Abstract
The current method of diagnosing diabetes mellitus (DM) using fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) without oral glucose tolerance tests (OGTT) results in inaccuracy and underdiagnosis of diabetes. This study aimed to investigate the mortality of individuals with improperly classified glycemic status and suggest new screening methods for diabetes. A total of 1935 subjects were prospectively followed for 10 years, with vital status obtained through linkage to the National Health Insurance Research Database (NHIRD). Hazard ratios (HR) of all-cause mortality were analyzed. The percentage of all-cause mortality was significantly higher in subjects with improperly classified glycemic status than those correctly classified (8.3% vs. 3.6%, p = 0.004; unadjusted HR 2.537, p = 0.002). The risk of mortality associated with underdiagnosis was highest in those over the age of 60 (adjusted HR 2.043, p = 0.036). Using components of metabolic syndrome, three screening strategies were developed to determine the need for OGTT in elderly subjects with improved sensitivity in diagnosing DM. Diagnosis of diabetes based solely on FPG and HbA1c leads to underdiagnosis of glycemic status and higher all-cause mortality in older adults. Age and the presence of components of metabolic syndrome-based screening strategies can improve diagnostic accuracy and reduce the need for OGTT.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a significant global health issue that results in a considerable health and economic burden1. The current standard method of DM diagnosis involves an oral glucose tolerance test (OGTT) and measurement of HbA1c2. DM is diagnosed when any one of the following criteria is met: fasting plasma glucose ≥ 126 mg/dL, 2-h plasma glucose (OGTT-2 h PG) ≥ 200 mg/dL during OGTT, HbA1c ≥ 6.5%, or random plasma glucose ≥ 200 mg/dL with classical symptoms of hyperglycemia. However, OGTT can be cumbersome in some clinical settings and is frequently omitted during the diagnostic process of DM3.
In a real-world observational study conducted in Europe, patients were screened for diabetes following an event of coronary artery disease4. A total of 22.2% of subjects did not receive OGTT during the DM screening. Among those diagnosed with DM using OGTT and HbA1c, around 20% of them would not have been detected if OGTT was not performed. Similarly, several studies report a high percentage (13.74 to 44%) of patients who did not perform OGTT during the screening of DM5,6.
The diagnosis of DM without OGTT leads to underdiagnosis of glycemic status and may result in poorer health consequences. In the literature, a high OGTT-2 h PG is associated with a poorer cardiovascular risk factor profile, larger carotid intima-media thickness, and a higher Framingham risk score for the risk of coronary arterial disease7. Besides, OGTT-2 h PG is a prognostic indicator for subjects with acute coronary syndrome8 and myocardial infarction9. A high OGTT-2 h PG is also a well-known risk factor for diabetic microvascular complications, including diabetic retinopathy and nephropathy10. Post-prandial hyperglycemia was associated with cancer incidence and mortality in the literature11. Furthermore, glucose variability (GV) is known to be associated with higher risk of stroke, myocardial infarction and all-cause mortality12, and it has also emerged as a recognized risk factor for diabetic complications13. The information of GV could be missed if OGTT is not performed. A recent Mendelian randomization study also suggests that FPG, OGTT-2 h PG and HbA1c had different effects on various health outcomes14. Specifically, only OGTT-2 h PG, but not FPG or HbA1c, was associated with small vessel stroke and aortic aneurysm. This emphasizes the importance of OGTT. However, it remains unclear whether misdiagnosis of glycemic status due to not performing OGTT is associated with higher mortality or not.
Therefore, we conducted a prospective cohort study where we collected OGTT and HbA1c results at enrollment and followed these subjects for an average of 10 years. The aim of this study is to elucidate the impact of underdiagnosed glycemic status on all-cause mortality. We also identified target populations that were more likely to be underdiagnosed and developed screening strategies to improve diagnostic performance while minimizing the need for OGTT.
Methods
Study populations
The participants of this research were prospectively enrolled in the Taiwan Lifestyle Study, a community-based cohort study15,16,17. We recruited individuals aged 18 or above who resided in Yun-Lin County, Taiwan between 2006 and 2019. The subjects were enrolled on a volunteer basis via recruitment posters on the bulletin board at the Yun-Lin branch of National Taiwan University. Those who agreed to participate and signed written informed consent were recruited consecutively. For every study participant, we collected their demographic data, personal and family histories, blood pressure (BP), body mass index (BMI) and waist circumference (WC) measurements, as well as laboratory tests, including oral glucose tolerance tests and hemoglobin A1c (HbA1c), by study nurses and physicians. Participants were informed about and consented to the linkage of their collected data with national databases, such as the National Health Insurance Research Database (NHIRD), as stated in the written informed consent. The study protocol was approved by the institutional review board of National Taiwan University Hospital (NTUH IRB numbers: 9461701008, 200705039R, and 201412122RINC) and was conducted in accordance with the Declaration of Helsinki.
Diabetes mellitus definition and insulin resistance measurements
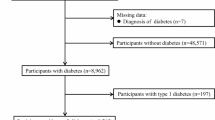
DM was defined according to the American Diabetes Association (ADA) recommendations using OGTT and HbA1c. DM was diagnosed by endocrinologists if any of the following criteria were met: fasting plasma glucose (FPG) ≥ 126 mg/dL, OGTT 2-h plasma glucose (OGTT-2 h PG) ≥ 200 mg/dL, or HbA1c ≥ 6.5%. There were 13 participants who did not complete the OGTT and HbA1c tests due to personal reasons (intolerable to OGTT in most subjects). Since we needed the complete data of OGTT and HbA1c to categorize the study subjects, these 13 subjects were excluded. Besides, since the primary objective of this study was to examine the significance of OGTT in individuals without diabetes at baseline and investigate whether undetected diabetes, resulting from the absence of OGTT, contributes to increased mortality rates, subjects with pre-existing diabetes, including those who received anti-diabetic medications (N = 41), were excluded. We also excluded 176 subjects diagnosed with DM by fasting plasma glucose ≥ 126 mg/dL and HbA1c ≥ 6.5%, as they would receive anti-diabetic medications after the diagnosis of DM, which may change the risk of further complications and mortality. Participants who were newly diagnosed with diabetes based on OGTT and/or HbA1c were referred to outpatient clinics for further evaluation and treatment in accordance with standard clinical care. Detailed study flowchart was illustrated in Fig. 1. Insulin resistance was estimated using the homeostatic model assessment (HOMA2-IR) based on plasma fasting glucose and insulin levels18.
Categorization of correctly or incorrectly classified glycemic status
Based on OGTT and HbA1c results, subjects were classified as normoglycemia, prediabetes, or DM. Among these groups, participants were categorized into the “misclassified glycemic status by FPG and HbA1c” group (the exposed group) if both FPG and HbA1c failed to identify a glycemic abnormality that was detected by the OGTT-2 h PG. For example, participants classified as normoglycemic or prediabetic by FPG and HbA1c alone, but meeting the criteria for prediabetes or diabetes based on OGTT 2 h-PG, were considered misclassified. In contrast, participants whose glycemic status was accurately identified using FPG and HbA1c, regardless of OGTT results, were assigned to the “correctly classified glycemic status” group (the reference group). In other words, if the glycemic classification based on FPG and HbA1c alone was consistent with the classification based on the combination of FPG, HbA1c, and OGTT 2 h-PG, the participant was considered “correctly classified”. If the glycemic classification based on FPG and HbA1c alone differed from the classification based on the combination of FPG, HbA1c, and OGTT 2 h-PG, the participant was considered “misclassified”.
Detailed categorization of these groups is provided in Supplementary Table 1.
Metabolic syndrome definition
Metabolic Syndrome was defined according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria, which included central obesity (≥ 90 cm or 80 cm in men or women respectively in Han Chinese populations), raised triglycerides (≥ 150 mg/dL), reduced HDL-cholesterol (< 40 mg/dL or 50 mg/dL in men or women, respectively), raised blood pressure (≥ 130/85 mmHg), and raised fasting plasma glucose (≥ 100 mg/dL)19. Metabolic Syndrome was diagnosed if 3 out of 5 components were present.
Assays for biochemical parameters
Plasma glucose and lipid profiles were measured using an automatic analyzer (Toshiba TBA 120FR, Toshiba Medical Systems Co., Ltd., Tokyo, Japan). Plasma insulin was tested using an automatic analyzer with microparticle enzyme immunoassay (Abbott AxSYM system; Abbott Laboratories, Abbott Park, IL). HbA1c was examined using an automatic analyzer (HLC-723 G7 HPLC system, Tosoh Corporation, Tokyo, Japan), which was certified by the National Glycohemoglobin Standardization Program (NGSP) and standardized to the Diabetes Control and Complications Trial (DCCT) reference assay.
Mortality record and data linkage to the NHIRD
The NHIRD was used to define mortality. The NHIRD was initiated in 1995 and utilized until present, which contains registered data and reimbursement claims of the National Health Insurance (NHI) program in Taiwan. The cause of death data (CDD) is one of the datasets in NHIRD, which contains all the registered death records in Taiwanese populations. Causes of death are coded using the International Classification of Diseases-9 or -10 (ICD-9 or ICD-10). The NHIRD contains only registered medical records, such as information on diagnoses, treatments, costs, and causes of death. We integrated the comprehensive demographic data, personal history, and laboratory data collected from the Taiwan Lifestyle Study at enrollment and linked this dataset with the NHIRD by anonymized identifiers to obtain the survival status of all the participants enrolled in the study until 30/08/2019. All enrolled subjects, including those classified as having normoglycemia, prediabetes, or DM, were followed for mortality outcomes. The follow-up duration was calculated from the time of enrollment until either the occurrence of death or the end of the follow-up period, which was 30/08/2019. The dataset of NHIRD was first accessed on 07/09/2020.
Development of screening strategies for diabetes mellitus in subjects aged over 60 years
It is widely recognized that advanced age is associated with a higher prevalence of metabolic syndrome20, and we did observe a significantly higher presence of metabolic syndrome components in individuals aged over 60 in the present study (Supplementary Table 4). Consequently, we developed three novel screening strategies for diabetes based on the presence of components of metabolic syndrome, in order to minimize the necessity of performing the OGTT while having a good diagnostic efficacy in individuals aged 60 and above.
In strategy 1, OGTT is recommended for individuals aged over 60 who exhibit any component of metabolic syndrome. In strategy 2, OGTT is suggested for individuals aged 60 and above who have either elevated blood pressure or increased waist circumference, which are the two components of metabolic syndrome that does not require blood tests. Therefore, this approach provides a more practical alternative for clinical use. Lastly, strategy 3 entails recommending an OGTT for individuals aged 60 and above who had metabolic syndrome, i.e. had three or more components of metabolic syndrome.
Statistical analyses
Categorical variables were presented as numbers (percentages), while continuous variables with normal distribution were shown as means ± standard deviation (SD). Continuous variables without normal distribution were reported as medians (inter-quartile ranges) and logarithmic transformation was performed for statistical analysis. Statistical differences were analyzed using t-tests or chi-squared tests. Cox proportional hazards models were used to estimate hazard ratios (HRs) for all-cause mortality. Significant differences between subjects with correct and incorrect glycemic status diagnoses, and between survivors and non-survivors, were considered potential confounders. Additionally, variables associated with mortality in the literature, including age21, gender21, BMI22, WC23, smoking24, alcohol25, hypertension26, HOMA2%-IR27, TG28, LDL-C29, HDL-C30, and hsCRP31, were considered confounders. Model 1 included only age. Model 2 used stepwise model selection for confounders, with or without age adjustment. Model 3 included all confounders, with or without age adjustment. Since age was a significant confounder influencing the relationship between misclassified glycemic status and mortality, a subgroup analysis was conducted for participants below and above 60 years old. The 60-year age threshold was based on WHO32 and United Nations33 definitions. Screening strategy performance was analyzed using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and numbers needed to screen (NNS). A two-tailed p-value below 0.05 was considered significant. Statistical analyses were performed using Stata/SE 14.0 for Windows (StataCorp LP, College Station, TX).
Results
We analyzed 1935 subjects over a median follow-up period of 10.2 years (interquartile range 8.7–11.7 years). Based on OGTT and HbA1c results, subjects were classified as normoglycemia, prediabetes, or DM (Supplementary Table 1). Of these groups, 156 subjects were incorrectly classified into normoglycemia (N = 101) or prediabetes (N = 55) if the diagnosis was made only by FPG and HbA1c, and these subjects were categorized as the “misclassified glycemic status by FPG and HbA1c” group (the exposed group). On the other hand, 1779 subjects whose glycemic status was correctly determined using only fasting plasma glucose and HbA1c were utilized as the reference group, the “glycemic status correctly classified” group. We compared baseline characteristics between these two groups (Table 1). The misclassified group had a significantly higher percentage of all-cause mortality compared to those correctly classified (8.3% vs. 3.6%, p = 0.004). Additionally, subjects with misclassified glycemic status had a shorter follow-up duration, were older, had a larger BMI and waist circumference, higher blood pressure, worse glycemic profiles including fasting and OGTT-2 h PG, higher fasting insulin, more insulin resistance, higher TG, higher hsCRP, and were more likely to have metabolic syndrome. Furthermore, comparison of subjects who died or survived throughout the follow-up period was presented in Supplementary Table 2. The subjects who died during follow-up exhibited a greater likelihood of misclassified glycemic status, a higher percentage of having metabolic syndrome, advanced age, a higher proportion of male gender, increased waist circumferences, elevated proportion of smoking, higher blood pressure levels, worse OGTT-2 h PG and HbA1c values, as well as elevated levels of hsCRP.
We analyzed hazard ratios (HRs) for all-cause mortality in those with misclassified glycemic status compared to those without (Supplementary Table 3). The unadjusted HR was 2.537 (95% confidence interval (CI) 1.397–4.607, p = 0.002), but after adjusting for age (Model 1), the HR decreased dramatically to 1.685 and was no longer statistically significant (95% CI 0.926–3.070, p = 0.088). In model 2, the HR of misdiagnosis of glycemic status for mortality was significantly higher after adjusted for gender, BMI, and WC (HR 2.597; 95% CI 1.421–4.747; p = 0.002). Similarly, in model 3, the HR for mortality remained statistically higher which involved multiple variables for adjustment including gender, BMI, WC, smoking, alcohol, hypertension, HOMA2%-IR, TG, LDL-C, HDL-C, and hsCRP (HR 2.451; 95% CI 1.301–4.613; p = 0.005). When age was included in Model 2 or Model 3, the HR for mortality reduced but was still in favor of increased risk based on the 95% CI, although the p value became non-significant (HR of misclassified glycemic status, in model 2 + age: 1.770 (95% CI 0.960–3.262, p = 0.067, in model 3 + age: 1.707, 95% CI 0.897–3.250, p = 0.104). These findings indicate that age is an important confounding factor in the relationship between misclassified glycemic status due to the lack of OGTT and mortality.
Therefore, we conducted a subgroup analysis by age and found that individuals over 60 were more likely to be misclassified in this age group and linked to a significantly increased risk of mortality (Table 2). The hazard ratios for mortality associated with misclassified glycemic status, stratified by age group, were illustrated in Fig. 2. In the ≥ 60 age group, those with misclassified glycemic status had higher hazard ratio of mortality (crude HR 2.082, 95% CI 1.098–3.946, p = 0.025). Multivariable adjustment analysis demonstrated that the HRs for mortality associated with misclassified glycemic status were significantly elevated in model 1, model 2, model 2 + age, model 3, and model 3 + age (HRs of 1.937, 2.505, 2.081, 2.268 and 2.043, respectively, all with p < 0.05). These findings suggest a positive association between misclassified glycemic status and increased risk of mortality in subjects with age over 60. Those aged more than 60 also had larger BMI, higher blood pressure, glucose levels, insulin resistance, triglyceride level, and hsCRP (Supplementary Table 4).
Age-stratified hazard ratios for mortality from crude (unadjusted) and adjusted models. Forest plots present hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality among individuals aged < 60 years (Panel A) and ≥ 60 years (Panel B). Model 1: adjusted for age. Model 2: adjusted for variables which were statistically significant in stepwise model selection, including gender, BMI and WC. Model 2 + age: adjusted for age, gender, BMI and WC. Model 3: adjusted for all potential confounders other than age, including gender, BMI, WC, smoking, alcohol, hypertension, HOMA2%-IR, TG, LDL-C, HDL-C and hsCRP. Model 3 + age: adjusted for age, gender, BMI, WC, smoking, alcohol, hypertension, HOMA2%-IR, TG, LDL-C, HDL-C and hsCRP.
To develop an effective screening strategy for correctly diagnosing DM and minimizing the need for OGTT, we developed three screening strategies based on age and the presence of metabolic syndrome (Fig. 3). Strategy 1 recommended OGTT and HbA1c tests for individuals aged over 60 who met any one component of metabolic syndrome. In strategy 2, individuals aged over 60 who met the blood pressure or waist circumference criteria of metabolic syndrome should undergo OGTT and HbA1c tests. Strategy 3 recommended OGTT and HbA1c tests for individuals aged over 60 who had metabolic syndrome (three or more components). Using only FPG and HbA1c for DM diagnosis had low sensitivity (74.0%). Strategies 1, 2, and 3 had higher sensitivity (89.9%, 88.7%, and 80.7%, respectively) and required fewer OGTTs to diagnose one individual with DM (4.0, 3.8, and 2.3, respectively) (Table 3).
Different screening strategies of diabetes in all study populations. (A) Strategy 1: use OGTT and HbA1c if age ≥ 60 years plus any one components of metabolic syndrome, while others using only FPG and HbA1c to diagnose DM. (B) Strategy 2: use OGTT and HbA1c if age ≥ 60 years plus either the presence of blood pressure or waist circumference component of metabolic syndrome, while others using only FPG and HbA1c to diagnose DM. (C) Strategy 3: age ≥ 60 years plus any three or more components of metabolic syndrome, while others using only FPG and HbA1c to diagnose DM. BP, blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; MS, metabolic syndrome, OGTT, oral glucose tolerance test; WC, waist circumference.
Discussion
In the present study, we showed that misclassification of glycemic status due to omission of OGTT was associated with higher mortality. Age played an important role and was associated with the percentage of incorrect diagnosis and mortality. Subjects aged over 60 had the highest percentage of incorrect diagnosis and mortality. In this age group, the presence of components of metabolic syndrome were significantly different between those with and without correct diagnosis of glycemic status. Therefore, three screening strategies to decide the need of OGTT were proposed based on the presence of components of metabolic syndrome. In strategy 1 and 2, OGTT and HbA1c were performed in those who met any components of metabolic syndrome (strategy 1) or those who met either blood pressure or waist circumference criterion (strategy 2), 19.4% and 17.6% of subjects should receive OGTT and HbA1c, and the sensitivity was 89.9% and 88.7%, respectively. In strategy 3, OGTT and HbA1c were done in those with 3 or more components of metabolic syndrome, which resulted in fewer OGTT needed (5.9%), but the sensitivity was lower (80.7%).
In the present study, we found that subjects whose glycemic status were misclassified by FPG and HbA1c alone had a higher mortality. This finding is supported by previous reports showing the prognostic value of post-load hyperglycemia during OGTT. A large cohort study in China disclosed that post-load hyperglycemia (OGTT-2 h PG ≥ 200 mg/dL) was more closely associated with incident cardiovascular events, cancers, and mortalities, compared with fasting hyperglycemia (FPG ≥ 126 mg/dL) or high HbA1c (≥ 6.5%)34. Two-hour post-load plasma glucose was used to improve the prognostic prediction in subjects with coronary artery disease or acute coronary syndrome in several studies8,35,36,37. Every one mmol/L (18 mg/dL) increase in OGTT-2 h PG, the risk of major cardiovascular events (MACE) increased by 8–11%8. In fact, the current diabetic diagnostic threshold of OGTT-2 h PG (≥ 200 mg/dL) adopted by the ADA was amended and established based on several studies regarding glycemic cut-off of two-hour post-load glucose and its relationship with risks of diabetic microvascular complications10.
The importance of OGTT-2 h PG during the screening of DM cannot be overemphasized. In our previous report, we screened subjects without a history of diabetes by OGTT and HbA1c38. Among the 8.3% of subjects with newly diagnosed DM, 52% of them had isolated post-load hyperglycemia. In other words, over half of them would not have been found to have diabetes if OGTT was not performed. Another observational study in subjects with coronary artery disease also revealed that 18.83% participants with newly diagnosed diabetes were found by OGTT-2 h PG, but not by FPG or HbA1c4. In the National Health and Nutrition Examination Surveys (NHANES), the overall prevalence of undiagnosed DM was 2.9% by FPG and HbA1c criteria39. This number increased 1.72-fold to 5% if FPG, OGTT-2 h PG and HbA1c were used to screen DM. In a recent Mendelian randomization study, it was found that FPG, OGTT-2 h PG, and HbA1c had varying effects on different atherosclerotic and thrombotic outcomes14. These findings suggest that these glycemic traits are distinct from each other, and OGTT-2 h PG may play a unique role in contributing to health outcomes, highlighting its significance and emphasizing that it should not be overlooked. Several studies have demonstrated that isolated post-load hyperglycemia is associated with significant insulin resistance, even when fasting glucose levels remain within normal or prediabetic ranges40. This supports our finding that elevated HOMA2%-IR in the misclassified group may be driven by disproportionately high fasting insulin levels in the context of early compensatory mechanisms. In the present study, 8.3% of participants would have been misclassified in terms of glycemic status if OGTT had not been performed. This misclassification may have led to delayed diagnosis and treatment, potentially contributing to the increased mortality observed in this group. These findings underscore the importance of including OGTT in glycemic screening strategies, particularly in high-risk populations. Since OGTT is time-consuming and may be cumbersome clinically, it is important to identify subjects who would benefit most if OGTT is performed.
Age was identified as an important variable affecting the accuracy to correctly classify one’s glycemic status by FPG and HbA1c only in this study. Nearly one-sixth (16.6%) of subjects had incorrect diagnosis of diabetes in elderly subjects aged over 60. Our findings were consistent with the results in the literature. Since 2010, the ADA recommended HbA1c to be one of the diagnostic criteria of glycemic status41. However, HbA1c has several limitations and can be incorrect in certain conditions, such as conditions that alter RBC’s life span10. One study in France reported a total of 18% of undiagnosed prediabetes and diabetes if only FPG and HbA1c were used in overweight or obese patients. Age and waist circumference were independently associated with high OGTT-2 h PG. In addition, with every 10-year increase in age, the risk of glycemic status misclassification due to the absence of OGTT-2 h PG increased by 28 to 78%42. Another study in the U.S. showed that aging was associated with increased HbA1c levels and decreased diagnostic specificity of HbA1c43. One recent study investigated the misclassification of prediabetes and diabetes in the National Health and Nutrition Examination Surveys (NHANES) from 2005 to 2016 using hemoglobin glycation index (HGI), the difference between one’s observed and predicted HbA1c. A total of 15% subjects showed clinically significant glycemic mismatches, which potentially lead to HbA1c-related misdiagnosis and inappropriate management, especially in older adults or non-Hispanic Black individuals44. Taken together, all these findings suggest that HbA1c and FPG alone are not sufficient to correctly diagnose glycemic status in older adults, and OGTT is needed. As compared with older adults, younger individuals are less likely to be underdiagnosed with diabetes (6.3% vs. 14.6% in our study) and tend to have a more favorable health status even if their diabetes remains undiagnosed. These factors may contribute to the lack of significance in mortality observed in our study. However, early identification of dysglycemia in younger population remains clinically important, as long-term complications may still develop without timely intervention.
Increasing the diagnostic accuracy while avoidance of unnecessary OGTT remains a challenge in the screening of diabetes. Several risk score-based strategies were proposed, such as the Finnish Diabetes Risk Score (FINDRISC)45, but the results were not good enough. In our previous report, we used FPG and A1c to determine the need of OGTT46,47. OGTTs were performed if FPG was between 100 and 125 mg/dL or HbA1c was between 6.1 and 6.4%. The sensitivity and specificity for this strategy were 85.2 and 100%, and the numbers of OGTT required were decreased by 81.8%. In the present study, we found that age was an important determinant for the correctness of the diagnosis of glycemic status. Therefore, we developed three screening strategies based on age and the presence of metabolic syndrome. Strategy 1 or strategy 2 showed high sensitivity (88.7 to 89.9%), reduced needs of OGTT (17.6 to 19.4%), and reasonable numbers needed to screen (NNS) of 3.8 to 4.0. In other words, one case of diabetes could be detected in every 3 to 4 OGTTs performed. Strategy 2 may be more feasible than strategy 1, since only blood pressure and waist circumference are needed, and blood tests are not required. For the same reason, strategy 1 needs two visits to complete the screening, whereas strategy 2 can be completed in a single visit. In strategy 3, the need of OGTT and NNS decreased to 5.9% and 2.3, respectively. However, the sensitivity also reduced to 80.7%. Since this strategy also requires two visits to be completed, it may be useful only when the need of OGTT should be minimized. Considering the potential complications and increased mortality resulted from underdiagnosed diabetes, the balance between OGTT percentage and sensitivity, and clinical feasibility, strategy 2 is more preferred to screen DM in elder subjects.
To our best knowledge, this is the first study unveiling that subjects with incorrect diagnosis of glycemic status due to lack of OGTT were associated with a higher all-cause mortality, and the consequences were more profound in subjects aged more than 60. In addition, age-specific screening strategy for diabetes was proposed for the first time in response to the fact that the percentage of underdiagnosis by merely FPG and HbA1c increased by age. Furthermore, components of metabolic syndrome were incorporated in designing the screening strategy of DM, and three strategies for screening of diabetes were proposed. One could balance the accuracy to diagnose DM and the percentage of OGTT required to choose the best screening strategy in different clinical settings and needs.
There were some limitations of this study. First, all participants in this study were Taiwanese, so the findings should be tested in other ethnic groups. Second, the recruitment of participants was based on a voluntary basis rather than random sampling, which may limit the generalizability of the results. Participants who volunteered for the study might have had a higher level of health awareness and healthier lifestyle compared to the general population. Third, only 77 out of 1935 participants (4.0%) died during the follow-up period, with 43.1% due to cancer, 12.8% due to cardiovascular disease, and 7.4% due to cerebrovascular disease. The relatively low mortality rate may limit the statistical power, especially the impact of glycemic misclassification on specific causes of death. Moreover, we were not able to obtain all comorbidities such as dementia or chronic obstructive pulmonary disease (COPD) for adjustment in every subject enrolled. Therefore, there may be some residual confounding effects from these unmeasured factors. However, it is worth noting that these differences in prevalence, health awareness and the presence of comorbidities did not impact the relationship between misclassified glycemic status and mortality, nor did they affect the sensitivity and specificity of the screening strategies. Besides, only baseline data were used. Therefore, changes of clinical factors were not considered in the analyses and in the development of screening strategy. However, using data in the baseline only has a better feasibility than using data both in the baseline and during follow-up. Last but not least, the high within-person variability of blood glucose may limit the diagnostic accuracy of the OGTT48. In addition, the one-hour OGTT has been proposed by the International Diabetes Federation (IDF) to diagnose intermediate hyperglycemia (IH) and diabetes, using OGTT-1 h PG cutoffs of 155 mg/dL and 209 mg/dL, respectively49. This approach may enable earlier detection of dysglycemia and facilitate timely intervention, owing to the relative simplicity and greater acceptability of the OGTT-1 h PG measurement, although it was not analyzed or compared in the current study.
In conclusion, we have demonstrated that diagnosis of diabetes without OGTT leads to underdiagnosed diabetes, which is associated with a higher mortality, especially in elderly adults aged over 60 years. We developed three screening strategies based on age and components of metabolic syndrome to determine if OGTT is required. These strategies could improve the accuracy on the diagnosis of DM and reduce the need for OGTT. Since subjects of diabetes identified by the strategies will be treated, it may reduce the incidence of complication by underdiagnosis by FPG and HbA1c only and improve their prognosis.
Data availability
Data and materials within this cohort study are available under request. Please contact with the corresponding author for further information.
References
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
Inzucchi, S. E. Clinical practice. Diagnosis of diabetes. N. Engl. J. Med. 367, 542–550. https://doi.org/10.1056/NEJMcp1103643 (2012).
Tomkins, M. & Smith, D. Should we continue to use the 75-g OGTT to diagnose diabetes?. Ir. J. Med. Sci. 189, 525–527. https://doi.org/10.1007/s11845-019-02134-0 (2020).
Gyberg, V. et al. Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: A report from EUROASPIRE IV–a survey from the European Society of Cardiology. Eur. Heart J. 36, 1171–1177. https://doi.org/10.1093/eurheartj/ehv008 (2015).
Drobek, N. et al. Undiagnosed diabetes and prediabetes in patients with chronic coronary syndromes-an alarming public health issue. J. Clin. Med. 10, 191. https://doi.org/10.3390/jcm10091981 (2021).
Bartnik, M. et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart 93, 72–77. https://doi.org/10.1136/hrt.2005.086975 (2007).
Marini, M. A. et al. Cardiometabolic risk profiles and carotid atherosclerosis in individuals with prediabetes identified by fasting glucose, postchallenge glucose, and hemoglobin A1c criteria. Diabetes Care 35, 1144–1149. https://doi.org/10.2337/dc11-2032 (2012).
Chattopadhyay, S., George, A., John, J. & Sathyapalan, T. Two-hour post-load plasma glucose, a biomarker to improve the GRACE score in patients without known diabetes. Cardiology 145, 553–561. https://doi.org/10.1159/000509180 (2020).
Chattopadhyay, S., George, A., John, J. & Sathyapalan, T. Two-hour post-challenge glucose is a better predictor of adverse outcome after myocardial infarction than fasting or admission glucose in patients without diabetes. Acta Diabetol. 55, 449–458. https://doi.org/10.1007/s00592-018-1114-2 (2018).
Bergman, M. et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 165, 108233. https://doi.org/10.1016/j.diabres.2020.108233 (2020).
Stattin, P. et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care 30, 561–567. https://doi.org/10.2337/dc06-0922 (2007).
Lee, D. Y. et al. Glucose variability and the risks of stroke, myocardial infarction, and all-cause mortality in individuals with diabetes: Retrospective cohort study. Cardiovasc. Diabetol. 19, 144. https://doi.org/10.1186/s12933-020-01134-0 (2020).
Ceriello, A., Monnier, L. & Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 7, 221–230. https://doi.org/10.1016/s2213-8587(18)30136-0 (2019).
Yuan, S., Mason, A. M., Burgess, S. & Larsson, S. C. Differentiating associations of glycemic traits with atherosclerotic and thrombotic outcomes: Mendelian randomization investigation. Diabetes 71, 2222–2232. https://doi.org/10.2337/db21-0905 (2022).
Lin, C. H. et al. Different cutoffs of hypertension, risk of incident diabetes and progression of insulin resistance: A prospective cohort study. J. Formos. Med. Assoc. https://doi.org/10.1016/j.jfma.2021.02.022 (2021).
Ma, W. Y. et al. Measurement of waist circumference: Midabdominal or iliac crest?. Diabetes Care 36, 1660–1666. https://doi.org/10.2337/dc12-1452 (2013).
Yu, T. Y. et al. The impact of gastric atrophy on the incidence of diabetes. Sci. Rep. 7, 39777. https://doi.org/10.1038/srep39777 (2017).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495 (2004).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752. https://doi.org/10.1161/circulationaha.105.169404 (2005).
Gouveia, É. et al. Predictors of metabolic syndrome in adults and older adults from amazonas, Brazil. Int. J. Environ. Res. Public Health 18, 1303. https://doi.org/10.3390/ijerph18031303 (2021).
Austin, P. C. & Walraven, C. The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med. Care 49, 940–947. https://doi.org/10.1097/MLR.0b013e318229360e (2011).
Bhaskaran, K., Dos-Santos-Silva, I., Leon, D. A., Douglas, I. J. & Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 6, 944–953. https://doi.org/10.1016/s2213-8587(18)30288-2 (2018).
Jacobs, E. J. et al. Waist circumference and all-cause mortality in a large US cohort. Arch. Intern. Med. 170, 1293–1301. https://doi.org/10.1001/archinternmed.2010.201 (2010).
Jacobs, D. R. Jr. et al. Cigarette smoking and mortality risk: twenty-five-year follow-up of the Seven Countries Study. Arch. Intern. Med. 159, 733–740. https://doi.org/10.1001/archinte.159.7.733 (1999).
Tian, Y. et al. Alcohol consumption and all-cause and cause-specific mortality among US adults: prospective cohort study. BMC Med. 21, 208. https://doi.org/10.1186/s12916-023-02907-6 (2023).
Aune, D., Huang, W., Nie, J. & Wang, Y. Hypertension and the risk of all-cause and cause-specific mortality: An outcome-wide association study of 67 causes of death in the national health interview survey. Biomed. Res. Int. 2021, 9376134. https://doi.org/10.1155/2021/9376134 (2021).
Ausk, K. J., Boyko, E. J. & Ioannou, G. N. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care 33, 1179–1185. https://doi.org/10.2337/dc09-2110 (2010).
Liu, J. et al. Effects of blood triglycerides on cardiovascular and all-cause mortality: A systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 12, 159. https://doi.org/10.1186/1476-511x-12-159 (2013).
Johannesen, C. D. L., Langsted, A., Mortensen, M. B. & Nordestgaard, B. G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 371, m4266. https://doi.org/10.1136/bmj.m4266 (2020).
Zhong, G. C. et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: A pooled analysis of 37 prospective cohort studies. Eur. J. Prev. Cardiol. 27, 1187–1203. https://doi.org/10.1177/2047487320914756 (2020).
Li, Y. et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 259, 75–82. https://doi.org/10.1016/j.atherosclerosis.2017.02.003 (2017).
Bautmans, I. et al. WHO working definition of vitality capacity for healthy longevity monitoring. Lancet Healthy Longev. 3, e789–e796. https://doi.org/10.1016/s2666-7568(22)00200-8 (2022).
Amuthavalli Thiyagarajan, J. et al. The UN Decade of healthy ageing: strengthening measurement for monitoring health and wellbeing of older people. Age Ageing https://doi.org/10.1093/ageing/afac147.10.1093/ageing/afac147 (2022).
Lu, J. et al. Predictive value of fasting glucose, postload glucose, and hemoglobin A(1c) on risk of diabetes and complications in Chinese adults. Diabetes Care 42, 1539–1548. https://doi.org/10.2337/dc18-1390 (2019).
Shahim, B. et al. The prognostic value of fasting plasma glucose, two-hour postload glucose, and HbA(1c) in patients with coronary artery disease: A report from EUROASPIRE IV: A survey from the european society of cardiology. Diabetes Care 40, 1233–1240. https://doi.org/10.2337/dc17-0245 (2017).
Wang, Y. et al. Postload plasma glucose but not fasting plasma glucose had a greater predictive value for cardiovascular disease in a large prospective cohort study in southwest China. Front. Cardiovasc. Med. 8, 815357. https://doi.org/10.3389/fcvm.2021.815357 (2021).
Chattopadhyay, S., George, A., John, J. & Sathyapalan, T. Postload glucose spike but not fasting glucose determines prognosis after myocardial infarction in patients without known or newly diagnosed diabetes. J. Diabetes 13, 191–199. https://doi.org/10.1111/1753-0407.13111 (2021).
Lai, Y. C. et al. Performance of homeostasis model assessment and serum high-sensitivity C-reactive protein for prediction of isolated post-load hyperglycaemia. Diabet. Med. 30, 318–325. https://doi.org/10.1111/dme.12008 (2013).
Cowie, C. C., Casagrande, S. S. & Geiss, L. S. in Diabetes in America (eds C. C. Cowie et al.) (National Institute of Diabetes and Digestive and Kidney Diseases (US), 2018).
Abdul-Ghani, M. A., Tripathy, D. & DeFronzo, R. A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29, 1130–1139. https://doi.org/10.2337/diacare.2951130 (2006).
Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2022. Diabetes Care 45, S17-s38. https://doi.org/10.2337/dc22-S002 (2022).
Cosson, E. et al. A large proportion of prediabetes and diabetes goes undiagnosed when only fasting plasma glucose and/or HbA1c are measured in overweight or obese patients. Diabetes Metab. 36, 312–318. https://doi.org/10.1016/j.diabet.2010.02.004 (2010).
Dubowitz, N. et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet. Med. 31, 927–935. https://doi.org/10.1111/dme.12459 (2014).
Staimez, L. R. et al. Potential misclassification of diabetes and prediabetes in the U.S.: Mismatched HbA1c and glucose in NHANES 2005–2016. Diabetes Res. Clin. Pract. 189, 109935. https://doi.org/10.1016/j.diabres.2022.109935 (2022).
Shahim, B. et al. Undetected dysglycaemia common in primary care patients treated for hypertension and/or dyslipidaemia: On the need for a screening strategy in clinical practice. A report from EUROASPIRE IV a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 17, 21. https://doi.org/10.1186/s12933-018-0665-4 (2018).
Li, H. Y. et al. Hemoglobin A1c for the diagnosis of diabetes: To replace or to guide oral glucose tolerance tests?. J Diabetes Investig 3, 259–265. https://doi.org/10.1111/j.2040-1124.2011.00181.x (2012).
Li, H. Y. et al. The performance of risk scores and hemoglobin A1c to find undiagnosed diabetes with isolated postload hyperglycemia. Endocr. J. 58, 441–448. https://doi.org/10.1507/endocrj.k11e-005 (2011).
Diagnosis and Classification of Diabetes. Standards of care in diabetes-2025. Diabetes Care 48, S27-s49. https://doi.org/10.2337/dc25-S002 (2025).
Bergman, M. et al. International diabetes federation position statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res. Clin. Pract. 209, 111589. https://doi.org/10.1016/j.diabres.2024.111589 (2024).
Acknowledgements
This study is partially based on the registry data from the National Health Insurance Research Database (NHIRD) provided by the Health and Welfare Data Science Center (HWDC) in the Ministry of Health and Welfare and managed by National Health Research Institutes. The authors would like to thank Miss Ying-Jhu Liao and the staff of the eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for their technical and computing assistance.
Funding
We wish to acknowledge National Science and Technology Council (NSTC) (grant number MOST 104-2314-B-002-124-MY3, MOST 106-2314-B-041-001, and NSTC 113-2314-B-002-317), National Taiwan University Hospital (grant number 114-O0032, 114-N0027), and National Taiwan University Hospital, Hsin-Chu branch (grant number 109-HCH051, 113-HCH063) for the funding support.
Author information
Authors and Affiliations
Contributions
H.-Y.L. and C.-H.L. developed the concept and design of the study. H.-Y.L., M.-S.L., S.-R.S. and C.-Y.Y. participated in the enrollment of study subjects and data acquisition. H.-Y.L. and C.-H.L. performed statistical analyses. K.-C.F., I-W.Y., S.-C.C., C.-Y.H., Y.-P.L. W.-C.W., C.-Y.Y., M.-S.L. and S.-R.S. researched data and provided critical thoughts to the discussion. C.-H.L. and H.-Y.L. drafted the manuscript. All authors contributed to the revision of the manuscript and approved the final version of the manuscript. H.-Y.L. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All enrolled subjects provided written informed consent before the study began, and the National Taiwan University Hospital’s institutional review board approved the study protocol (NTUH IRB number: 9461701008, 200705039R, and 201412122RINC).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, CH., Fan, KC., Yen, IW. et al. Diagnosis of diabetes without oral glucose tolerance test is associated with increased mortality in older adults. Sci Rep 15, 22109 (2025). https://doi.org/10.1038/s41598-025-03988-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03988-4