-induced anxiety-like behavior, cortical synaptogenesis deficits and serotonergic neurotransmission decline. Neuropsychopharmacology 47, 1188–1198. https://doi.org/10.1038/s41386-022-01301-9 (2022).
Liu, M. Y. et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 13, 1686–1698. https://doi.org/10.1038/s41596-018-0011-z (2018).
He, L. W. et al. Optimization of food deprivation and sucrose preference test in SD rat model undergoing chronic unpredictable mild stress. Anim. Model. Exp. Med. 3, 69–78. https://doi.org/10.1002/ame2.12107 (2020).
Chesworth, R., Watt, G. & Karl, T. in Handbook of Behavioral Neuroscience Vol. 27 (eds Abdel Ennaceur & Maria Angelica de Souza Silva) 461–488 (Elsevier, 2018).
Akkerman, S. et al. Object recognition testing: methodological considerations on exploration and discrimination measures. Behav. Brain Res. 232, 335–347. https://doi.org/10.1016/j.bbr.2012.03.022 (2012).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. https://doi.org/10.1007/s10339-011-0430-z (2012).
Correa-Netto, N. F. et al. An ontogenic study of the behavioral effects of chronic intermittent exposure to Ayahuasca in mice. Braz J. Med. Biol. Res. 50, e6036. https://doi.org/10.1590/1414-431x20176036 (2017).
Walf, A. A. & Frye, C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328. https://doi.org/10.1038/nprot.2007.44 (2007).
Yankelevitch-Yahav, R., Franko, M., Huly, A. & Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. https://doi.org/10.3791/52587 (2015).
Slattery, D. A. & Cryan, J. F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 7, 1009–1014. https://doi.org/10.1038/nprot.2012.044 (2012).
Bogdanova, O. V., Kanekar, S., D’Anci, K. E. & Renshaw, P. F. Factors influencing behavior in the forced swim test. Physiol. Behav. 118, 227–239. https://doi.org/10.1016/j.physbeh.2013.05.012 (2013).
Fenton, E. Y. et al. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behavior, hippocampal reelin expression, and neuronal maturation. Prog Neuropsychopharmacol. Biol. Psychiatry. 60, 52–59. https://doi.org/10.1016/j.pnpbp.2015.02.001 (2015).
Commons, K. G., Cholanians, A. B., Babb, J. A. & Ehlinger, D. G. The rodent forced swim test measures Stress-Coping strategy, not Depression-like behavior. ACS Chem. Neurosci. 8, 955–960. https://doi.org/10.1021/acschemneuro.7b00042 (2017).
Griebel, G., Rodgers, R. J., Perrault, G. & Sanger, D. J. Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol. Biochem. Behav. 57, 817–827. https://doi.org/10.1016/s0091-3057(96)00402-9 (1997).
Cruz, A. P., Frei, F. & Graeff, F. G. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 49, 171–176. https://doi.org/10.1016/0091-3057(94)90472-3 (1994).
Inserra, A., De Gregorio, D. & Gobbi, G. Psychedelics in psychiatry: neuroplastic, Immunomodulatory, and neurotransmitter mechanisms. Pharmacol. Rev. 73, 202–277. https://doi.org/10.1124/pharmrev.120.000056 (2021).
Hesselgrave, N., Troppoli, T. A., Wulff, A. B., Cole, A. B. & Thompson, S. M. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci. U S A. 118 https://doi.org/10.1073/pnas.2022489118 (2021).
Husain, M. I. et al. Psilocybin for treatment-resistant depression without psychedelic effects: study protocol for a 4-week, double-blind, proof-of-concept randomised controlled trial. BJPsych Open. 9, e134. https://doi.org/10.1192/bjo.2023.535 (2023).
Madsen, M. K. et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma Psilocin levels. Neuropsychopharmacology 44, 1328–1334. https://doi.org/10.1038/s41386-019-0324-9 (2019).
Grandjean, J. et al. Psilocybin exerts distinct effects on resting state networks associated with serotonin and dopamine in mice. Neuroimage 225, 117456. https://doi.org/10.1016/j.neuroimage.2020.117456 (2021).
Schank, J. R., Liles, L. C. & Weinshenker, D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol. Psychiatry. 63, 1007–1012. https://doi.org/10.1016/j.biopsych.2007.10.018 (2008).
Rodgers, R. J., Nikulina, E. M. & Cole, J. C. Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus-maze. Pharmacol. Biochem. Behav. 49, 985–995. https://doi.org/10.1016/0091-3057(94)90253-4 (1994).
Belujon, P. & Grace, A. A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046. https://doi.org/10.1093/ijnp/pyx056 (2017).
Der-Avakian, A. & Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35, 68–77. https://doi.org/10.1016/j.tins.2011.11.005 (2012).
Herman, J. P. et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical stress response. Compr. Physiol. 6, 603–621. https://doi.org/10.1002/cphy.c150015 (2016).
Hill, M. N. et al. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. 30, 14980–14986. https://doi.org/10.1523/jneurosci.4283-10.2010 (2010).
Oster, H. et al. The functional and clinical significance of the 24-Hour rhythm of Circulating glucocorticoids. Endocr. Rev. 38, 3–45. https://doi.org/10.1210/er.2015-1080 (2017).
Stephens, M. A. & Wand, G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 34, 468–483 (2012).
Mikulska, J., Juszczyk, G., Gawrońska-Grzywacz, M. & Herbet, M. HPA Axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. 11 https://doi.org/10.3390/brainsci11101298 (2021).
Hannibal, K. E. & Bishop, M. D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 94, 1816–1825. https://doi.org/10.2522/ptj.20130597 (2014).
Vashist, S. K., Schneider, E. M. & Depression An insight and need for personalized psychological stress monitoring and management. J. Basic. Appl. Sci. 10, 177–182 (2014).
Fekete, C. & Lechan, R. M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194. https://doi.org/10.1210/er.2013-1087 (2014).
Medici, M. et al. Thyroid function within the normal range and the risk of depression: a population-based cohort study. J. Clin. Endocrinol. Metab. 99, 1213–1219. https://doi.org/10.1210/jc.2013-3589 (2014).
Khaleghzadeh-Ahangar, H., Talebi, A. & Mohseni-Moghaddam, P. Thyroid disorders and development of cognitive impairment: A review study. Neuroendocrinology 112, 835–844. https://doi.org/10.1159/000521650 (2022).
Nuguru, S. P. et al. Hypothyroidism and depression: A narrative review. Cureus 14, e28201. https://doi.org/10.7759/cureus.28201 (2022).
Helmreich, D. L. & Tylee, D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm. Behav. 60, 284–291. https://doi.org/10.1016/j.yhbeh.2011.06.003 (2011).
Jonsdottir, I. H., Sjörs & Dahlman A. MECHANISMS IN ENDOCRINOLOGY: endocrine and immunological aspects of burnout: a narrative review. Eur. J. Endocrinol. 180, R147–r158. https://doi.org/10.1530/eje-18-0741 (2019).
Cai, R. et al. Association between thyroid function and serum cortisol in cortisol-producing adenoma patients. Endocrine 69, 196–203. https://doi.org/10.1007/s12020-020-02278-5 (2020).
Toll, A. et al. Relationship between thyroid-stimulating hormone, BDNF levels, and hippocampal volume in antipsychotic-naïve first-episode psychosis patients. Front. Psychiatry. 14, 1301714. https://doi.org/10.3389/fpsyt.2023.1301714 (2023).
Ling, S. et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. 36, 17–30. https://doi.org/10.1007/s40263-021-00877-y (2022).
Moliner, R. et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 26, 1032–1041. https://doi.org/10.1038/s41593-023-01316-5 (2023).
Cameron, L. P. et al. Beyond the 5-HT(2A) receptor: classic and nonclassic targets in psychedelic drug action. J. Neurosci. 43, 7472–7482. https://doi.org/10.1523/jneurosci.1384-23.2023 (2023).
Fries, G. R., Saldana, V. A., Finnstein, J. & Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry. 28, 284–297. https://doi.org/10.1038/s41380-022-01806-1 (2023).
Ye, X. et al. Low-intensity pulsed ultrasound enhances neurite growth in serum-starved human neuroblastoma cells. Front. Neurosci. 17, 1269267. https://doi.org/10.3389/fnins.2023.1269267 (2023).
Ignácio, Z. M. et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br. J. Clin. Pharmacol. 82, 1280–1290. https://doi.org/10.1111/bcp.12845 (2016).
Sun, Z. et al. Deep brain stimulation improved depressive-like behaviors and hippocampal synapse deficits by activating the BDNF/mTOR signaling pathway. Behav. Brain Res. 419, 113709. https://doi.org/10.1016/j.bbr.2021.113709 (2022).
Kato, T. Role of mTOR1 signaling in the antidepressant effects of ketamine and the potential of mTORC1 activators as novel antidepressants. Neuropharmacology 223, 109325. https://doi.org/10.1016/j.neuropharm.2022.109325 (2023).
Hansen, J. Y. et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 25, 1569–1581. https://doi.org/10.1038/s41593-022-01186-3 (2022).
Tafet, G. E. & Nemeroff, C. B. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 28, 77–88. https://doi.org/10.1176/appi.neuropsych.15030053 (2016).
Godoy, L. D., Rossignoli, M. T., Delfino-Pereira, P. & Garcia-Cairasco, N. Lima Umeoka, E. H. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 12, 127. https://doi.org/10.3389/fnbeh.2018.00127 (2018). de.
Yang, Y. & Wang, J. Z. From structure to behavior in basolateral Amygdala-Hippocampus circuits. Front. Neural Circuits. 11, 86. https://doi.org/10.3389/fncir.2017.00086 (2017).
Arnsten, A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422. https://doi.org/10.1038/nrn2648 (2009).
Zhong, P. et al. Monoacylglycerol lipase Inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 39, 1763–1776. https://doi.org/10.1038/npp.2014.24 (2014).
Maldonado, R., Cabañero, D. & Martín-García, E. The endocannabinoid system in modulating fear, anxiety, and stress. Dialogues Clin. Neurosci. 22, 229–239. https://doi.org/10.31887/DCNS.2020.22.3/rmaldonado (2020).
Bouter, Y. et al. Chronic psychosocial stress causes increased Anxiety-Like behavior and alters endocannabinoid levels in the brain of C57Bl/6J mice. Cannabis Cannabinoid Res. 5, 51–61. https://doi.org/10.1089/can.2019.0041 (2020).
Marguilho, M., Figueiredo, I. & Castro-Rodrigues, P. A unified model of ketamine’s dissociative and psychedelic properties. J. Psychopharmacol. 37, 14–32. https://doi.org/10.1177/02698811221140011 (2023).
Gobira, P. H. et al. CB1 receptor Silencing attenuates Ketamine-Induced hyperlocomotion without compromising its Antidepressant-Like effects. Cannabis Cannabinoid Res. 8, 768–778. https://doi.org/10.1089/can.2022.0072 (2023).
Inserra, A. et al. Effects of repeated lysergic acid diethylamide (LSD) on the mouse brain endocannabinoidome and gut Microbiome. Br. J. Pharmacol. 180, 721–739. https://doi.org/10.1111/bph.15977 (2023).
Schmid, Y. & Bershad, A. K. Altered States and social bonds: effects of MDMA and serotonergic psychedelics on social behavior as a mechanism underlying Substance-Assisted therapy. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 9, 490–499. https://doi.org/10.1016/j.bpsc.2024.02.001 (2024).
Puxty, D. J. et al. MDMA-Induced dissociative state not mediated by the 5-HT(2A) receptor. Front. Pharmacol. 8, 455. https://doi.org/10.3389/fphar.2017.00455 (2017).
Robledo, P. Cannabinoids, opioids and MDMA: neuropsychological interactions related to addiction. Curr. Drug Targets. 11, 429–439. https://doi.org/10.2174/138945010790980330 (2010).
Funding
This study was supported by the Mitacs Accelerate Project (IT23510) to ZW and TS and the start-up fund (RES0052505) from the University of Alberta to YZ.
Author information
Authors and Affiliations
Contributions
ZW conducted the experiments, analyzed and interpreted the data, and wrote the manuscript. The use of RvB and TS helped to establish the methodologies. BR contributed to the behavioral tests and animal sacrifice. RZ assisted with some of the biochemical tests. BR, RZ, RvB, and TS reviewed and revised the manuscript. YZ and XML guided the study, supervised the research project, and provided funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have nothing to disclose.
Ethical Statement
All animal procedures strictly complied with the Guidelines of the Canadian Council on Animal Care (CCAC). All treatment and testing procedures were approved by the Animal Care and Use Committees (ACUC) of the Health Sciences Laboratory Animal Services (HSLAS) of the University of Alberta. All experiments were conducted in compliance with the ARRIVE guidelines to enhance transparency, rigor, and reproducibility. This included detailed reporting on study design, experimental conduction, and data analysis to ensure methodological consistency and reliability.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Robbins, B., Zhuang, R. et al. Psilocybin mitigates behavioral despair and cognitive impairment in treatment-resistant depression model using wistar kyoto rats. Sci Rep 15, 18432 (2025). https://doi.org/10.1038/s41598-025-03383-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03383-z